Abstract
Cerebral blood flow (CBF) and cerebral metabolic rate are normally coupled, that is an increase in metabolic demand will lead to an increase in flow. However, during functional activation, CBF and glucose metabolism remain coupled as they increase in proportion, whereas oxygen metabolism only increases to a minor degree—the so-called uncoupling of CBF and oxidative metabolism. Several studies have dealt with these issues, and theories have been forwarded regarding the underlying mechanisms. Some reports have speculated about the existence of a potentially deficient oxygen supply to the tissue most distant from the capillaries, whereas other studies point to a shift toward a higher degree of non-oxidative glucose consumption during activation. In this review, we argue that the key mechanism responsible for the regional CBF (rCBF) increase during functional activation is a tight coupling between rCBF and glucose metabolism. We assert that uncoupling of rCBF and oxidative metabolism is a consequence of a less pronounced increase in oxygen consumption. On the basis of earlier studies, we take into consideration the functional recruitment of capillaries and attempt to accommodate the cerebral tissue's increased demand for glucose supply during neural activation with recent evidence supporting a key function for astrocytes in rCBF regulation.
Keywords: BOLD (blood oxygenation level dependent), CBF (cerebral blood flow), fMRI (functional magnetic resonance imaging), glucose brain metabolism, neurovascular coupling, oxidative brain metabolism
Introduction
More than 100 years ago, Roy and Sherrington (1890) came to the conclusion that the vascular supply of the brain can be varied locally in correspondence with local variations in functional activity. In the early 1960s, Lassen and Ingvar introduced a methodology for measurement of regional cerebral blood flow (rCBF) in humans, and during the 1970s, once it was more widely acknowledged that rCBF increases in response to cerebral activation, these methods were applied to functional brain mapping (Lassen et al, 1978). Although it had already been known for a while that cortical oxygen tension increases in response to activation (Cooper et al, 1966), it was only somewhat later realized that regional cerebral oxygen consumption does not increase to the same extent as does rCBF and rCMRglc (Fox et al, 1988). This important physiologic phenomenon, commonly referred to as the uncoupling of flow and oxidative metabolism during functional activation, forms the basis for the well-known BOLD (blood oxygenation level dependent) magnetic resonance imaging method for functional magnetic resonance imaging (fMRI). In short, when brain tissue is activated, the magnetic resonance imaging signal increases in response to a decreased amount of deoxyhemoglobin in that particular region. In other words, although brain oxygen metabolism does change in same direction as glucose metabolism and rCBF, this happens disproportionately, resulting in an increased level of oxygenation.
The cause of the rCBF increase during activation is still not entirely clear. Some authors have argued that, because of diffusion limitations, there will be limitations in oxygen availability in the tissue and no reserve pool. For this reason, the rCBF increases must always be disproportionate to changes in oxidative metabolism, to the extent that the increases in capillary PO2 needed to support an increased oxidative metabolism under these conditions can only be achieved through a large flow increase (Gjedde, 1991; Buxton and Frank, 1997). In contrast with the excess increase in rCBF compared with oxygen consumption, there is a very close coupling between rCBF and CMRglc during activation. This might suggest that rCBF increase during functional activation is a consequence of the increased demand for glucose supply to the cerebral tissue. From a physiologic point of view, it makes sense to assume that there is a mechanism responsible for the coupling between rCBF and glucose consumption to ensure the activated tissue a sufficient glucose supply. But why should the brain switch to anaerobic glucose consumption during activation? Is it because of limitations in oxygen transport across the blood–brain barrier (BBB) or because of diffusion limitations from the capillary bed to the neuronal mitochondria? Discussion on this topic has been ongoing for decades, but recent evidence has shed some light on these issues. In the following sections, we will consider the older literature, in conjunction with more recent studies, with the main focus on the rCBF response and the subsequent availability of glucose and oxygen for energy production during activation.
