Abstract
Rationale
The neuropeptide galanin and its receptors are expressed in brain regions implicated in the rewarding effects of natural stimuli and drugs of abuse. Galanin has been shown to attenuate neurochemical, physiological and behavioral signs of opiate and amphetamine reinforcement.
Objective
In the current study, we present evidence that galanin modulates neurochemical and behavioral correlates of response to exposure to cocaine.
Methods
Mice lacking the neuropeptide galanin (Gal −/−) and wild type (Gal +/+) controls were used to analyze the effects of galanin in an unbiased conditioned place preference paradigm. We then examined cocaine-induced activation of extracellular signal-0regulated kinase (ERK) activity as a marker of intracellular signaling in the mesolimbic dopaminergic pathway induced by acute cocaine administration.
Results
Gal −/− mice showed significantly greater conditioned place preference to the cocaine-paired chamber at a threshold dose of cocaine (3 mg/kg) than Gal +/+ mice, and this was reversed by administration of the galanin agonist galnon. Consistent with the results of behavioral experiments, there was a significant increase in ERK activation in the ventral tegmental area (VTA) and nucleus accumbens (NAc) of Gal −/− mice but not Gal +/+ mice following acute, systemic cocaine injection at the threshold dose. In the NAc, but not VTA, this effect was reversed by administration of galnon.
Conclusions
These data, coupled with previous studies on the effects of morphine and amphetmaine, demonstrate that galanin normally attenuates drug reinforcement, potentially via modulation of the mesolimbic dopamine system.
Keywords: galanin, addiction, cocaine, ERK, place preference
Introduction
The neuropeptide galanin decreases behavioral and neurochemical responses to morphine and amphetamine in mice (Hawes et al. 2008; Kuteeva et al. 2005a; b; Zachariou et al. 2003; Zachariou et al. 1999). Central infusion of galanin in C57BL/6J mice attenuates morphine-induced place preference (Zachariou et al. 1999), whereas morphine conditioned place preference and locmotor activation are increased in galanin null mutant (Gal −/−) mice (Hawes et al. 2008). Furthermore, overexpression of galanin or administration of galnon attenuates behavioral signs of opiate withdrawal whereas Gal−/− mice show increased opiate withdrawal signs (Zachariou et al. 2003). In addition, transgenic mice that overexpress galanin are less sensitive to the locomotor stimulatory effects of an acute injection of amphetamine (Kuteeva et al. 2005a; b). These data suggest that galanin may be an endogenous protective factor against the progression to addiction.
The mesolimbic dopamine system is critical for the rewarding properties of drugs of abuse (Koob 1992). In addition, it is clear that the extracellular signal-regulated kinase (ERK) signaling pathway is induced by administration of a number of drugs of abuse (Berhow et al. 1996; Valjent et al. 2000; Valjent et al. 2004; Valjent et al. 2005). Blocking ERK activation has been shown to block the rewarding effects of drugs of abuse, including cocaine (Girault et al. 2007; Valjent et al. 2000). Galanin and its receptors (GalR1, GalR2 and GalR3) are expressed in the mesolimbic dopamine system, as well as many other brain areas (Burgevin et al. 1995; Gustafson et al. 1996; Hawes and Picciotto 2004; Kolakowski et al. 1998; Waters and Krause 2000) and signaling through GalRs can modulate the activity of ERK in cultured neurons (Hawes et al. 2006a; b; Wittau et al. 2000) and in vivo (Elliott-Hunt et al. 2007; Hawes et al. 2008; Hobson et al. 2006). We have shown previously that mice lacking the gene encoding the galanin peptide exhibit higher levels of ERK activation in response to morphine in the ventral tegmental area (VTA), nucleus accumbens (NAc) and amygdala as compared to their wild type (Gal +/+) controls (Hawes et al. 2008). Galanin has been shown to regulate release of classical neurotransmitters, including dopamine, acetylcholine, and glutamate, in mesolimbic brain regions (Antoniou et al. 1997; Ellis and Davies 1994; Ogren et al. 1993; Tsuda et al. 1998).
In the current study, we wished to determine whether this interaction is restricted to opiates, or whether galanin could also modulate the rewarding effects of cocaine. We therefore investigated whether cocaine-mediated place preference (CPP) or induction of ERK signaling was altered in mice lacking the neuropeptide galanin. We hypothesized that mice lacking galanin would be more sensitive to cocaine CPP and would show enhanced ERK activation in the mesolimbic circuit compared to Gal +/+ mice.
Materials and Methods
Galanin wild type (Gal +/+) and null mutant (Gal −/−) mice on the 129/OlaHsd background (Wynick et al. 1998) were used for all experiments. All mice were housed 2 – 5 per cage of the same genotype in standard plastic mouse cages (Allentown Inc, Allentown, NJ). Mice had ad libitum access to rodent chow (Harlan Teklad #2018) and water. The female mice used in these studies were generated by crossing homozygous Gal +/+ or Gal −/− animals derived from Gal+/− matings. All mice were 6 – 8 months old and experimentally naïve at the start of testing. All animal studies were conducted in accordance with guidelines from the National Institutes of Health and approved by the Yale Animal Care and Use Committee.
