Abstract
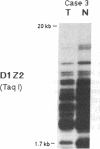
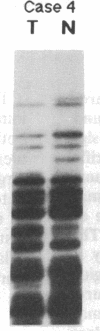
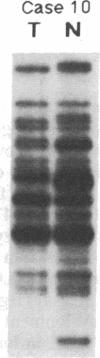
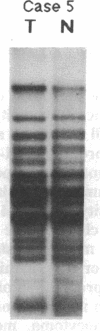
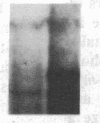
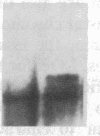
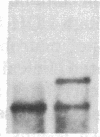
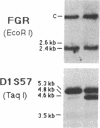
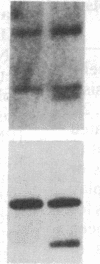
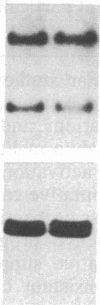
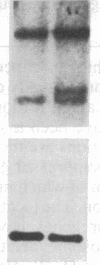
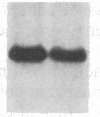
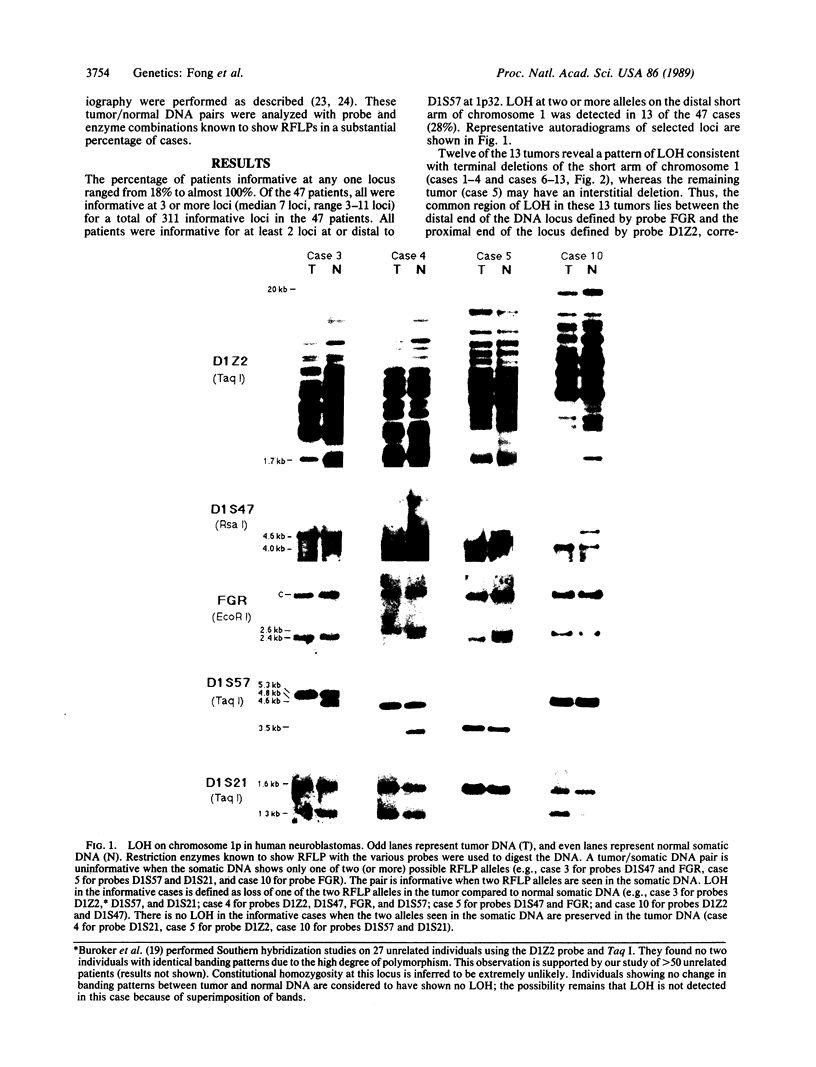
Partial monosomy of the short arm of chromosome 1 is the most consistent cytogenetic abnormality found in human neuroblastomas, but its overall frequency and significance are unclear. Using a panel of chromosome-1-specific DNA probes that identify restriction fragment length polymorphisms, we demonstrate that 13 of 47 human neuroblastomas (28%) have somatic loss of heterozygosity (LOH) at one or more loci on the distal short arm of chromosome 1. the chromosomal region that shows LOH most consistently is between 1p36.1 and 1p36.3; loss of a gene or genes in this region may be critical for the development or progression of neuroblastomas. The region of LOH in human neuroblastoma may resemble that described for pheochromocytoma, medullary thyroid carcinoma, and melanoma, which are also tumors of neural-crest origin. Although LOH for distal chromosome 1p can occur in early stages of neuroblastoma, the loss usually occurs in tumors of advanced clinical stages. LOH for the short arm of chromosome 1 correlates significantly with N-myc amplification, suggesting that these two genetic events are related. Indeed, these two lesions appear to characterize a genetically distinct subset of particularly aggressive neuroblastomas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Lidereau R., Theillet C., Callahan R. Reduction to homozygosity of genes on chromosome 11 in human breast neoplasia. Science. 1987 Oct 9;238(4824):185–188. doi: 10.1126/science.3659909. [DOI] [PubMed] [Google Scholar]
- Brauch H., Johnson B., Hovis J., Yano T., Gazdar A., Pettengill O. S., Graziano S., Sorenson G. D., Poiesz B. J., Minna J. Molecular analysis of the short arm of chromosome 3 in small-cell and non-small-cell carcinoma of the lung. N Engl J Med. 1987 Oct 29;317(18):1109–1113. doi: 10.1056/NEJM198710293171803. [DOI] [PubMed] [Google Scholar]
- Breakefield X. O., Orloff G., Castiglione C., Coussens L., Axelrod F. B., Ullrich A. Structural gene for beta-nerve growth factor not defective in familial dysautonomia. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4213–4216. doi: 10.1073/pnas.81.13.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Fong C. T., Morita M., Griffith R., Hayes F. A., Seeger R. C. Molecular analysis and clinical significance of N-myc amplification and chromosome 1p monosomy in human neuroblastomas. Prog Clin Biol Res. 1988;271:3–15. [PubMed] [Google Scholar]