Capillary heterogeneity
As the concept of capillary heterogeneity is of relevance for discussion of both glucose and oxygen transport in the brain, a brief overview will be given here. Recruitment of capillaries with opening of closed non-perfused capillaries, as it takes place in muscles during exercise, will increase the effective capillary surface area and thereby the flux of substances across the capillary as long as permeability remains unchanged. A functional recruitment with slowly perfused capillaries becoming rapidly perfused may be a physiologic phenomenon of significant importance for substrate transport across the BBB (Hertz and Paulson, 1980; Knudsen et al, 1990; Kuschinsky and Paulson, 1992). Thus, in baseline conditions, the cerebral microcirculation is relatively heterogeneous, but it becomes gradually more homogeneous as CBF increases (Kuschinsky and Paulson, 1992).
The notion of capillary heterogeneity in the brain is strongly supported by studies of direct microscopic observation of the cerebral microcirculation (Pawlik et al, 1981) and in vivo staining of perfused capillaries (Vetterlein et al, 1990). Further, when the oxygen carrying capacity is reduced with unchanged arterial oxygen (by CO inhalation), the number of perfused capillaries in the brain increases (Sinha et al, 1991). The concept of functional recruitment is also supported by the observation that an rCBF increase is not accompanied by a significant reduction of the transit time as measured by reflectance spectrophotometry (Shockley and LaManna, 1988).
Still the question of capillary recruitment is often considered controversial (Buxton, 2002) and other mechanisms have been or are still debated, for example increased velocity without recruitment.
Glucose consumption—cerebral blood flow coupling
Earlier studies conducted in humans in resting state have found a quite consistent ratio of about 5.6 between glucose and oxygen brain uptake (Madsen et al, 1995; Hasselbalch et al, 1996). These studies suggest that glucose is almost completely oxidized to CO2 and H2O through glycolysis and the subsequent tricarboxylic acid (TCA) cycle. Glucose consumption seems tightly coupled to neurotransmitter recycling and restoration of neuronal membrane potentials through conversion of glucose to lactate in astrocytes, and shuttling of lactate to neurons for oxidation (Rothman et al, 1999; Magistretti and Pellerin, 1999; Pellerin and Magistretti, 2004; Pellerin et al, 2007). Thus, in the resting state, coupling of CMRglc and neural activity seems to be regulated by the need for energy substrates to maintain synaptic transmission.
Further, a tight coupling between CBF and glucose consumption during functional activation was established by several earlier studies. During epileptic seizure, both CBF and glucose uptake increase by at least a factor of 2 (Brodersen et al, 1973). In studies of functional activation in humans, parallel increases in CBF and glucose consumption have been found (Fox et al, 1988; Madsen et al, 1995; Newberg et al, 2005) (Figure 1A). In addition, in experimental studies in rats, the CBF increase correlated better to the increase in glucose uptake than to the increase in oxygen uptake, although the CBF increase was more marked (Madsen et al, 1998). However, the exact coupling (% increase) of flow to the glucose consumption seen in the human studies was not observed in the experimental study in which flow increased by 68% and glucose consumption only by 35%, as the (a-v)glu dropped by 20%. We consider this to reflect a statistical variation and not a biological phenomenon. Thus, in a subsequent study using exactly the same activation paradigms in the same laboratory, a drop in (a-v)glu could not be confirmed, but in contrast, an increase by 13% was observed.
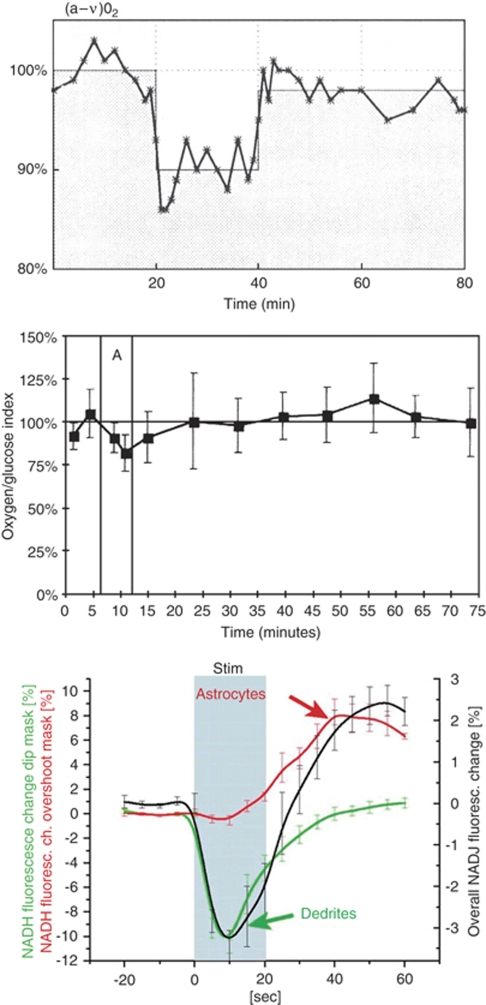
Figure 1.
