Abstract
The Inhibitor of Growth (ING) gene family encodes structurally related proteins that alter chromatin to regulate gene expression and cell growth. The initial member, ING1, has also been proposed to function as a tumor suppressor in human cancer based on its ability to suppress cell growth and transformation in vitro. Mouse Ing1 produces two proteins (p31 and p37) from differentially spliced transcripts. We have recently generated p37Ing1b-null mice and observed spontaneous follicular B-cell lymphomagenesis in this model to show that ING proteins can function in vivo as tumor suppressors. In this present report, we examine the role of p37Ing1b in the regulation of B-cell growth and explore the relationship between p37Ing1b and p53-mediated tumor suppression. Our results indicate that p37Ing1b inhibits the proliferation of B cells and follicular B cells regardless of p53 status, and loss of p53 greatly accelerates the rate of B-cell lymphomagenesis in p37Ing1b-null mice. However, in contrast to the highly penetrant follicular B-cell lymphomas observed in p37Ing1b-null mice, mice lacking both p37Ing1b and p53 typically present with aggressive diffuse large B-cell lymphomas (DLBL). Analysis of marker gene expression in p37Ing1b/p53 null tumors indicates that the double-null mice develop both nongerminal center and germinal center B-cell–like DLBL, and also documents up-regulation of nuclear factor-κB activity in p37Ing1b/p53-null B cells and B-cell tumors. These results confirm that p53 mutation is an important mechanistic step in the formation of diffuse large B-cell lymphomas and reveals a p53-independent role for Ing1b in suppressing B-cell tumorigenesis.
Introduction
Follicular B-cell lymphoma (FL) and diffuse large B-cell lymphoma (DLBL) account for approximately half of all malignant, non–Hodgkin lymphomas in adults (1–3). Patients with FL typically display microscopic accumulations (follicles) of CD45R/B220+ B cells in lymph nodes. The median survival time for FL patients is 8 to 10 years with a variable disease course that is usually protracted with multiple relapses after treatment (1, 4). Eventually, the tumor becomes resistant to chemotherapy and can undergo transformation to a more aggressive phase that is often fatal to the patient. In contrast, DLBL is a more aggressive type of lymphoma but with a more varied clinical course (5, 6). Patients with DLBL display large, CD45R/B220+ lymphocytes in their tumor masses and a relatively high frequency of widespread organ involvement. DLBL can arise either de novo from mature germinal center (GC) B cells (GCB) or via transformation from a less aggressive B-cell lymphoma, such as FL (7). Although the disease responds initially to chemotherapy, a durable remission occurs in fewer than half of treated DLBL patients.
Several groups have used either cDNA microarrays or immunohistochemistry to divide DLBL into three subgroups with prognostic significance; GCB-like, activated B-cell–like (ABC), or type 3 expression profile (8, 9). The expression of CD10, BCL-6, and IRF-4 (MUM1) are used to differentiate the GCB from ABC subtypes. GCB-like DLBL usually expresses CD10 but can also have a CD10-, BCL-6+, IRF-4– signature (10). This subgroup has the best prognosis with 60% of patients surviving for 5 years or more (11). ABC DLBL, also called non-GCB, can have several different expression signatures, such as CD10–, BCL-6–, or CD10–, BCL-6+, IRF-4+. ABC-DLBL has a less favorable prognosis with only ~35% of patients surviving to 5 years (11). The third subgroup of DLBL encompasses those cases that do not express genes characteristic of either the ABC or GCB types. It also has a poor prognosis similar to ABC-DLBL but is less well-studied (12).
Despite the morphologic heterogeneity of human B-cell lymphomas, a number of common genetic alterations have been observed. The most prevalent is a t(14;18) translocation seen in human FL, which results in BCL-2 overexpression and protection of cells from apoptosis. Because the t(14:18) translocation is also detected in healthy human B cells, Bcl-2 overexpression is thought to be necessary but not sufficient to induce FL. Furthermore, mice harboring an Eμ promoter-Bcl-2 transgene fail to develop spontaneous lymphoma (13), and overexpression of BCL-2 is not observed in all human FL. These data suggest that an alternative prosurvival mechanism may exist in these tumors. In support of this finding, overexpression of MCL-1 has also been detected in human B-cell lymphomas (14). MCL-1 is an antiapoptotic BCL-2 family member required for survival of both T and B cells (15), and MCL-1 transgenic mice develop widely disseminated B-cell lymphomas displaying a variety of histologic subtypes, including FL and DLBL (16). In addition, BCL-6, a critical regulator of the GC response wherein B-cells undergo antigen-driven somatic hypermutation to generate high affinity antibodies, is another prosurvival BCL-2 family member often dysregulated in B-cell lymphoma. Chromosomal translocations involving BCL-6 have been observed in 15% to 40% of human DLBL and 5 to 10% of FL cases (17), and transgenic mice expressing exogenous Bcl-6 in B-cells developed a GCB-like subtype of DLBL (18). Collectively, these findings support a role for overexpression of various BCL-2 family members in the pathogenesis of B-cell lymphomas.
Mutations in the p53 tumor suppressor gene have been observed in many types of human lymphomas and often correlate with a poor patient prognosis. Furthermore, mutations in p53 have been proposed to play a role in the transformation of FL to a more aggressive DLBL (19–21). However, recent work suggests that p53 may have a limited role in the transformation of FL to DLBL (22), and mice either deficient for p53 or bearing a transgene encoding the p53-inhibitor Mdm2 do not develop FL or DLBL. Rather, these mouse models develop CD4+/CD8+ T-cell lymphomas, B220+ marginal zone B-cell lymphomas, or mixed lineage T-cell and B-cell lymphomas (23–26). Thus, the precise role of p53 in suppressing the formation or transformation of FL and DLBL is unclear.
Recently, studies of Ing1-mutated mice have revealed a role for Inhibitor of Growth (ING) proteins in suppressing B-cell lymphomagenesis (27, 28). ING1, a member of the ING gene family, has been proposed to regulate a number of biological processes, including nucleotide excision repair, chromatin remodeling, proliferation, apoptosis, and senescence (29). In addition, ING1 has been proposed to function as a tumor suppressor by regulating p53 activity (29–32). Mouse Ing1 encodes two different proteins (p31 and p37) from differentially spliced transcripts (33). Approximately 20% of mice lacking both p31 and p37 Ing1 proteins developed a B220+, B-cell lymphoma by age 23 months (28). A small percentage of these tumors were biphenotypic, expressed both B220 and CD3 surface antigens, and were classified as follicular (FO) center B-cell lymphomas (28). We have previously generated mice deficient solely for p37Ing1b, the most prevalent isoform of Ing1. These mice developed spontaneous FO B220+ lymphomas between ages 1 and 2 years, and analysis of the growth and response of p37Ing1b-deficient mouse embryonic fibroblasts (MEF) and thymocytes to DNA damaging agents revealed that p37Ing1b inhibited MEF proliferation and suppressed thymocyte apoptosis in a p53-independent manner (27). In the present study, we examined the role of p37Ing1b in B-cell growth and apoptosis, and explore the relationship between p37Ing1b and p53 in tumor suppression. Our data indicate that p37Ing1b inhibits FO B cell proliferation regardless of p53 status and that p37Ing1b and p53 cooperate to suppress the formation of non-GC and GCB-like DLBL. In addition, we find that nuclear factor-κB (NF-κB) activity is up-regulated in the absence of p37Ing1b, supporting a role for mouse p37Ing1b in regulating NF-κB signaling. These results reveal a p53-independent role for p37Ing1b in suppressing B-cell lymphomagenesis and show that p53 deletion facilitates the formation of DLBL in a mouse model of FL.