- Brodeur G. M., Green A. A., Hayes F. A., Williams K. J., Williams D. L., Tsiatis A. A. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981 Nov;41(11 Pt 1):4678–4686. [PubMed] [Google Scholar]
- Brodeur G. M., Hayes F. A., Green A. A., Casper J. T., Wasson J., Wallach S., Seeger R. C. Consistent N-myc copy number in simultaneous or consecutive neuroblastoma samples from sixty individual patients. Cancer Res. 1987 Aug 15;47(16):4248–4253. [PubMed] [Google Scholar]
- Brodeur G. M. Molecular correlates of cytogenetic abnormalities in human cancer cells: implications for oncogene activation. Prog Hematol. 1986;14:229–256. [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Barrett A., Berthold F., Castleberry R. P., D'Angio G., De Bernardi B., Evans A. E., Favrot M., Freeman A. I. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988 Dec;6(12):1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M. The involvement of oncogenes and suppressor genes in human neoplasia. Adv Pediatr. 1987;34:1–44. [PubMed] [Google Scholar]
- Brodeur G. M., Tsiatis A. A., Williams D. L., Luthardt F. W., Green A. A. Statistical analysis of cytogenetic abnormalities in human cancer cells. Cancer Genet Cytogenet. 1982 Oct;7(2):137–152. doi: 10.1016/0165-4608(82)90010-3. [DOI] [PubMed] [Google Scholar]
- Buroker N., Bestwick R., Haight G., Magenis R. E., Litt M. A hypervariable repeated sequence on human chromosome 1p36. Hum Genet. 1987 Oct;77(2):175–181. doi: 10.1007/BF00272388. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Christiansen H., Lampert F. Tumour karyotype discriminates between good and bad prognostic outcome in neuroblastoma. Br J Cancer. 1988 Jan;57(1):121–126. doi: 10.1038/bjc.1988.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby J. K., Johnsen J., Nakashima P., Willems P. J., O'Brien J. S., Fowler M. L., Shows T. B., Shooter E. M., Cavalli-Sforza L. L. Pvu II RFLP at the human chromosome 1 alpha-L-fucosidase gene locus (FUCA1). Nucleic Acids Res. 1986 Dec 9;14(23):9543–9543. doi: 10.1093/nar/14.23.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Green P., Helms C., Cartinhour S., Weiffenbach B., Stephens K., Keith T. P., Bowden D. W., Smith D. R., Lander E. S. A genetic linkage map of the human genome. Cell. 1987 Oct 23;51(2):319–337. doi: 10.1016/0092-8674(87)90158-9. [DOI] [PubMed] [Google Scholar]
- Dracopoli N. C., Stanger B. Z., Ito C. Y., Call K. M., Lincoln S. E., Lander E. S., Housman D. E. A genetic linkage map of 27 loci from PND to FY on the short arm of human chromosome I. Am J Hum Genet. 1988 Oct;43(4):462–470. [PMC free article] [PubMed] [Google Scholar]
- Dracopoli N. C., Stanger B. Z., Lager M., Housman D. E. Localization of the FGR protooncogene on the genetic linkage map of human chromosome 1p. Genomics. 1988 Aug;3(2):124–128. doi: 10.1016/0888-7543(88)90142-5. [DOI] [PubMed] [Google Scholar]