(A) Experiment in humans with a global maximal activation period of 10 mins (from 20–30 mins) induced by the Wisconsin card sorting test. Values are expressed as the percentage of the baseline measurements. Note the continuous resetting of the oxygen to glucose ratio, which may be accounted for by a prolonged phase of non-oxidative glucose metabolism. Measurements were performed as arterio-venous (jugular) differences of glucose and oxygen combined with CBF measurement by the Kety–Schmidt method (Madsen et al, 1995). (B) The cerebral oxygen-glucose index before, during, and after maximal global activation of rats (duration 6 mins, period ‘A') induced by opening the sheltering box for 6 m. Values represent the mean of global cerebral arterio-venous determinations in a group of six animals. Vertical bars represent s.d. Note the decreased oxygen to glucose uptake ratio, signifying non-oxidative glucose consumption during and immediately after activation, and the reversal of that ratio after 35 mins, in which more oxygen than glucose is taken up by the brain (Madsen et al, 1998). A and B reprinted with permission from NPG. (C) Metabolic changes in hippocampal slice preparations after brief 20 secs stimulation. The biphasic NADH response represent early oxidative metabolism in neuronal dendrites followed by late activation of glycolysis in astrocytes (Kasischke et al, 2004). Reprinted with permission from AAAS.
During activation, the oxygen/glucose uptake ratio decreased by 20–25% (Madsen et al, 1998; Madsen et al, 1999) indicating that the excess CBF and glucose uptake during activation is not used for increased oxidative glucose metabolism (Figure 1B). Correspondingly, there is a modest increase in brain lactate concentration in the activated areas (Prichard et al, 1991; Sappey-Marinier et al, 1992; Frahm et al, 1996; Urrila et al, 2003; Mangia et al, 2007), and an increase in extracellular lactate concentration has been observed during functional activation (Hu and Wilson, 1997). Furthermore, a slight excess lactate efflux from the brain takes place during activation (Madsen et al, 1999). But oxidative metabolism accounts only for 60% of the glucose taken up by the brain, and of the non-oxidized glucose, only ∼50% seems to be metabolized to lactate escaping from the brain (Madsen et al, 1999). Further, by increasing blood lactate levels during maximal exercise in humans, lactate influx increases, but lactate seems not to be oxidized during the experimental time (Dalsgaard et al, 2004). All these early studies would, at first glance, seem to be compatible with the notion that once taken up by astrocytes, glucose is converted to lactate without being oxidized—at least not during the experimental time. More recent data support the astrocyte–neuron lactate shuttle hypothesis: neurons and astrocytes have comparable uptakes of glucose (Nehlig et al, 2004), but 90–95% of the energy coming from the glucose uptake is consumed by neurons (Attwell and Laughlin, 2001), suggesting that astrocytes deliver glucose (mostly in the form of lactate) to neurons for energy production. Further, neurons have a preference for metabolizing lactate over glucose (Smith et al, 2003). However, as covered in a recent review (Hertz et al, 2007), these concepts are open to challenge. As described in the section on the astrocyte–arteriole connection, the tight coupling between rCBF and rCMRglc during activation seems well established, and earlier data support the idea that lactate, whether produced peripherally and taken up by the brain, or produced by astrocytes, is accumulating in the brain during activation. Further, as brain lactate concentration and oxygen consumption do not increase markedly during activation, lactate must be metabolized by other pathways than through the TCA cycle. In a recent elegant study, Cruz et al (2007) used labeled deoxyglucose and glucose during functional activation to study local glucose uptake and spreading of labeled glucose metabolites, respectively. They found that during rest and activation, glucose metabolites including lactate, had a spatial spread 1.5–2.2-fold larger than deoxyglucose, and the tissue volume labeled by glucose metabolites increased 20–50% during activation compared with the resting condition. These results may explain the local and temporary decrease in the oxygen to glucose metabolic ratio. Thus, glucose metabolites are quickly being dispersed from the activated area, and subsequently, either released from the brain, entering metabolite pools or oxidized in the ‘recovering phase' of activation, as suggested by an increase in the oxygen/glucose metabolic ratio after activation (Madsen et al, 1998; Madsen et al, 1999).