Drugs
Galnon, a small non-peptide galanin receptor agonist that has the ability to cross the blood brain barrier (Saar et al. 2002), was purchased from Bachem (Torrance, CA). Cocaine was obtained from the National Institute on Drug Abuse (Baltimore, MD).
Cocaine Place Preference
Gal −/− and Gal +/+ mice were transported to the facility where behavioral training would take place at least two weeks prior to behavioral testing and were habituated to experimenter handling for a minimum of three days. Med Associates CPP boxes (ENV-256C Med Associates Inc, St. Albans, VT) were modified for a non-biased CPP procedure. Two conditioning chambers with retractable doors were separated by a grey neutral chamber with a grey plexiglas floor. One conditioning chamber had a wire mesh floor. The second conditioning chamber had a grid floor. The location of each animal was recorded by photocell beam breaks and time spent in each chamber was calculated using Med-PC IV software.
Mice were transferred to the testing room at least 30 min prior to behavioral testing to allow mice to acclimate to the testing room. The methods used to assess unbiased cocaine CPP were similar to those that our lab used to assess morphine CPP in these animals (Hawes et al. 2008). Subjects were injected with saline or cocaine (3 or 10 mg/kg, doses chosen to be below and above the threshold for cocaine CPP in wild type mice based on pilot testing in our laboratory) just prior to placement into the CPP apparatus. To assess baseline preference mice were injected with saline and placed inside the neutral chamber and allowed to explore both conditioning chambers for 15 min. During the conditioning phase of the experiment, mice received two conditioning sessions per day for three consecutive days. During the AM session (beginning at approximately 1000 h), mice were isolated in one conditioning chamber for 30 min following vehicle injection. During the PM session (beginning at approximately 1400 h) animals were isolated in the opposite conditioning chamber following an injection of cocaine. Animals were counterbalanced for drug-paired chamber according to genotype and baseline preference. On the day after completing the conditioning sessions, animals were tested for their preference of the two conditioning cambers identical to how baseline preference was assess. The baseline and test sessions took place at an intermediate time between the AM and PM training sessions (approximately 1200 h). Total time spent in each conditioning chamber during the baseline and test sessions was observed and cocaine CPP was measured as the amount of time spent in the drug-paired chamber on the test day relative to the amount of time spent in the drug-paired chamber during the baseline session for each individual animal.
To determine the effects of galanin in adulthood and to rule out developmental changes in Gal −/− mice, both Gal +/+ and Gal −/− mice were administered saline or 2 mg/kg galnon 15 min before receiving a saline or cocaine injection at a threshold dose (3 mg/kg).
Given the a priori hypothesis based on our prior work with morphine (Hawes et al. 2008) that the Gal −/− mice would be more sensitive to the conditioned rewarding effects of cocaine, data from the CPP experiments were analyzed using one-tailed t-tests. To determine if there was a statistically significant place preference within each genotype we analyzed data from each genotype using a single-sample t-test tested against a mean of zero. The level of significance was set at p < 0.05.
Western Blot Analysis
Gal −/− and Gal +/+ mice were either administered i.p. injections of saline or 2 mg/kg galnon 15 minutes prior to an injection of saline or 3 mg/kg cocaine. Five min after injection, brains were harvested by rapid decapitation and tissue punches of VTA and NAc were isolated and immediately frozen on dry ice (within 15 min of injection). These times were chosen based on our prior studies examining the effects of morphine on ERK phosphorylation in these mice (Hawes et al. 2008) as well as studies that have examined phosporylated ERK (P-ERK) following cocaine administration (Valjent et al. 2000). To assess levels of P-ERK western blots were performed following procedures standard in our laboratory (Hawes et al. 2008). Briefly, cell lysis buffer (50 mM Tris, 1 mM EDTA, 1 mM EGTA, 1% SDS, and 1 mM PMSF) was added to the frozen tissue punches, immediately pulse sonicated for 5 seconds. Lowry reagents (Bio-Rad, Hercules, CA) were used to determine protein concentrations according to manufacturer’s instructions. For ERK/P-ERK immunoblots, 10 µg of protein for each sample was separated on 10% poly-acrylamide gel and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Blots were blocked in 5% Milk/TBST for 1 hr, washed with TBST, and incubated overnight in primary antibody diluted in TBST. Polyclonal antisera specific for p42/p44-MAPK (ERK1/2) and the phosphorylated form of ERK1/2 (Cell Signaling, Beverley, MA) were used at a dilution of 1:1000 for both antibodies. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) immunoreactivity was used as an internal standard, by incubating the blots for 30 min at room temperature in anti-GAPDH monoclonal primary antibody (Advanced ImmunoChemical Inc., Long Beach, CA) diluted 1:5000 in TBS following the overnight incubation with a cocktail of the ERK/P-ERK antibodies. After washing 3 times for 5 min with TBST blots were incubated with secondary antibodies for 1 hr at room temperature. The blots were incubated in IR Dye 800 conjugated anti-rabbit IgG (Rockland Inc., Gilbertsville, PA) and Alexa fluor 680 conjugated anti-mouse IgG (Molecular Probes, Eugene, OR) for 1 hr at room temperature. Bands were visualized using the LI-COR Odyssey imager (LI-COR Biosciences, Lincoln, NE). Bands were quantified using the Odyssey imaging software. Levels of protein phosphorylation were determined by calculating the ratio of phosphorylated band intensity to total protein band intensity. Data were normalized to the Gal +/+ saline group to allow comparison across multiple blots by dividing each value by the average of the Gal +/+ saline group and multiplying by 100. Data were analyzed using a 3-way ANOVA (genotype X pretreatment X challenge) followed by the Newman-Keuls post hoc test. An alpha < 0.05 was considered to be significant.