Materials and Methods
Mice and tumor assay
The generation of p37Ing1b-null mice and p37/p53-double null mice was described previously (27). The p37Ing1b-null mice were backcrossed to C57Bl/6 strain for six generations, and cohorts of these mice and of p37/p53-double null mice, wild-type (wt) mice, and p53-null mice (34) all on a C57Bl/6 inbred background were collected and aged to perform a tumor assay. Mice displaying obvious tumors or signs of reduced vitality were euthanized and tissues were harvested for fluorescence-activated cell sorting (FACS) analysis or fixed in 10% formalin for immunohistochemistry. Samples were also flash frozen in liquid nitrogen for use in quantitative reverse transcription-PCR (qRT-PCR) analysis. Formalin fixed, paraffin-embedded sections were stained with either H&E or with an antibody to CD45R/B220 (BD PharMingen; 1:50) or to Bcl-6 (Santa Cuz; 1:50) before being analyzed by microscopy. All mice were maintained and used in accordance with both federal guidelines and those established by the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School.
Cell sorting
Single cell suspensions of spleens harvested from p37/p53 double null mice at ages 6 to 8 wk were stained with CD45R/B220-PE (eBiosciences; 1:200) or IgD-PE (eBiosciences; 1:200) and FACS sorted. Resulting purity of either total B-cell (B220+) or FO B-cell (IgDHi) populations were >95%. B-cells were maintained in RPMI 1640 supplemented with 5% FCS, 2 mmol/L l-glutanine, 100 μg/mL streptomycin, 100 U/mL penicillin, and 50 μmol/L 2-mercaptoethanol (Life Technologies-Bethesda Research Laboratories) and cultured at 37°C and 10% CO2. Double positive (DP) thymocytes were sorted using CD4-PE (BD PharMingen; 1:250) and CD8-APC (BD PharMingen; 1:100) antibodies and sorted by FACS. RNA from sorted DP T-cells was purified using Trizol reagent from Invitrogen according to the manufacturer's instructions, and cDNA was generated using a first-strand cDNA synthesis kit from Invitrogen.
Analysis of B-cell subsets
Spleens were processed to single-cell suspension by pressing between frosted glass slides, and filtered through nylon mesh. RBC were lysed using brief hypotonic shock and the remaining cells were resuspended in biotin-, flavin-, and phenol red–deficient RPMI 1640 (Invitrogen) staining medium containing 10 mmol/L Hepes (pH 7.2), 0.02% sodium azide, 1 mmol/L EDTA, 3% newborn calf serum, and treated 10 min on ice with 2.4G2 Fc block (BD Biosciences). Cells were incubated for 20 min on ice with primary antibodies, washed, and biotin-stained cells were incubated with streptavidin-pacific blue (Molecular Probes/Invitrogen) for 15 min on ice. After two washes, cells were resuspended in 1 mg/mL propidium-iodide to exclude dead cells. Primary antibodies included antibodies specific for: CD24 (clone 30-F1) FITC or PE, CD45R/B220 (RA3-6B2) APC, IgM (II/41) biotin or FITC, IgD (11–26) PE, CD93/AA4.1 APC, and CD23 (B3B4) biotin. All reagents except when noted were from BD Biosciences, eBiosciences, Southern Biotechnology Associates, Inc., or CALTAG Laboratories/Invitrogen. Flow cytometry was performed on a 3-laser 12-color LSR II (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Proliferation and apoptosis assays
Incorporation of [3H] thymidine was used as a measure of proliferation as described previously (35). In brief, sorted 2 × 105 B cells were plated into wells of a 96-well plate with 200 μL of RPMI. After 24 h of culture, cells were pulsed with [3H] thymidine (1 μCi per well) for 16 h before harvest. Incorporated radioactivity was quantified with a Liquid Scintillation and Luminescence Counter (Perkin-Elmer Wallace, Inc.). Apoptosis assays were described previously (36). In brief, B-cells were plated as before and then treated with either increasing amounts of anti-IgM F(ab’)2 (Jackson Immunoresearch), 250 rads γ-irradiation, 100 nmol/L dexamethasone (Sigma), or allowed to die by neglect. After 24 h after IR or 100 nmol/L dexamethasone treatment or 16 h after IgM treatment, cells were collected, stained for propidium iodide, and analyzed by FACS. Additionally, mice were treated with 10Gy IR and spleens were harvested 8 h later. Single-cell suspensions of splenocytes were stained with B220, Annexin V, and 7-AAD to measure apoptosis as previously described (36).
qRT-PCR
QRT-PCR was done as previously described (37). The primers used were as follows: Mcl-1, AGTCAGCACAGCTTTCCTGTCAGA and TGCCAATCCAAGAATGCCAATCCC; Bcl-2, CTCGTCGCTACCGTCGTGACTTCG and GTGGCCCAGGTATGCACCCAG; Bcl-6, GCAACGAATGTGACTGCCGTTTCT and TTTCTCACCCGTGTGGACAGTCTT; CD10, TTCTGTGGCCAGACTGATTCGTCA and GCAGCATTGGGTCATTTCGGTCTT; Irf4, GAGCTGCAAGTGTTTGCTCACCAT and ACAGTTGTCTGGCTAGCAGAGGTT; RelA (p65), TGTGTTGATAGCTCCTGCTTCGGT and ATCAAGGTGTACAGGAATCCGCGT; Il-6, AGTCACAGAAGGAGTGGCTAAGGA and TCTGACCACAGTGAGGAATGTCCA; and Ef1α, AGCTTCTCTGACTACCCTCCACTT and GACCGTTCTTCCACCACTGATT. All primers were amplified using 62°C for an annealing temperature and 24 cycles to keep the products in the linear range. PCR products were run on a 2% agarose gel with SYBR green to visualize the bands.