In a recent study, fludeoxyglycose (FDG) uptake after 1 mins of visual stimulation was higher than after 15 mins of continuous stimulation, in which it was attenuated by 28%. This suggests a shift from glycolytic to oxidative glucose metabolism with continued activation (Vlassenko et al, 2006). This may be considered as compatible with the observation that only part of the excess glucose uptake can be accounted for by a release from the brain to the blood of lactate (discussed above, Madsen et al, 1999). Thus, the excess glucose uptake cannot accumulate forever in the brain and a new balance must be achieved. In this context, we have to recall that what one measures is in most instances a deviation of a balance between metabolism and delivery of substances triggered by metabolic changes, and such a deviation may have a time profile by its own. Mintun et al (2004) and Vlassenko et al (2006) hypothesized that, in activated brain, blood flow is modulated by changes in the cytosolic-free nicotinamide adenine dinucleotide (NADH)/NAD+ ratio, related to increased glycolysis —a theory in accord with our hypothesis of the flow increase being driven by an excess demand for glucose.
The Activation-Induced Cerebral Blood Flow Response During Hypoglycemia
If the functionally most important consequence of the rCBF response to activation is an increased glucose supply, hypoglycemia could be expected to augment the CBF response. Using PET and 15O2, Powers et al (1996) found similar increases in the CBF in response to activation (instead of augmentation), when comparing subjects exposed to acute moderate hypoglycemia with normoglycemic subjects. On the basis of their findings, the authors concluded that the increase in rCBF during physiologic brain activation was not regulated by a mechanism that matches local cerebral glucose supply to local cerebral glucose demand. However, there are some inherent limitations with the study by Powers et al (1996). First, no measurements of CMRglc were obtained. This is a key piece of information for judging whether coupling may be present or lacking. There is evidence from human subjects that CMRglc begins to decline at blood glucose levels of ∼3 mmol/L (Lubow et al, 2006). Second, no confirmation was obtained that the stimulus (fingertip vibration) elicited identical electrical responses (equal magnitude neural activations) in the cortex in normoglycemic versus hypoglycemic states. This has some relevance, as even moderate hypoglycemia may alter cerebral electrophysiologic functions (Rosenthal et al, 2001). In fact, Powers et al (1996) reported significant neurobehavioral changes in a group of hypoglycemic subjects (plasma glucose=2.8 mmol/L). This could have affected cortical reactions to somatosensory stimuli, including CMRglc responses. Despite the caveats, these findings seem to imply that the generally close coupling between CBF and glucose utilization may begin to fail when glucose supply becomes limiting and interferes with the capacity to increase the cerebral glucose consumption rate, under conditions of neural activation. Further support for hypoglycemia (plasma glucose=2.2 mmol/L) interfering with sensory processing and brain activity/CBF relationships in human subjects was provided in a recent report by Bie-Olsen et al (2009). Here, the authors concluded that hypoglycemia induces changes in sensory processing in a cognition-independent manner, whereas activation of areas of higher order functions is influenced by cognitive load as well as hypoglycemia.
Recent fMRI studies have indicated that functional activation during hypoglycemia does not augment, but rather attenuates, the BOLD response (Rosenthal et al, 2001; Kennan et al, 2005; Anderson et al, 2006). In the study by Kennan et al (2005), baseline CBF increased 14%, when going from euglycemia to moderate hypoglycemia (plasma glucose=3.6 mmol/L). It has been earlier shown that increases in baseline CBF can decrease the BOLD signal through limiting the CBF response during subsequent activations (Vazquez et al, 2006; Cohen et al, 2002). This might, in part, explain the lack of neural activation-induced CBF augmentation during hypoglycemia. Nevertheless, while the results of Kennan et al do not support the concept of an increased glucose demand as the triggering factor for the flow increase during activation, one might also view those findings as yet another indication that hypoglycemia represents one condition in which coupling between CBF and glucose metabolism begins to fall apart.