Results
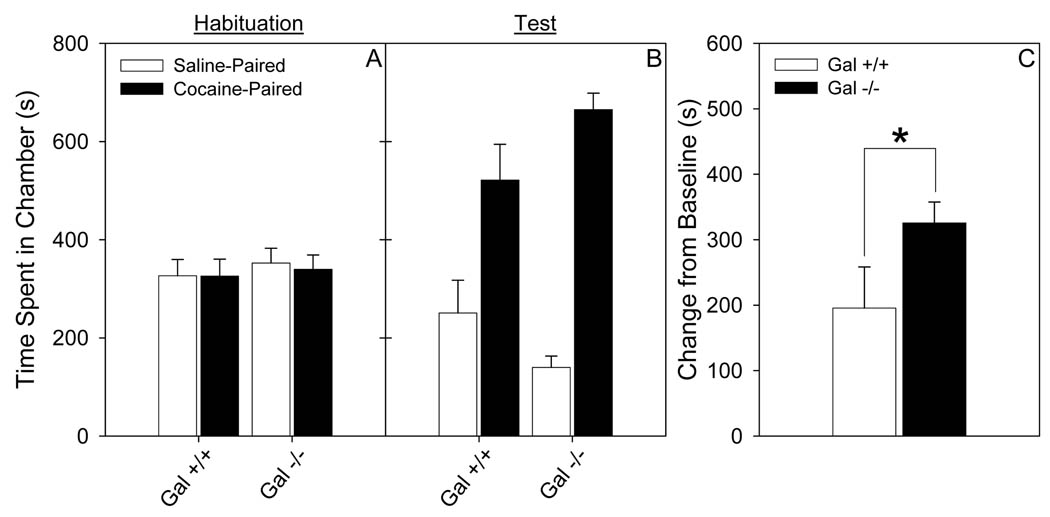
Cocaine conditioned place preference was evaluated in female Gal −/− and Gal +/+ mice. In all experiments Gal +/+ and Gal −/− mice spent equal time in both the drug- and saline-paired compartments on the baseline day. Because the mice spent equal time in both compartments on the baseline day in this unbiased procedure, the difference between time spent in the drug paired compartment on the baseline day compared to the test day was used as the primary measure of place conditioning (Cunningham et al. 2003). Gal −/− mice showed greater CPP to a threshold dose of cocaine (3 mg/kg), compared to Gal +/+ mice (t31 = 1.87, p < 0.05; Fig 1). Both genotypes of mice did show a significant place preference for 3 mg/kg cocaine (Gal +/+: t15 = 3.1, p < 0.01; Gal −/−: t16 = 10.2, p < 0.001). Gal +/+ and −/− mice spent a similar amount of time in the grey chamber on both the baseline and test days (data not shown).
Figure 1. A threshold dose of cocaine (3 mg/kg) induces significantly more cocaine place preference in Gal −/− compared to Gal +/+ mice.
Data (mean ± SEM) from conditioned place preference for cocaine (3 mg/kg) in Gal +/+ and Gal −/− mice. a. Total time spent in the drug-paired and saline-paired chambers during baseline testing. b. Total time spent in the drug-paired and saline-paired chambers during the test session. c. Change from baseline preference for the drug-paired chamber. N = 16–17. * p value < 0.05.
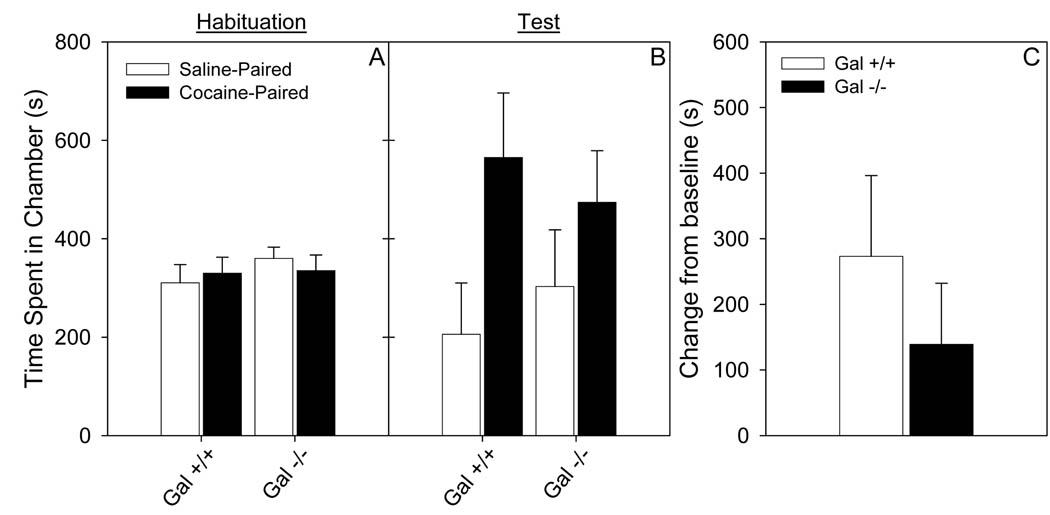
Administration of galnon (2 mg/kg) blocked the preference for a threshold dose of cocaine (3 mg/kg; Fig 2) in Gal −/− mice. There was no difference between Gal +/+ and Gal −/− mice when galnon was administered prior to cocaine. A significant place preference was observed in Gal +/+ mice (t10 = 3.0, p < 0.01), but not in Gal −/− mice, treated with galnon (p > 0.05). There was no significant difference between the Gal +/+ and Gal −/− in time spent in the grey chamber on either the baseline or test session (data not shown). These data suggest that developmental compensation does not explain the increased sensitivity to cocaine in the Gal −/− mice.