Results
Mice deficient for p37Ing1b have an expanded FO B-cell compartment
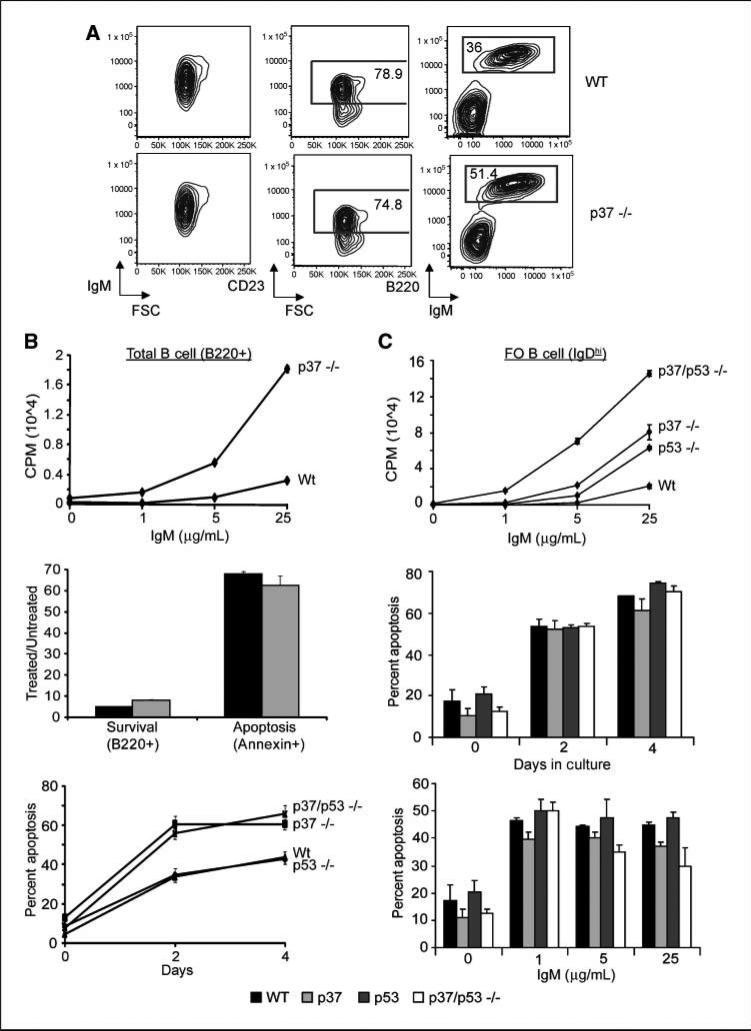
To characterize the B-cell compartment in p37Ing1b-deficient mice, single-cell suspensions were prepared from spleens isolated from 6-month-old, asymptomatic mice and FACS analysis was performed. CD45R/B220 staining revealed a subtle expansion of splenic B-cell lymphocytes in p37Ing1b null mice compared with age-matched wt mice (51.4% versus 36% in wt), with a majority of the B220+ cells also staining positive for both IgM and CD23, a marker of FO cells (Fig. 1A). These data suggest that loss of p37Ing1b leads to an increase in the number of mature FO B cells in adult mice, consistent with a role for p37Ing1b in regulating the growth of these cells. Spleens harvested from mice deleted for both p37Ing1b and p53 revealed a similar expansion of these mature B cells (data not shown).
Figure 1.
Proliferation and apoptosis in p37-null and p37/p53-double null total B cells and FO B cells. A, FACS analysis of spleen cells from a healthy 8-mo-old wt control mouse and matched p37-null mouse. FSC is forward scatter (proportional to cell size). Left and right plots, all live spleen cells within the lymph scatter gate; middle plots, data from CD23+ B cells. B, [3H] thymidine uptake assay of Wt, p53-null, p37-null, and p37/p53-double null purified total B cells (CD45R/B220+; top). Assay was performed in triplicate and repeated twice. B cells were treated with the indicated amount of anti-IgM and harvested 24 h for [3H] thymidine uptake assay. Middle, mice were treated with 10Gy ionizing radiation and spleens were harvested 8 h later. Single cell suspensions were then stained with B220, Annexin V, and 7-AAD before being analyzed by FACS. Shown is the ratio of treated versus untreated cells that were positive for B220 or Annexin V. Experiment was done in triplicate and repeated at least thrice. Bottom, apoptosis assay on purified total B cells (B220+) from Wt, p53-null, p37-null, and p37/p53-double null mice treated with 100 nmol/L dexamethasone. Plots show percent of sub-G1. Assay was done in triplicate and repeated twice. C, [3H] thymidine uptake assay of Wt, p53-null, p37-null, and p37/p53-double null–purified FO B cells (IgDhi; top). Assay was done as in B. Middle and bottom, apoptosis assay on purified FO B cells (IgDhi) from wt, p53-null, p37-null, and p37/p53-double null mice. FO B cells were either left in prolonged culture for 4 d, with cells being harvested and stained with propidium iodide every other day, or FO B cells were treated with increasing amounts of IgM and harvested 16 h later. Plots show percent of sub-G1. Assays were done in triplicate and repeated twice.
Proliferation, but not apoptosis, is altered in p37Ing1b and p37/p53 deficient B cells
To further investigate the role of p37Ing1b in B-cell growth, we isolated either total CD45R/B220+ B cells or FO (IgDhi) B cells from the spleens of adult wt, p37Ing1b-null, p53-null, and p37/p53-double null mice by FACS sorting using either anti-B220 or IgD-PE antibodies. Because anti-IgM F(ab’)2 antibodies activate B cells by cross-linking of the B-cell receptor complex (BCR) to initiate cell proliferation and death, we treated either total B cells or FO B cells with increasing amounts of anti-IgM antibody for 16 hours and measured cell proliferation via [3H] thymidine incorporation (Fig. 1B and C). Both total B cells (Fig. 1B, top) and FO B cells (Fig. 1C, top) isolated from p37Ing1b-deficient mice displayed increased proliferation relative to their wt counterparts. Although deletion of p53 also increased the proliferative response of FO B cells, codeletion of both p37Ing1b and p53 further increased the proliferation of the IgDhi, FO B cells in response to BCR activation. As FO B cells from p37/p53-double null mice displayed even greater proliferation than observed in either p37-null or p53-null cells, these data indicate that p37Ing1b does not require functional p53 to inhibit FO B cell growth. Rather, the p37 and p53 tumor suppressors cooperate in negatively regulating the proliferation of these cells.
To confirm that the increased numbers of FO B cells observed both in vivo in p37 Ing1b-null mice and in the in vitro proliferation assays were due to increased cell proliferation and not a reflection of altered cell death, we examined apoptosis in p37 Ing1b-null cells. Wt or p37Ing1b-null mice were mock treated or exposed to 10 Gy ionizing radiation. Eight hours after IR treatment, single-cell suspensions of splenocytes were prepared from the mice and stained with B220, Annexin V, and 7-AAD to determine the percent of cells undergoing apoptosis. No difference in cell death was observed between wt and p37Ing1b-null splenocytes, as measured by depletion of B220+ cells or by increased Annexin V + staining (Fig. 1B, middle). To confirm these in vivo results, total B-cells (B220+) and FO B-cells (IgDhi) were isolated from the spleens of adult wt, p37Ing1b-null, p53-null, and p37/p53-double null mice and grown in prolonged culture or treated with increasing amounts of anti-IgM or dexamethasone to induce cell death. Sub-G1 content after staining of the B220+ or IgDhi cells with propidium iodide was used to identify the percentage of apoptotic cells in each population (Fig. 1B, bottom; Fig. 1C, middle and bottom). Although prolonged culture in vitro or activation of BCR by anti-IgM treatment increased apoptosis in FO B cells, the presence or absence of p37Ing1b did not alter the apoptotic response of the FO B cells. In addition, there was no difference in the apoptotic response of wt, p37 Ing1b-null, p53-null, or p37/p53-double null FO B-cells to treatment in vitro with either 2.5 Gy ionizing radiation or with 100 nmol/L dexamethasone (data not shown). Thus, although p37Ing1b does alter apoptosis in total B cells when treated with dexamethasone, a p53-independent inducer of cell death, p37Ing1b does not seem to play a role in regulating apoptosis in FO B cells in response to these stimuli. The FO B-cell data contrast with the increased apoptosis we observed previously in p37Ing1b-null thymocytes (27), and confirm that the increase in FO B-cell numbers observed in adult p37 Ing1b-null mice is likely due to increased proliferation of these cells.