In addition, there are other conditions associated with CMRglc/CBF uncoupling. Thus, unlike the hypoglycemia findings, seizure-prone mice displayed increased rCMRglc, in the absence of rCBF changes (Hosokawa et al, 1997), whereas in rats subjected to cortical impact trauma, rCMRglc/rCBF ratios were substantially elevated (Chen et al, 2004).
In conclusion, the studies of hypoglycemia do not support the concept of glucose lack, as the triggering mechanism for the flow increase occurring during functional activation. Despite these and additional findings, evidence of an apparent uncoupling of CBF/CMRglc under mild (hypoglycemia) to severe (seizure; neurotrauma) perturbations does not negate the presence of close coupling under normal physiologic circumstances. Such findings may only be taken as indicating that there are constraints on that linkage.
The Significance of Capillary Heterogeneity for Glucose Transport
For glucose, an increase in CBF in a homogenous vascular bed is an inefficient way to increase its transport across the BBB. Under normal physiologic conditions, the arterio-venous difference for glucose is about 10% and the unidirectional flux close to 20%. That is, approximately half of the glucose entering the brain from the capillaries diffuses back to the blood (Knudsen et al, 1990). With an arterio-venous difference of 10%, the average capillary concentration of glucose would be close to 95% of the arterial concentration. Thus, even an infinitely high increase in CBF, with preserved heterogeneity, would increase the average capillary concentration and thereby the glucose transfer from blood to brain by only 5%. Functional recruitment, in contrast, would be a much more efficient way to increase glucose supply. This is because in capillaries with a low flow, the average concentration of nutrients will approach the tissue level, and net transport will be minimal. Thus, if half of the capillaries had a high and the other half a low-linear velocity, then recruitment with homogenization of CBF to the high value would double the potential for glucose transfer from the blood to the brain. When discussing glucose transfer across the BBB, back flux from the tissue to the blood has also to be taken into consideration. Thus, a drop in peri-capillary glucose concentration might increase the net glucose uptake. Such a drop has been observed in activation studies using magnetic resonance spectroscopy (Chen et al, 1993; Frahm et al, 1996). However, in studies using direct measurements of brain tissue glucose and lactate, an essentially constant glucose concentration was found during activation, whereas lactate increased (Madsen et al, 1999). Thus, it seems that a CBF increase with capillary recruitment could be a key factor responsible for an increased glucose uptake during activation, although models postulating translocation of the endothelial glucose transporter (GLUT1) from intracellular sites to the plasma membrane need to be considered (Cornford et al, 2000).
Oxygen Metabolism—Cerebral Blood Flow Uncoupling
During functional activation studies, using positron emission tomography and the Kety–Schmidt technique did not reveal any major increases in CMRO2 (Fox et al, 1988; Madsen et al, 1995). In contrast, studies using the magnetic resonance BOLD methods has revealed a CMRO2 increase of about a third of the increase in CBF (Davis et al, 1998; Kim et al, 1999). Animal studies have shown that oxygen consumption not only increases during activation, but also decreases during deactivation (Hyder et al, 2001).
It has been hypothesized that this more modest increase in oxygen consumption still can explain the uncoupling of CBF and oxidative metabolism during functional activation because of diffusion limitations for oxygen diffusion limiting oxygen availability in the tissue (Buxton and Frank, 1997; Kuwabara et al, 1992), either because of limited diffusion across the BBB or diffusion limitations within the brain tissue. Below, we will look at these aspects separately and add new evidence from recent studies to classic models of oxygen transport and diffusion (Uludağ et al, 2004; Zheng et al, 2002; Kuschinsky and Paulson, 1992).