Figure 2. Galnon (2 mg/kg) decreased cocaine CPP at the threshold dose (3 mg/kg) in Gal −/− mice.
Data (mean ± SEM) from conditioned place preference for cocaine (3 mg/kg) following an injection of galnon (2 mg/kg) in Gal +/+ and Gal −/− mice. a. Total time spent in the drug-paired and saline-paired chambers during baseline testing. b. Total time spent in the drug-paired and saline-paired chambers during the test session. c. Change from baseline preference for the drug-paired chamber. N = 8–11.
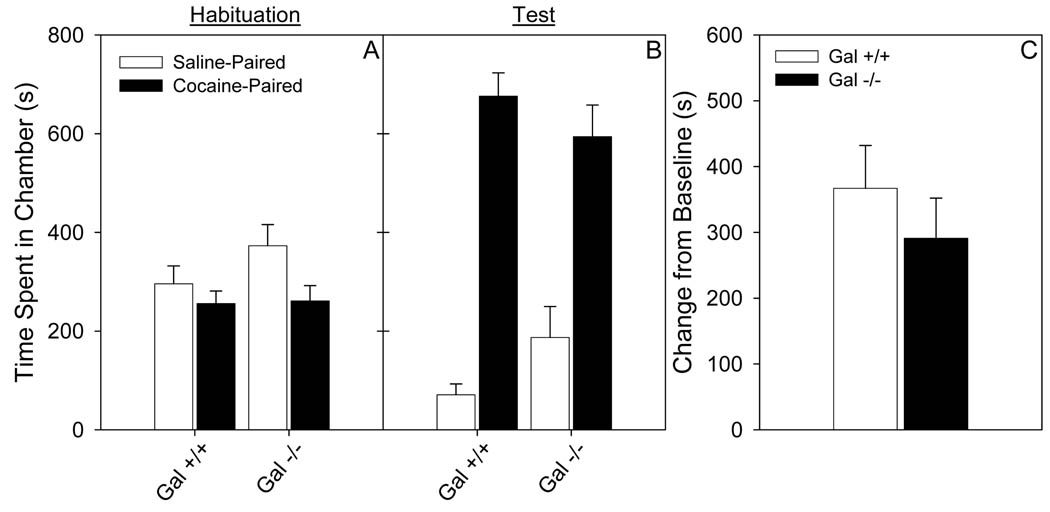
At a higher dose of cocaine (10 mg/kg), both Gal +/+ and Gal −/− mice showed a strong preference (t6 = 9.4, p < 0.001, t6 = 7.4, p < 0.001, respectively) for the cocaine-paired chamber that was not statistically different between the two genotypes (Fig 3), demonstrating that the effect of galanin on place preference is most pronounced at a threshold dose of cocaine. On the baseline and test days Gal +/+ and Gal −/− mice spent a similar amount of time the grey chamber.
Figure 3. Place preference for a supra-threshold dose of cocaine (10 mg/kg) is equivalent in Gal +/+ and Gal −/− mice.
Data (mean ± SEM) from conditioned place preference for cocaine (10 mg/kg) in Gal +/+ and Gal −/− mice. a. Total time spent in the drug-paired and saline-paired chambers during baseline testing. b. Total time spent in the drug-paired and saline-paired chambers during the test session. c. Change from baseline preference for the drug-paired chamber. N = 7/genotype.