p37/p53 double null mice primarily develop aggressive B-cell lymphomas
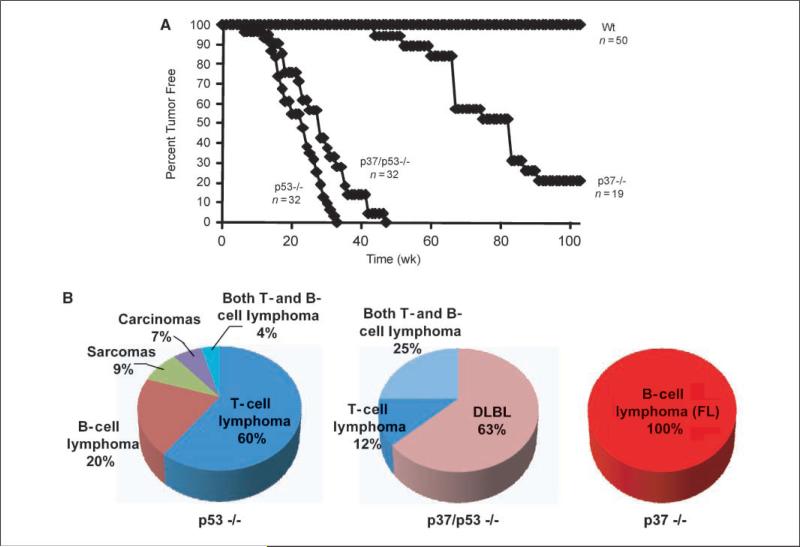
The increase in FO B-cell proliferation when codeleted for p37Ing1b and p53 suggests that p37Ing1b and p53 may cooperate to suppress B-cell tumorigenesis. To test this hypothesis, we collected cohorts of wt mice, p37Ing1b-null mice, p53-null mice, and p37/p53 double-null mice and compared the rate of spontaneous tumor formation (Fig. 2A) and the spectrum of tumor types (Fig. 2B) in these mice. The p37Ing1b-null mice presented with FL between ages 1 and 2 years, in agreement with our previous report (27). Three quarters of the p37Ing1b-null cohort developed spontaneous cancer, whereas wt mice did not develop tumors during this time. Tumors arising in p37Ing1b-null mice were predominantly localized to the lymph or spleen, with little or no involvement of peripheral tissues. As expected, all p53-null mice succumbed to spontaneous tumorigenesis within 10 months, with 75% of the tumors classified as lymphomas and the remainder a mix of sarcomas and carcinomas. As previously reported, two-thirds of the p53-null lymphomas formed in the thymus and were composed of CD4+CD8+ T cells, whereas the remaining third of the lymphomas formed in the spleen and were classified as marginal zone B-cell lymphomas or as polymorphic tumors containing both B cells and T cells (23–25, 34, 38). Similarly, p53-null mice codeleted for p37 rapidly formed spontaneous tumors by age 45 weeks (Fig. 2A). However, all of the p37/p53-double null mice presented with lymphoma, with three quarters (17/23) of the p37/p53-null mice displaying enlarged spleens, discolored and enlarged livers, and lymph node involvement. Microscopic analysis of H&E-stained tumor samples isolated from p37/p53-double-null mice revealed evidence of a lymphoblastic lymphoma involving spleen, liver, and/or mesenteric lymph node (Fig. 3A). In contrast to tumorigenesis in p53-null mice, only a small subset (6 of 23) of lymphomas arising in p37/p53-double null mice formed in the thymus (Fig. 2B).
Figure 2.
Deletion of p53 alters the rate and spectrum of lymphomagenesis in p37-null mice. A, tumor curves of a cohort of mice that were deficient for p53, p37, or both tumor suppressors. Wt mice did not present with tumors during this time period. B, relative percentage of different tumor types arising spontaneously in p53-null, p37/p53-double null, and p37-null mice. Whereas 100% of tumors arising in p37-null mice were classified as FL, a majority of the tumors present in p37-null mice codeleted for p53 were DLBL. No tumors other than lymphomas were present in p37/p53-double null mice.
Figure 3.
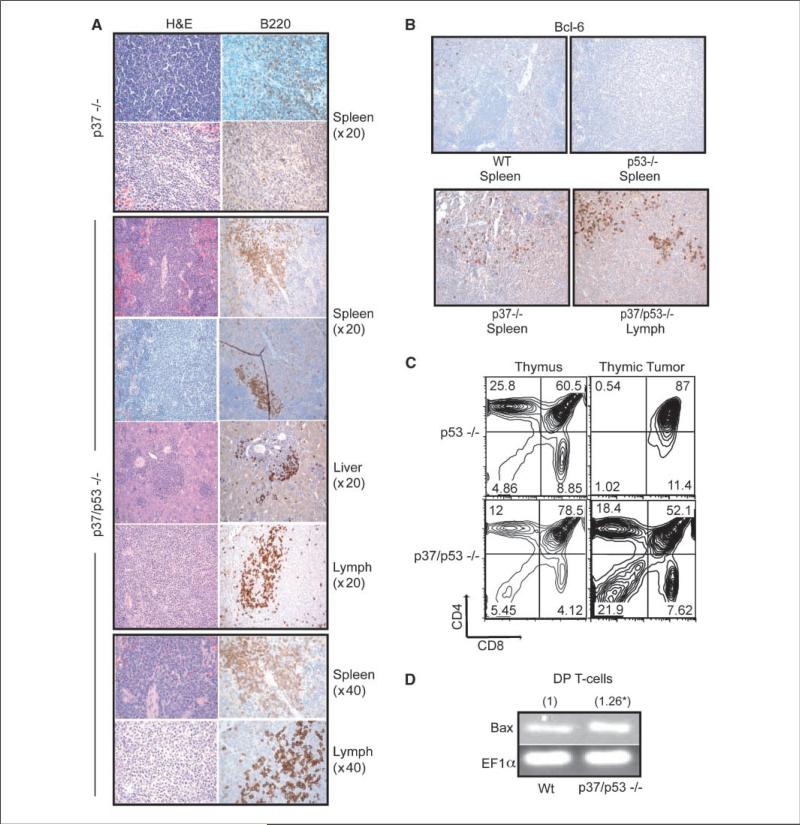
Characterization of tumors from p37/p53-double null mice. A, representative H&E or CD45R/B220-stained tumor sections from p37-null or p37/p53-double null mice. The p37/p53-double null liver, lymph node, and first spleen sections are from one mouse. All pictures were taken at ×20 magnification unless otherwise indicated. B, representative Bcl-6 stained wt, p53-null, p37-null, and p37/p53-double null spleenic or lymph node tumors. All pictures were taken at ×20 magnification. C, FACS plot of CD4 and CD8-stained thymic tumors from p53-null or p37/p53-double null mice. D, relative fold induction of Bax expression by quantitative real-time RT-PCR on CD4/CD8 DP sorted T cells from wt or p37/p53-double null thymii.