- Dumanski J. P., Carlbom E., Collins V. P., Nordenskjöld M. Deletion mapping of a locus on human chromosome 22 involved in the oncogenesis of meningioma. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9275–9279. doi: 10.1073/pnas.84.24.9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Feinberg A. P., Hamilton S. H., Vogelstein B. Loss of genes on the short arm of chromosome 11 in bladder cancer. 1985 Nov 28-Dec 4Nature. 318(6044):377–380. doi: 10.1038/318377a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Hamilton S. R., Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987 Oct 9;238(4824):193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Horowitz J. M., Gerber M. R., Wang X. F., Bogenmann E., Li F. P., Weinberg R. A. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossard P. M., Coleman R. T. Human atrial natriuretic peptides (ANP) gene locus: BglI RFLP. Nucleic Acids Res. 1986 Nov 25;14(22):9223–9223. doi: 10.1093/nar/14.22.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gilbert F., Feder M., Balaban G., Brangman D., Lurie D. K., Podolsky R., Rinaldt V., Vinikoor N., Weisband J. Human neuroblastomas and abnormalities of chromosomes 1 and 17. Cancer Res. 1984 Nov;44(11):5444–5449. [PubMed] [Google Scholar]
- Goddard A. D., Balakier H., Canton M., Dunn J., Squire J., Reyes E., Becker A., Phillips R. A., Gallie B. L. Infrequent genomic rearrangement and normal expression of the putative RB1 gene in retinoblastoma tumors. Mol Cell Biol. 1988 May;8(5):2082–2088. doi: 10.1128/mcb.8.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. F., Koufos A., Gallie B. L., Phillips R. A., Fodstad O., Brøgger A., Gedde-Dahl T., Cavenee W. K. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobart P. M., Fogliano M., O'Connor B. A., Schaefer I. M., Chirgwin J. M. Human renin gene: structure and sequence analysis. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5026–5030. doi: 10.1073/pnas.81.16.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Kok K., Osinga J., Carritt B., Davis M. B., van der Hout A. H., van der Veen A. Y., Landsvater R. M., de Leij L. F., Berendsen H. H., Postmus P. E. Deletion of a DNA sequence at the chromosomal region 3p21 in all major types of lung cancer. Nature. 1987 Dec 10;330(6148):578–581. doi: 10.1038/330578a0. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Kovacs G., Erlandsson R., Boldog F., Ingvarsson S., Müller-Brechlin R., Klein G., Sümegi J. Consistent chromosome 3p deletion and loss of heterozygosity in renal cell carcinoma. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1571–1575. doi: 10.1073/pnas.85.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C., Skogseid B., Oberg K., Nakamura Y., Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988 Mar 3;332(6159):85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Lundberg C., Skoog L., Cavenee W. K., Nordenskjöld M. Loss of heterozygosity in human ductal breast tumors indicates a recessive mutation on chromosome 13. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2372–2376. doi: 10.1073/pnas.84.8.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew C. G., Smith B. A., Thorpe K., Wong Z., Royle N. J., Jeffreys A. J., Ponder B. A. Deletion of genes on chromosome 1 in endocrine neoplasia. Nature. 1987 Aug 6;328(6130):524–526. doi: 10.1038/328524a0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Nau M. M., Brooks B. J., Battey J., Sausville E., Gazdar A. F., Kirsch I. R., McBride O. W., Bertness V., Hollis G. F., Minna J. D. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985 Nov 7;318(6041):69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Johnson B. E., Minna J. D., Sakaguchi A. Y. Loss of heterozygosity of chromosome 3p markers in small-cell lung cancer. Nature. 1987 Oct 1;329(6138):451–454. doi: 10.1038/329451a0. [DOI] [PubMed] [Google Scholar]
- Nishide T., Emi M., Nakamura Y., Matsubara K. Corrected sequences of cDNAs for human salivary and pancreatic alpha-amylases [corrected]. Gene. 1984 May;28(2):263–270. doi: 10.1016/0378-1119(84)90265-8. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Sasaki M., Sugio K., Sato C., Iwama T., Ikeuchi T., Tonomura A., Sasazuki T., Miyaki M. Loss of constitutional heterozygosity in colon carcinoma from patients with familial polyposis coli. Nature. 1988 Jan 21;331(6153):273–277. doi: 10.1038/331273a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Markham A. F., Orkin S. H. Isolation of a cDNA clone for human antithrombin III. J Biol Chem. 1983 Jul 10;258(13):8389–8394. [PubMed] [Google Scholar]
- Scrable H. J., Witte D. P., Lampkin B. C., Cavenee W. K. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987 Oct 15;329(6140):645–647. doi: 10.1038/329645a0. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Rouleau G., Ozelius L. J., Lane A. H., St George-Hyslop P., Huson S., Gusella J. F., Martuza R. L. Common pathogenetic mechanism for three tumor types in bilateral acoustic neurofibromatosis. Science. 1987 Apr 17;236(4799):317–319. doi: 10.1126/science.3105060. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., de la Monte S., Atkins L., Gusella J. F., Martuza R. L. Molecular genetic approach to human meningioma: loss of genes on chromosome 22. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5419–5423. doi: 10.1073/pnas.84.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Skolnick M. H., Pearson P. L., Mandel J. L. Report of the Committee on Human Gene Mapping by Recombinant DNA Techniques. Cytogenet Cell Genet. 1985;40(1-4):360–489. doi: 10.1159/000132180. [DOI] [PubMed] [Google Scholar]
- Zbar B., Brauch H., Talmadge C., Linehan M. Loss of alleles of loci on the short arm of chromosome 3 in renal cell carcinoma. 1987 Jun 25-Jul 1Nature. 327(6124):721–724. doi: 10.1038/327721a0. [DOI] [PubMed] [Google Scholar]