Effects of galanin on neurochemical changes in response to cocaine
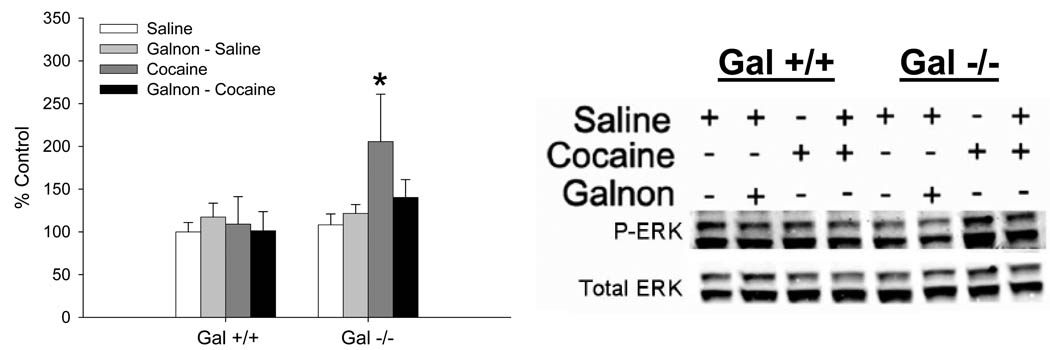
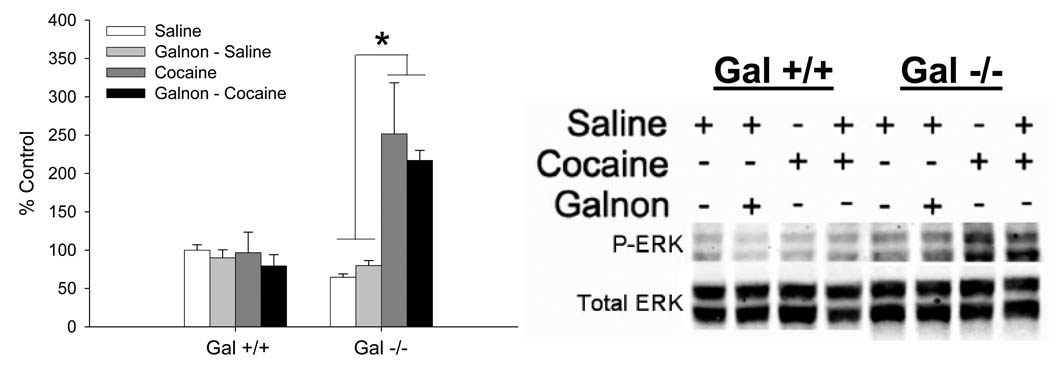
The effect of cocaine or galnon plus cocaine on the levels of P-ERK, a signaling molecule known to be regulated by cocaine, was measured. In the NAc, cocaine elevated P-ERK only in Gal −/− mice, and this was reversed by systemic administration of galnon (Fig 4). A 3-way ANOVA (genotype X pretreatment X challenge) was used to examine these data. In the NAc, there was a significant genotype X challenge interaction, therefore these data were examined separately in each genotype. There were no significant effects in Gal +/+ mice, providing evidence that 3 mg/kg cocaine does not increase P-ERK levels in Gal +/+ mice. In contrast, in Gal −/− mice there was a significant pretreatment X challenge interaction (F1,12 = 5.5, p < 0.05). Gal −/− mice that received saline prior to an acute injection of cocaine had significantly more P-ERK in the NAc compared to all other groups (p’s < 0.05), total ERK levels were not statistically different (data not shown). These data provide evidence that an acute injection of cocaine increases the level of activated ERK, and that the increase in ERK activity in the NAc can be reversed by administration of galnon.
Figure 4. Changes in ERK activation in the NAc following administration of acute cocaine (3 mg/kg) alone or galnon (2 mg/kg) prior to cocaine.
Data are presented as mean ± SEM, relative to the Gal +/+ saline group. N = 4/genotype and drug condition. * = significantly greater (p’s < 0.05) than all other Gal −/− treatment groups.
In the VTA cocaine increased P-ERK in only Gal −/− mice, but this effect was not reversed by the systemic administration of galnon (Fig 5). Since genotype significantly interacted with challenge injection, the data were considered separately for each genotype. In Gal +/+ mice there were no significant main effects or interactions. In contrast, in Gal −/− mice there was a significant main effect of challenge injection (F1,12 = 164.8, p < 0.001) that did not interact with galnon pretreatment. Gal −/− mice that received cocaine had higher levels of P-ERK than mice that received saline, independent of galnon pretreatment. No significant differences in total ERK levels were detected (data not shown).
Figure 5. Changes in ERK activation in the VTA following administration of acute cocaine (3 mg/kg) alone or galnon (2 mg/kg) prior to cocaine.
Data are presented as mean ± SEM, relative to the Gal +/+ saline group. N = 4/genotype and drug condition. * = p < 0.05.
Discussion
Drugs of abuse have different primary molecular targets in the brain, but converge to increase extracellular dopamine in the NAc (Di Chiara and Imperato 1988). Acute administration of the psychostimulant cocaine induces long-lasting electrophysiological, neurochemical and behavioral changes (Saal et al. 2003; Ungless et al. 2001; Valjent et al. 2001; Valjent et al. 2004; Valjent et al. 2005; Vanderschuren et al. 1999). In the current study, we demonstrate that knockout of the neuropeptide galanin increases susceptibility to cocaine place preference and enhances cocaine-mediated ERK activation in the mesolimbic system. The increased sensitivity to place preference is reduced by administration of the small molecular galanin agonist galnon in the NAc, but not VTA. Prior studies have shown that galanin normally reduces morphine place preference and signs of morphine withdrawal as well as amphetamine-induced locomotor activity (Hawes et al. 2008; Kuteeva et al. 2005a; b; Zachariou et al. 2003; Zachariou et al. 1999). Taken together, these studies suggest that galanin decreases the rewarding effects of different classes of abused drugs, likely through effects on the mesolimbic dopamine pathway.