The nonthymic p37/p53-double null tumors were characterized by a uniform population of large, malignant lymphocytes with round, vesicular nuclei with frequent membrane indentations, occasional centrally placed nucleoli, and scanty cytoplasm. Lymphoblasts completely effaced lymph node architecture, diffusely involved the splenic white pulp, and infiltrated periportal and sinusoidal regions in the liver. Immunohistochemistry performed on select sections of paraffin-embedded tumors isolated from p37Ing1b mice and from p37/p53-double null mice using antibodies against CD45R/B220 and CD3 antigens confirmed that the predominant tumor type was a CD45R/B220+ lymphoma (data not shown; Fig. 3A). As multiple sites within the same p37/p53-double null mouse contained tumor nodules, in contrast to the typical single site in p37-null mice, and because p37/p53-double null mice present tumors faster and die much earlier than p37-null mice, deletion of both tumor suppressor genes seems to lead to the formation of a more aggressive B-cell tumor.
Although deletion of p53 greatly accelerated tumorigenesis in p37Ing1b-null mice (P < 0.05), codeletion of p37Ing1b and p53 seemed to also delay slightly tumor onset in p53-null mice (Fig. 2A), although the difference in the tumor curves was subtle (P < 0.1). This may reflect the reduced incidence in p37/p53-double null mice of the highly aggressive thymic lymphomas typically seen in p53-null mice (Fig. 2B). Furthermore, the cellular characteristics of the thymic lymphomas arising in the p37/p53-double null mice seemed different from those arising in p53-null mice. FACS analysis of thymii isolated from p53-null and p37/p53-double null mice revealed that the CD4+CD8+ population was somewhat larger in p37/p53-double null mice relative to p53-null mice (Fig. 3C). Analysis of a thymic tumor originating in p37/p53-double null mice revealed an expansion of the non-CD4+CD8+ compartments. In contrast, FACS analysis of p53-null thymic tumors indicated that these tumors originated in CD4+/8+ DP T-cell compartment (Fig. 3C), in agreement with earlier studies (25).
We previously reported that thymocytes lacking p37Ing1b display up-regulated levels of proapoptotic Bax expression and possess an increased susceptibility to cell death induced by various stimuli (27). To confirm that the p37Ing1b null CD4+CD8+ cells codeleted for p53 also have increased Bax expression, we isolated total RNA from CD4+CD8+ thymocytes harvested from p37/p53-double null mice and performed qPCR to measure Bax transcripts. A modest increase in Bax mRNA (1.26 ± 0.02) was observed relative to wt CD4+CD8+ Bax transcript levels (Fig. 3D). The increase in Bax expression in p37/p53-double null CD4+CD8+ cells correlates with the decrease incidence of CD4+CD8+ thymic lymphomagenesis observed in the double null mice and may account for the overall slight delay in the rate of tumorigenesis in p53-null mice codeleted for p37Ing1b.
DLBL in p37/p53 double null mice
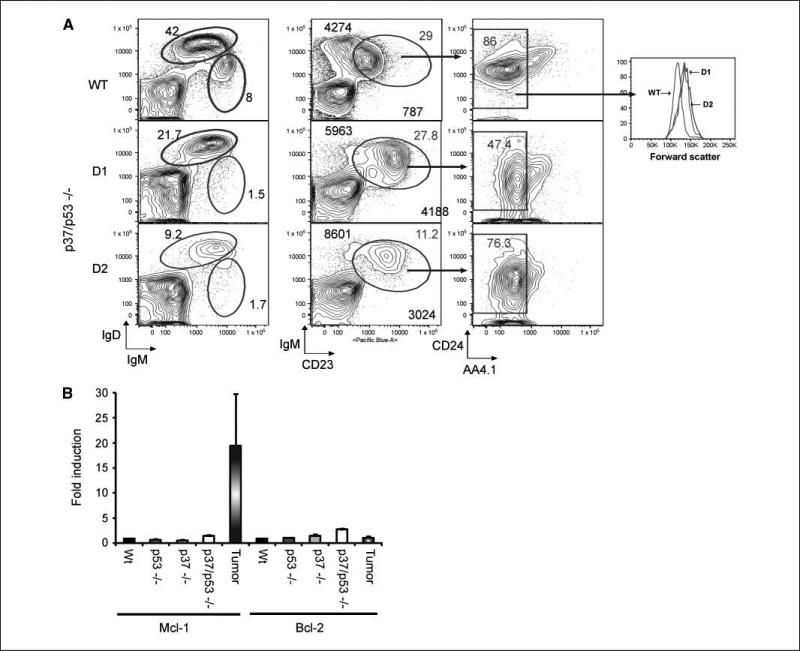
FACS analysis was performed on the B-cell compartments of two tumor-bearing mice to further characterize the tumor phenotype of the p37/p53-double null mice (Fig. 4A). Several distinct splenic B-cell populations were readily observed in nontumor-bearing mice, including FO B cells (IgD++ IgMlo CD23+), immature T1 (IgDlo IgM++ CD23–) and T2 (IgDlo IgM++ CD23+ CD24++ AA4.1++) B cells. However, the splenic B cells of tumor-bearing p37/p53-double null mice were almost exclusively composed of FO B cells (IgD++ IgMlo CD23+ CD24lo AA4.1–). In addition, the forward scatter distribution of this FO B population is shifted (Fig. 4A, right), indicating that the majority of the FO B cells are larger, blast stage cells rather than resting cells. The phenotype of FO B cells in p37/p53 tumorigenic mice was distinguished by elevated levels of IgM and CD23 staining, and reduced amounts of CD24 staining relative to levels observed in wt FO B cells (Fig. 4A). Similar results were obtained in a repeat of this experiment (data not shown). The difference in the staining patterns of the p37Ing1b-null tumor cells and p37/p53-double null tumor cells, coupled with the widespread organ involvement and larger size of the tumor cells arising in p37/p53-double null mice, classifies these CD45R/B220+ tumors as DLBL rather than FL.
Figure 4.
Tumors from p37/p53-double null mice are derived from FO B cells and have elevated Mcl-1 expression. A, representative FACS analysis of spleen cells from a healthy wt control mouse (WT) and spleen cells from two different p37/p53-double null mice with tumors (D1 and D2). Middle column, the larger bold numbers report the geometric mean fluorescence intensity values for IgM (y axis) and CD23 (x axis) for the IgM+CD23+CD24+/–AA4.1– population. This experiment was repeated with similar results. B, expression levels of Bcl-2 and Mcl-1 assayed by quantitative real-time RT-PCR in spleens from wt, p53-null, p37-null, and p37/p53-double null mice or on p37/p53-double null splenic tumors. Real-time PCR of normal spleens was done in triplicate; columns, mean of tumor samples from three independent mouse tumors; bars, SD.
To determine if there was increased expression of prosurvival genes in lymphomas arising in the double null mice as had been previously observed in human FL and DLBL (11, 13, 39), real-time PCR was performed on total RNA isolated from the spleens and tumors of various mice. Mcl-1 expression seemed unaltered in wt, p37Ing1b-null, and in p53-null cells but was slightly up-regulated in p37/p53 double-null spleens (Fig. 4B). However, Mcl-1 expression levels were greatly increased in tumors arising in p37/p53-double null mice. In contrast, Bcl-2 was not elevated in splenic tissues or in tumors, regardless of the genotype of the mice.
Approximately one-third of all NHL display translocations involving BCL-6, a transcriptional repressor required for the formation of GCB cells. Overexpression of BCL-6 has been proposed to block the terminal differentiation of GC B cells by inhibiting the expression of the B lymphocyte maturation protein 1, resulting in prolonged exposure of the GC B cells to the mutagenic effects of the somatic hypermutation machinery (40). In addition, Bcl-6 has been proposed to suppress DNA damage-induced apoptosis caused by the hypermutation apparatus by suppressing p53 expression (41). Thus, Bcl-6 expression serves as a marker for DLBL that are derived from GC-like B cells. To determine if Bcl-6 expression was up-regulated in DLBL in p37/p53-double null mice, representative tissue sections were fixed, sectioned, and stained for Bcl-6 (Fig. 3B). Bcl-6 was not detected in wt tissue, or in a B-cell lymphoma arising in the spleen of a p53-null mouse (Fig. 3B). In contrast, varying levels of Bcl-6 expression could be detected in some but not all tumor tissues harvested from p37/p53-double null mice (Fig. 3B). To confirm up-regulation of Bcl-6 expression in a subset of p37/p53-tumors, total RNA was harvested from 13 p37/p53-double null DLBL and assayed for Bcl-6 transcript levels by qRT-PCR. In addition, the expression levels of the CD10 surface marker, Irf-4 transcription factor, and the NF-κB subunit Rela (p65) were also assayed by qRT-PCR (Table 1). Irf-4 is a transcription cofactor expressed in plasma cells that silences BCL-6 expression and the GC transcriptional program, and is critical for GC to plasma cell differentiation (42). Bcl-6 was up-regulated in 6 of 13 tested DLBL that arose in p37/p53-double null mice, marking these tumors as GCB-like DLBL. CD10, another marker for GCB (43), was elevated in two other tumors, indicating that 8 of the 13 tumors were the GC subtype of DLBL. The remaining 5 DLBL tumors did not display elevated levels of CD10, Bcl-6, or Irf-4. Lack of CD10, Bcl-6, or Irf-4 up-regulation in the remaining five DLBL indicates that these are non–GCB-like DLBL tumors.
Table 1.
Expression of DLBL markers (fold induction)
| Tumor | CD10 | Bcl-6 | lrf-4 | Classification | RelA |
|---|---|---|---|---|---|
| T1 | — | 27.7 | GCB | 72.6 | |
| T2 | — | — | — | Non-GC | 73.9 |
| T3 | — | — | — | Non-GC | 4.5 |
| T4 | 2.3 | — | — | GCB | 2.5 |
| T5 | 306.4 | — | — | GCB | 10.4 |
| T6 | — | 12.5 | — | GCB | 4.2 |
| T7 | — | 86.9 | — | GCB | 21.5 |
| T8 | — | — | — | Non-GC | — |
| T9 | — | 13.2 | — | GCB | 31.5 |
| T10 | 6.7 | 29.6 | — | GCB | 53.5 |
| T11 | — | — | — | Non-GC | 6.8 |
| T12 | — | — | — | Non-GC | — |
| T13 | — | 3.6 | — | GCB | 3.3 |
NOTE: p37/p53-double null mice develop predominately GCB DLBL and have elevated RelA expression and activity. Table shows classification of 13 independent p37/p53-double null spleenic tumors based on relative fold induction of CD10, lrf-4, and Bcl-6 by quantitative real-time RT-PCR. Also shown is the relative fold induction of p65/RelA in the same 13 spleenic tumor samples. Values are averages of relative fold induction of three independent experiments for each tumor. SDs were within 10% of the mean value for each sample.
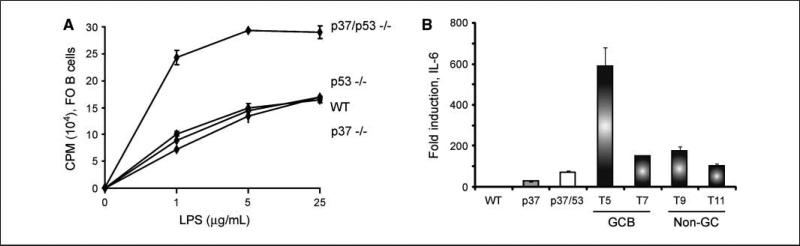
NF-κB activity is often increased in human lymphomas due to chromosomal translocations, mutation of NF-κB inhibitors such as IκB, or through other, less well-characterized processes (44, 45). Furthermore, NF-κB activation is also observed in subtypes of non-GC DLBL such as in peripheral (or activated) B cell–like DLBL. Recently, the ING1 protein has been proposed to inhibit NF-κB signaling in human fibroblasts by up-regulating HSP70 expression (46). To examine NF-κB activity in cells deleted for p37Ing1b, qPCR was performed on total RNA isolated from the 13 DLBL samples. Analysis of RelA expression levels in the DLBL revealed that the RelA (p65) subunit expression was elevated in nearly all of the tumors (Table 1, right column) relative to RelA expression in wt splenic tisssue. To determine if NF-κB activity is up-regulated in p37/p53-double null cells, FO B cells were isolated from the spleens of wt, p37Ing1b-null, p53-null, or p37/p53 double-null mice, and cell proliferation was measured in response to treatment of the cells with lipopolysaccharide (LPS), an established inducer of NF-κB activity (Fig. 5A). Although deletion of p53 or p37Ing1b did not result in a greater proliferative response of FO B cells to LPS, codeletion of p53 and p37Ing1b did result in increased proliferation of FO B cells to varying dosages of LPS. To confirm that the increase in RelA expression observed in the DLBL samples correlated with increased NF-κB activity in the tumors, qPCR was used to measure expression of the Interleukin 6 gene (IL-6), a classic target gene up-regulated by NF-κB signaling (Fig. 5B). Increased IL-6 transcript levels were observed in the spleens of p37-null mice, regardless of p53 status, and very large increases (100- to 600-fold) in IL-6 expression levels were observed in p37/p53 double-null tumors, regardless of their classification as GCB-like DLBL (tumor #5 and #7) or as non-GC DLBL (tumor #9 and #11). These results confirm that loss of p37Ing1b up-regulates NF-κB activity in p37/p53 double-null mice and in tumors, supporting a role for p37Ing1b in inhibiting NF-κB signaling in suppression of DLBL.
Figure 5.
FO B cells from p37/p53-double null mice show elevated NF-κB activity. A, [3H] thymidine uptake assay of Wt, p53-null, p37-null, and p37/p53-double null purified FO B cells (IgDHi). Assay was done in triplicate and repeated twice. B cells were treated with the indicated amount of LPS and harvested 24 h later for [3H] thymidine uptake assay. B, relative fold induction of IL-6 in Wt, p37-null, p37/p53-double null spleens, and representative p37/p53-double null GCB (n = 2) and non–GC-DLBL (n = 2) tumors by quantitative real-time RT-PCR. Columns, mean of quantitative real-time RT-PCR performed in triplicate; bars, SD.