While it has been shown that galanin can attenuate the responses to morphine and psychostimulants (Hawes et al. 2008; Kuteeva et al. 2005a; b; Zachariou et al. 2003; Zachariou et al. 1999), there is accumulating data that galanin increases ethanol intake (Lewis et al. 2005; Rada et al. 2004; Schneider et al. 2007). Additionally, polymorphisms in the galanin and GalR3 genes have been associated with alcoholism (Belfer et al. 2007; Belfer et al. 2006). We speculate that this effect of galanin may be due to alcohol’s caloric content (Picciotto 2008). It would be interesting to see if galanin modulates the conditioned reward effects of ethanol. It is possible that galanin may influence this behavior differently from alcohol consumption because it occurs in the absence of the caloric confound.
Galanin binds with similar affinity to all three known G-protein coupled receptors GalR1, GalR2 and GalR3. While GalR1 and GalR2 are widely expressed in the brain, there is a lower level of expression of the GalR3 receptor (Lang et al. 2007). Activation of GalR2 can increase activity of ERK as measured by a change in P-ERK in the absence of a change in total ERK, likely through activation of PKC (Elliott-Hunt et al. 2007; Hawes et al. 2006a; Zubrzycka and Janecka 2008). In contrast, activation of GalR1 decreases adenylate cyclase, cAMP, PKA, and CREB activation (Hawes et al. 2005; Hawes et al. 2006a; Lang et al. 2007). Since cocaine exposure results in P-ERK but not total ERK in the mesolimbic dopaminergic pathway (Valjent et al. 2001; Valjent et al. 2005) and inhibition of ERK activation prevents behavioral changes mediated by cocaine (Valjent et al. 2006), it seems possible that the increase in cocaine-mediated ERK activation in Gal −/− mice could be due to loss of GalR1 signaling in dopamine-responsive neurons. A decrease in GalR1 signaling would be expected to attenuate cAMP levels, resulting in a concomitant decrease in ERK activation (Vossler et al. 1997).
In the current study, galnon normalized P-ERK levels in the NAc, but not VTA, following treatment with 3 mg/kg cocaine in Gal −/− mice. These results are similar to those observed with morphine (Hawes et al. 2008). An acute injection of galnon completely reversed the activation of ERK in the nucleus accumbens, but only partially reversed this effect in the VTA (Hawes et al. 2008). Similar to the current study, Hawes et al. (2008) observed differences in P-ERK, not total ERK levels. The agonist activity of galnon appears to be somewhat more selective for GalR1 compared to GalR2 or GalR3 (Saar et al. 2002; Wu et al. 2003). It is possible that regional differences in GalR1 expression may account for differences in galnon’s ability to reverse the P-ERK in the NAc, but not VTA. Alternatively, the difference in P-ERK levels in the VTA may be due to developmental alterations in this brain area in galanin knockout mice that are not reversible in adulthood.
One mechanism that could explain the ability of galanin to decrease sensitivity for the behavioral effects of drugs of abuse is its ability to modulate dopamine release. Galanin can decrease dopamine release in striatal slices (Tsuda et al. 1998). Endogenous galanin or exogenous galnon may thus attenuate dopamine release, leading to decreased effects of cocaine. This could be due to a direct effect of galanin on dopamine terminals, or an indirect effect through modulation of other neurotransmitter systems in the mesolimbic circuit.
Galanin regulates the release of a number of neurotransmitters such as acetylcholine, glutamate, serotonin and norepinephrine in addition to dopamine (Kinney et al. 1998; Ogren et al. 1998; Pieribone et al. 1995; Tsuda et al. 1998; Wang et al. 1999). The effect of galanin on these different neurotransmitters has, in most cases, been studied in the hippocampus. In brain regions known to be important in the rewarding effect of drugs of abuse less is known. Galanin has been shown to decrease striatal acetylcholine levels using microdialysis studies in awake rats (Antoniou et al. 1997); although in the same brain region it can increase acetylcholine release in anesthetized rats (Antoniou et al. 1997; Ogren et al. 1993), suggesting that this effect is modulated by arousal. Accordingly, galanin also decreases glutamate, but not GABA, release in striatal slices (Ellis and Davies 1994). These data suggest that galanin normally decreases acetylcholine and glutamate release in the striatum. While little data is available on the effects of galanin on the VTA, it is plausible that similar effects occur in this brain area. For example, local infusion of galanin into the VTA but not the NAc modulates dopamine synthesis (Ericson and Ahlenius 1999). Therefore, effects on neurons in the VTA and/or the NAc may be responsible for the effects of galanin in the mesolimbic system. Galanin has also been shown to be involved in a number of other behavioral tasks in mice such as anxiety and depression (for review see Holmes and Picciotto 2006) which may also influence drug traits, potentially through actions in these mesolimbic brain regions.
The increased cocaine preference seen in Gal −/− mice, and the ability of the galanin agonist galnon to decrease cocaine CPP in those mice, make galanin and galanin receptors promising candidates for the development of novel therapeutics for preventing progression from drug exposure to drug abuse. The current findings, in combination with previous data showing that galanin modulates responses to morphine and amphetamine (Hawes et al. 2008; Kuteeva et al. 2005a; b; Zachariou et al. 2003; Zachariou et al. 1999), demonstrate that galanin modulates responses to drugs of abuse from different classes. It is therefore possible that genetic polymorphisms in galanin and its receptors could result in protection, or susceptibility, to drug abuse. A recent study has found an association with a SNP found in the galanin gene and heroin addiction (Levran et al. 2008). It will be interesting to determine whether genes in the galanin pathway contribute to the development of psychostimulant abuse in future candidate gene studies.