Discussion
We have previously described the generation of mice deleted for the p37Ing1b isoform of Ing1 (27). Loss of p37Ing1b increased the proliferation of MEF regardless of p53 status, and the p53-mediated response of cells to DNA damage or to overexpression of oncogenic Ras was unaltered in p37Ing1b deficient MEFs. In addition, deletion of p37Ing1b up-regulated Bax gene expression in the presence or absence of functional p53, and increased the apoptotic response of thymocytes to DNA damage. A majority of p37Ing1b-deficient mice spontaneously developed enlarged spleens between ages 1 and 2 years. Histopathology revealed a CD45R/B220+ CD3– lymphocytic hypercellularity in spleens isolated from p37Ing1b-deficient mice, and all of the tumors arising in p37Ing1b-null mice were classified as FL (27). Although our results indicated that p37Ing1b can inhibit cell growth and cell death in a p53-independent manner, in MEFs and in thymocytes, respectively, it was unclear from these data if p37Ing1b alters p53 functions to regulate tumorigenesis. In this present study, we crossed p37Ing1b mice with p53-null mice to generate p37/p53-double null mice and explored the relationship between p37Ing1b and p53 tumor suppression. Deletion of p53 greatly accelerated the rate of tumor formation in p37Ing1b-null mice, an unexpected finding if p37Ing1b and p53 were functionally redundant, or if p37Ing1b suppressed tumorigenesis by signaling through p53. Thus, cooperation observed between the two tumor suppressors in preventing B-cell lymphomagenesis indicates that p37Ing1b does not suppress tumors by activating p53. Cooperation between p37Ing1b and p53 in tumor suppression is also in agreement with the increased rate of cell proliferation in FO B cells when codeleted for p37Ing1b and p53.
Interestingly, there was a reduction in the incidence of thymic lymphomas in the p37/p53-double null mice relative to the high incidence CD4+CD8+ thymomas in p53-null mice. This reduction correlates with an increase in proapoptotic Bax expression observed in CD4+CD8+ T cells isolated from p37/p53-double null mice, in keeping with our previous finding of a dramatic increase in Bax mRNA in total thymus when treated with γ-irradiation (27). The reduced incidence of thymic lymphoma, typically an aggressive cancer resulting in rapid morbidity and mortality in p53-null mice, may account for the slight delay in the lethality of the p37/p53-double null mice. We also observed a slight decrease in expression of Mcl-1 in thymii from p37/p53-null mice (data not shown). The elevation of Mcl-1 in p37/p53-null spleens and reduction of Mcl-1 in T cells might also explain the shift from a predominately T-cell lymphoma in p53-null mice to a more B-cell lymphoma in p37/p53-null mice. Approximately half of the tested DLBL were found to have elevated Bcl-6 expression levels and/or increased CD10 expression, indicating a GCB-like subtype of DLBL. In addition, nearly all of the tumors displayed elevated RelA expression and, in some cases, increased NF-κB activity. It is yet unclear how p37Ing1b is regulating the expression of Bcl-6, CD10, or RelA in DLBL, or the expression of Mcl-1 and Bax in B and T cells. Further experimentation is needed to determine if this effect is indirect or is a direct effect of altered factor binding at the promoters of these genes.
A few studies have examined the expression of ING1 in human primary hematopoetic malignancies and found no mutation of ING1 or alterations in ING1 expression in myeloid leukemias (28, 47–49). However, down-regulation of ING1 expression has been reported in human mantle cell lymphomas in both primary tumors and cell lines (48, 49). In addition, expression of ING1 seems to be down-regulated in gene expression profiling data performed on human DLBL samples, although the reduction in ING1 expression was not enough to highlight ING1 as a prognostic indicator for the disease in these studies (8, 9). However, the results of our analysis of p37Ing1b-deficient mice indicate that loss of p37Ing1b induces the highly penetrant formation of spontaneous FL and DLBL in mice, especially in mice codeleted for p53. Thus, the role of p37Ing1b in suppressing human B-cell lymphomas, especially DLBL, should be evaluated further.
Acknowledgments
Grant support: NIH grants RO1AI43534 (R. M. Gerstein) and RO1CA77735 (S. N. Jones).
We thank the University of Massachusetts Medical School (UMMS) Morphology Core for assistance with tissue preparation, Erin Cloherty, Richard Konz, and the UMMS Flow Cytometry Core for help with FACS analyses, Charlene Baron for assistance with manuscript figure preparation, and Huiling Liang for advice and helpful discussions. Core facilities used in this study were supported in part by Program Project grant P30DK32529 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hiddemann W, Buske C, Dreyling M, Weigert O, Lenz G, Unterhalt M. Current management of follicular lymphomas. Br J Haematol. 2007;136:191–202. doi: 10.1111/j.1365-2141.2006.06378.x. [DOI] [PubMed] [Google Scholar]
- 2.Lichtman MA. Battling the hematological malignancies: the 200 years’ war. Oncologist. 2008;13:126–38. doi: 10.1634/theoncologist.2007-0228. [DOI] [PubMed] [Google Scholar]
- 3.Ng AK. Diffuse large B-cell lymphoma. Semin Radiat Oncol. 2007;17:169–75. doi: 10.1016/j.semradonc.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Hiddemann W, Unterhalt M. Current treatment strategies in follicular lymphomas. Ann Oncol. 2006;17(Suppl 10):x155–9. doi: 10.1093/annonc/mdl253. [DOI] [PubMed] [Google Scholar]
- 5.Hunt KE, Reichard KK. Diffuse large B-cell lymphoma. Arch Pathol Lab Med. 2008;132:118–24. doi: 10.5858/2008-132-118-DLBL. [DOI] [PubMed] [Google Scholar]
- 6.Abramson JS, Shipp MA. Advances in the biology and therapy of diffuse large B-cell lymphoma: moving toward a molecularly targeted approach. Blood. 2005;106:1164–74. doi: 10.1182/blood-2005-02-0687. [DOI] [PubMed] [Google Scholar]
- 7.Armitage JO. How I treat patients with diffuse large B-cell lymphoma. Blood. 2007;110:29–36. doi: 10.1182/blood-2007-01-041871. [DOI] [PubMed] [Google Scholar]
- 8.Monti S, Savage KJ, Kutok JL, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–61. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 9.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 10.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using atissue microarray. Blood. 2004;103:275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 11.Lossos IS, Morgensztern D. Prognostic biomarkers in diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:995–1007. doi: 10.1200/JCO.2005.02.4786. [DOI] [PubMed] [Google Scholar]
- 12.De Paepe P, De Wolf-Peeters C. Diffuse large B-cell lymphoma: a heterogeneous group of non-Hodgkin lymphomas comprising several distinct clinicopathological entities. Leukemia. 2007;21:37–43. doi: 10.1038/sj.leu.2404449. [DOI] [PubMed] [Google Scholar]
- 13.Bende RJ, Smit LA, van Noesel CJ. Molecular pathways in follicular lymphoma. Leukemia. 2007;21:18–29. doi: 10.1038/sj.leu.2404426. [DOI] [PubMed] [Google Scholar]
- 14.Michels J, Foria V, Mead B, et al. Immunohistochemical analysis of the antiapoptotic Mcl-1 and Bcl-2 proteins in follicular lymphoma. Br J Haematol. 2006;132:743–6. doi: 10.1111/j.1365-2141.2005.05954.x. [DOI] [PubMed] [Google Scholar]
- 15.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–6. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 16.Zhou P, Levy NB, Xie H, et al. MCL1 transgenic mice exhibit a high incidence of B-cell lymphoma manifested as a spectrum of histologic subtypes. Blood. 2001;97:3902–9. doi: 10.1182/blood.v97.12.3902. [DOI] [PubMed] [Google Scholar]
- 17.Saito M, Gao J, Basso K, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–92. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Cattoretti G, Pasqualucci L, Ballon G, et al. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell. 2005;7:445–55. doi: 10.1016/j.ccr.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa A, Kinoshita T, Watanabe T, et al. Mutations of the p53 gene as a prognostic factor in aggressive B-cell lymphoma. N Engl J Med. 1997;337:529–34. doi: 10.1056/NEJM199708213370804. [DOI] [PubMed] [Google Scholar]
- 20.Lo Coco F, Gaidano G, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. p53 mutations are associated with histologic transformation of follicular lymphoma. Blood. 1993;82:2289–95. [PubMed] [Google Scholar]
- 21.Sander CA, Yano T, Clark HM, et al. p53 mutation is associated with progression in follicular lymphomas. Blood. 1993;82:1994–2004. [PubMed] [Google Scholar]
- 22.Davies AJ, Lee AM, Taylor C, et al. A limited role for TP53 mutation in the transformation of follicular lymphoma to diffuse large B-cell lymphoma. Leukemia. 2005;19:1459–65. doi: 10.1038/sj.leu.2403802. [DOI] [PubMed] [Google Scholar]
- 23.Harvey M, McArthur MJ, Montgomery CA, Jr., Butel JS, Bradley A, Donehower LA. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet. 1993;5:225–9. doi: 10.1038/ng1193-225. [DOI] [PubMed] [Google Scholar]
- 24.Ward JM, Tadesse-Heath L, Perkins SN, Chattopadhyay SK, Hursting SD, Morse HC., III Splenic marginal zone B-cell and thymic T-cell lymphomas in p53-deficient mice. Lab Invest. 1999;79:3–14. [PubMed] [Google Scholar]
- 25.Jacks T, Remington L, Williams BO, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Lushnikova T, Odvody J, Greiner TC, Jones SN, Eischen CM. Elevated Mdm2 expression induces chromosomal instability and confers a survival and growth advantage to B cells. Oncogene. 2007;27:1590–8. doi: 10.1038/sj.onc.1210788. [DOI] [PubMed] [Google Scholar]
- 27.Coles AH, Liang H, Zhu Z, et al. Deletion of p37Ing1 in mice reveals a p53-independent role for Ing1 in the suppression of cell proliferation, apoptosis, and tumor-igenesis. Cancer Res. 2007;67:2054–61. doi: 10.1158/0008-5472.CAN-06-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kichina JV, Zeremski M, Aris L, et al. Targeted disruption of the mouse ing1 locus results in reduced body size, hypersensitivity to radiation and elevated incidence of lymphomas. Oncogene. 2006;25:857–66. doi: 10.1038/sj.onc.1209118. [DOI] [PubMed] [Google Scholar]
- 29.Campos EI, Chin MY, Kuo WH, Li G. Biological functions of the ING family tumor suppressors. Cell Mol Life Sci. 2004;61:2597–613. doi: 10.1007/s00018-004-4199-4. [DOI] [PubMed] [Google Scholar]
- 30.Gong W, Suzuki K, Russell M, Riabowol K. Function of the ING family of PHD proteins in cancer. Int J Biochem Cell Biol. 2005;37:1054–65. doi: 10.1016/j.biocel.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Nouman GS, Anderson JJ, Lunec J, Angus B. The role of the tumour suppressor p33 ING1b in human neoplasia. J Clin Pathol. 2003;56:491–6. doi: 10.1136/jcp.56.7.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soliman MA, Riabowol K. After a decade of study-ING, a PHD for a versatile family of proteins. Trends Biochem Sci. 2007;32:509–19. doi: 10.1016/j.tibs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Zeremski M, Hill JE, Kwek SS, et al. Structure and regulation of the mouse ing1 gene. Three alternative transcripts encode two phd finger proteins that have opposite effects on p53 function. J Biol Chem. 1999;274:32172–81. doi: 10.1074/jbc.274.45.32172. [DOI] [PubMed] [Google Scholar]
- 34.Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 35.Liang H, Chen Q, Coles AH, et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–60. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 36.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A. 2004;101:6164–9. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marfella CG, Ohkawa Y, Coles AH, Garlick DS, Jones SN, Imbalzano AN. Mutation of the SNF2 family member Chd2 affects mouse development and survival. J Cell Physiol. 2006;209:162–71. doi: 10.1002/jcp.20718. [DOI] [PubMed] [Google Scholar]
- 38.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–8. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 39.de Jong D. Molecular pathogenesis of follicular lymphoma: a cross talk of genetic and immunologic factors. J Clin Oncol. 2005;23:6358–63. doi: 10.1200/JCO.2005.26.856. [DOI] [PubMed] [Google Scholar]
- 40.Pasqualucci L, Neumeister P, Goossens T, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–6. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 41.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–9. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 42.Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- 43.Bai M, Skyrlas A, Agnantis NJ, et al. B-cell differentiation, apoptosis and proliferation in diffuse large B-cell lymphomas. Anticancer Res. 2005;25:347–62. [PubMed] [Google Scholar]
- 44.Houldsworth J, Olshen AB, Cattoretti G, et al. Relationship between REL amplification, REL function, and clinical and biologic features in diffuse large B-cell lymphomas. Blood. 2004;103:1862–8. doi: 10.1182/blood-2003-04-1359. [DOI] [PubMed] [Google Scholar]
- 45.Jost PJ, Ruland J. Aberrant NF-nB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–7. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 46.Feng X, Bonni S, Riabowol K. HSP70 induction by ING proteins sensitizes cells to tumor necrosis factor alpha receptor-mediated apoptosis. Mol Cell Biol. 2006;26:9244–55. doi: 10.1128/MCB.01538-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohmori M, Nagai M, Tasaka T, et al. Decreased expression of p33ING1 mRNA in lymphoid malignancies. Am J Hematol. 1999;62:118–9. doi: 10.1002/(sici)1096-8652(199910)62:2<118::aid-ajh11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 48.Schraders M, Pfundt R, Straatman HM, et al. Novel chromosomal imbalances in mantle cell lymphoma detected by genome-wide array-based comparative genomic hybridization. Blood. 2005;105:1686–93. doi: 10.1182/blood-2004-07-2730. [DOI] [PubMed] [Google Scholar]
- 49.Ripperger T, von Neuhoff N, Kamphues K, et al. Promoter methylation of PARG1, a novel candidate tumor suppressor gene in mantle-cell lymphomas. Haematologica. 2007;92:460–8. doi: 10.3324/haematol.10337. [DOI] [PubMed] [Google Scholar]