Acknowledgements
This work was supported by grants DA15425 and DA00436 from the National Institutes of Health.
Literature Cited
- Antoniou K, Kehr J, Snitt K, Ogren SO. Differential effects of the neuropeptide galanin on striatal acetylcholine release in anaesthetized and awake rats. Br J Pharmacol. 1997;121:1180–1186. doi: 10.1038/sj.bjp.0701233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfer I, Hipp H, Bollettino A, McKnight C, Evans C, Virkkunen M, Albaugh B, Max MB, Goldman D, Enoch MA. Alcoholism is associated with GALR3 but not two other galanin receptor genes. Genes Brain Behav. 2007;6:473–481. doi: 10.1111/j.1601-183X.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- Belfer I, Hipp H, McKnight C, Evans C, Buzas B, Bollettino A, Albaugh B, Virkkunen M, Yuan Q, Max MB, Goldman D, Enoch MA. Association of galanin haplotypes with alcoholism and anxiety in two ethnically distinct populations. Mol Psychiatry. 2006;11:301–311. doi: 10.1038/sj.mp.4001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgevin MC, Loquet I, Quarteronet D, Habert-Ortoli E. Cloning, pharmacological characterization, and anatomical distribution of a rat cDNA encoding for a galanin receptor. J Mol Neurosci. 1995;6:33–41. doi: 10.1007/BF02736757. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott-Hunt CR, Pope RJ, Vanderplank P, Wynick D. Activation of the galanin receptor 2 (GalR2) protects the hippocampus from neuronal damage. J Neurochem. 2007;100:780–789. doi: 10.1111/j.1471-4159.2006.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis Y, Davies JA. The effect of neuropeptides on the release of neurotransmitter amino acids from rat striatum. Neuropeptides. 1994;26:65–69. doi: 10.1016/0143-4179(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Ericson E, Ahlenius S. Suggestive evidence for inhibitory effects of galanin on mesolimbic dopaminergic neurotransmission. Brain Res. 1999;822:200–209. doi: 10.1016/s0006-8993(99)01144-0. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Smith KE, Durkin MM, Gerald C, Branchek TA. Distribution of a rat galanin receptor mRNA in rat brain. Neuroreport. 1996;7:953–957. doi: 10.1097/00001756-199603220-00025. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Brunzell DH, Narasimhaiah R, Langel U, Wynick D, Picciotto MR. Galanin protects against behavioral and neurochemical correlates of opiate reward. Neuropsychopharmacology. 2008;33:1864–1873. doi: 10.1038/sj.npp.1301579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes JJ, Brunzell DH, Wynick D, Zachariou V, Picciotto MR. GalR1, but not GalR2 or GalR3, levels are regulated by galanin signaling in the locus coeruleus through a cyclic AMP-dependent mechanism. J Neurochem. 2005;93:1168–1176. doi: 10.1111/j.1471-4159.2005.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes JJ, Narasimhaiah R, Picciotto MR. Galanin and galanin-like peptide modulate neurite outgrowth via protein kinase C-mediated activation of extracellular signal-related kinase. Eur J Neurosci. 2006a;23:2937–2946. doi: 10.1111/j.1460-9568.2006.04828.x. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Narasimhaiah R, Picciotto MR. Galanin attenuates cyclic AMP regulatory element-binding protein (CREB) phosphorylation induced by chronic morphine and naloxone challenge in Cath.a cells and primary striatal cultures. J Neurochem. 2006b;96:1160–1168. doi: 10.1111/j.1471-4159.2005.03613.x. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Picciotto MR. Characterization of GalR1, GalR2, and GalR3 immunoreactivity in catecholaminergic nuclei of the mouse brain. J Comp Neurol. 2004;479:410–423. doi: 10.1002/cne.20329. [DOI] [PubMed] [Google Scholar]
- Hobson SA, Holmes FE, Kerr NC, Pope RJ, Wynick D. Mice deficient for galanin receptor 2 have decreased neurite outgrowth from adult sensory neurons and impaired pain-like behaviour. J Neurochem. 2006;99:1000–1010. doi: 10.1111/j.1471-4159.2006.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Picciotto MR. Galanin: a novel therapeutic target for depression, anxiety disorders and drug addiction? CNS Neurol Disord Drug Targets. 2006;5:225–232. doi: 10.2174/187152706776359600. [DOI] [PubMed] [Google Scholar]
- Kinney GA, Emmerson PJ, Miller RJ. Galanin receptor-mediated inhibition of glutamate release in the arcuate nucleus of the hypothalamus. J Neurosci. 1998;18:3489–3500. doi: 10.1523/JNEUROSCI.18-10-03489.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakowski LF, Jr, O'Neill GP, Howard AD, Broussard SR, Sullivan KA, Feighner SD, Sawzdargo M, Nguyen T, Kargman S, Shiao LL, Hreniuk DL, Tan CP, Evans J, Abramovitz M, Chateauneuf A, Coulombe N, Ng G, Johnson MP, Tharian A, Khoshbouei H, George SR, Smith RG, O'Dowd BF. Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. J Neurochem. 1998;71:2239–2251. doi: 10.1046/j.1471-4159.1998.71062239.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Hokfelt T, Ogren SO. Behavioural characterisation of transgenic mice overexpressing galanin under the PDGF-B promoter. Neuropeptides. 2005a;39:299–304. doi: 10.1016/j.npep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Hokfelt T, Ogren SO. Behavioural characterisation of young adult transgenic mice overexpressing galanin under the PDGF-B promoter. Regul Pept. 2005b;125:67–78. doi: 10.1016/j.regpep.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Levran O, Londono D, O'Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction; a candidate-gene association study. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Rada P, Johnson DF, Avena NM, Leibowitz SF, Hoebel BG. Galanin and alcohol dependence: neurobehavioral research. Neuropeptides. 2005;39:317–321. doi: 10.1016/j.npep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Pramanik A, Land T, Langel U. Differential effects of the putative galanin receptor antagonists M15 and M35 on striatal acetylcholine release. Eur J Pharmacol. 1993;242:59–64. doi: 10.1016/0014-2999(93)90010-f. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Schott PA, Kehr J, Yoshitake T, Misane I, Mannstrom P, Sandin J. Modulation of acetylcholine and serotonin transmission by galanin. Relationship to spatial and aversive learning. Ann N Y Acad Sci. 1998;863:342–363. doi: 10.1111/j.1749-6632.1998.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Galanin and addiction. Cell Mol Life Sci. 2008;65:1872–1879. doi: 10.1007/s00018-008-8151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, Hokfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64:861–874. doi: 10.1016/0306-4522(94)00450-j. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Leibowitz SF, Hoebel BG. Ethanol intake is increased by injection of galanin in the paraventricular nucleus and reduced by a galanin antagonist. Alcohol. 2004;33:91–97. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Saar K, Mazarati AM, Mahlapuu R, Hallnemo G, Soomets U, Kilk K, Hellberg S, Pooga M, Tolf BR, Shi TS, Hokfelt T, Wasterlain C, Bartfai T, Langel U. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc Natl Acad Sci U S A. 2002;99:7136–7141. doi: 10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Tsuda S, Nishio I, Masuyama Y, Goldstein M. Effects of galanin on dopamine release in the central nervous system of normotensive and spontaneously hypertensive rats. Am J Hypertens. 1998;11:1475–1479. doi: 10.1016/s0895-7061(98)00168-x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Valjent E, Caboche J, Vanhoutte P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory? Mol Neurobiol. 2001;23:83–99. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schoffelmeer AN, Mulder AH, De Vries TJ. Dopaminergic mechanisms mediating the long-term expression of locomotor sensitization following pre-exposure to morphine or amphetamine. Psychopharmacology (Berl) 1999;143:244–253. doi: 10.1007/s002130050943. [DOI] [PubMed] [Google Scholar]
- Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Wang HY, Wild KD, Shank RP, Lee DH. Galanin inhibits acetylcholine release from rat cerebral cortex via a pertussis toxin-sensitive G(i)protein. Neuropeptides. 1999;33:197–205. doi: 10.1054/npep.1999.0024. [DOI] [PubMed] [Google Scholar]
- Waters SM, Krause JE. Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience. 2000;95:265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- Wittau N, Grosse R, Kalkbrenner F, Gohla A, Schultz G, Gudermann T. The galanin receptor type 2 initiates multiple signaling pathways in small cell lung cancer cells by coupling to G(q), G(i) and G(12) proteins. Oncogene. 2000;19:4199–4209. doi: 10.1038/sj.onc.1203777. [DOI] [PubMed] [Google Scholar]
- Wu WP, Hao JX, Lundstrom L, Wiesenfeld-Hallin Z, Langel U, Bartfai T, Xu XJ. Systemic galnon, a low-molecular weight galanin receptor agonist, reduces heat hyperalgesia in rats with nerve injury. Eur J Pharmacol. 2003;482:133–137. doi: 10.1016/j.ejphar.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Wynick D, Small CJ, Bloom SR, Pachnis V. Targeted disruption of the murine galanin gene. Ann N Y Acad Sci. 1998;863:22–47. doi: 10.1111/j.1749-6632.1998.tb10681.x. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Brunzell DH, Hawes J, Stedman DR, Bartfai T, Steiner RA, Wynick D, Langel U, Picciotto MR. The neuropeptide galanin modulates behavioral and neurochemical signs of opiate withdrawal. Proc Natl Acad Sci U S A. 2003;100:9028–9033. doi: 10.1073/pnas.1533224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Parikh K, Picciotto MR. Centrally administered galanin blocks morphine place preference in the mouse. Brain Res. 1999;831:33–42. doi: 10.1016/s0006-8993(99)01476-6. [DOI] [PubMed] [Google Scholar]
- Zubrzycka M, Janecka A. Interactions of galanin with endomorphin-2, vasopressin and oxytocin in nociceptive modulation of the trigemino-hypoglossal reflex in rats. Physiol Res. 2008;57:769–776. doi: 10.33549/physiolres.931287. [DOI] [PubMed] [Google Scholar]