Oxygen Limitation Concept
Buxton and Frank (1997) formulated a model, which assumed a low tissue oxygen concentration and no capillary recruitment. This model showed a non-linear relationship between CBF and oxygen extraction, and, as a consequence, O2 extraction increased to a proportionately smaller degree than CBF did. Similar conclusions were reached by Vafaee and Gjedde (2000). In addition, in a more complex model with non-negligible tissue oxygen tension, thus allowing for back flux from tissue to blood by Valabrègue et al (2003), a non-linear relationship was found. Although these models have attracted much attention, they may still be challenged. In addition, in the following discussion, we will focus on two limitations, which may represent an oversimplification: the assumption of lack of capillary heterogeneity and of lack or limitation of back flux of oxygen from the tissue to the blood.
Oxygen Diffusion Across the Blood–Brain Barrier
To what extent capillary recruitment is capable of increasing tissue oxygen supply is not as simple to quantify as for glucose. An increased effective capillary surface area as well as an increase in oxygen delivery through an appropriate CBF increase would facilitate oxygen transfer across the BBB. Further, functional capillary recruitment will lead to an increased number of transport efficient capillaries in the tissue and thereby to shorter diffusion distances from the capillaries to the more remote part of the tissue. The latter might be of major significance and account for the increased cortical oxygen tension observed during functional activation (Cooper et al, 1966). Only a few studies have measured oxygen transport in the brain. Using the double indicator method, Grieb et al (1983) found no significant diffusion limitation for oxygen from blood to brain tissue. In a similar setup, however, Kassissia et al (1995) could not confirm the observation of Grieb et al, as they surprisingly did not observe back diffusion from brain tissue to blood according to their Figure 1 (Kassissia et al, 1995). Kassissia et al further used a mathematical model to analyze their data based on the assumption that there was no diffusion between capillaries. It was further assumed that capillary transit times were linearly related to their large vessel transit times. These assumptions are likely to be violated. Shunting by diffusion of rapidly diffusible substances from the arterial to the venous end of the cerebrovascular bed is well described in the literature (Brodersen et al, 1973) and several studies directly observing cerebral microcirculation microscopically have revealed a marked heterogeneity of capillary perfusion directions and velocities (Pawlik et al, 1981).
Thus, the physiologic validity of the model used in the study of Kassissia et al (1995) may be questioned. Their calculation of a capillary permeability surface area product for oxygen of 70 mL/g/min may, therefore, represent a gross underestimation, and oxygen is highly diffusible and rapidly released from hemoglobin. In conclusion, diffusion across the BBB seems not in any likelihood to explain the uncoupling of oxygen metabolism and CBF during activation.
Oxygen Diffusion in Brain Tissue
The oxygen limitation concept states that because of the diffusion gradient in the brain tissue, an increased oxygen metabolism associated with functional activation would, in the absence of a sufficient increase in rCBF, lead to an insufficient oxygen tension in the least oxygenated parts of the cerebral tissue, that is the mitochondria. The oxygen limitation hypothesis has been addressed through sophisticated modeling of cerebral oxygen consumption and delivery (Buxton and Frank, 1997; Zheng et al, 2002). In both models, recruitment or capillary heterogeneity as well as back diffusion of oxygen were not, however, taken into account. Accordingly, Hyder et al (1998) have used another model for oxygen delivery to the brain, in which they include a variable for increased diffusibility of oxygen with increased CBF. With this model, oxygen delivery to the brain is not critical during functional activation.
The old observation of an increased tissue oxygen tension in activated regions (Cooper et al, 1966) would seem non-compatible with the theory of deficient oxygen supply in functional activation. However, the models referred to imply a steeper gradient of the oxygen tension in the tissue, and this may lead to decreased oxygen tension in the cerebral tissue most distant from the capillaries concomitant with an increase of the average tissue oxygen tension. Recent studies have shed some light on the oxygen limitation hypothesis. In hypoxia, both acute and after adaptation to high altitude, the relative rCBF increase during functional activation remains unaltered (Mintun et al, 2001; Law et al, 2002; Tuunanen and Kauppinen, 2006), even though the BOLD response is diminished (Rostrup et al, 2005; Tuunanen and Kauppinen, 2006; Ho et al, 2008). Further, the same degree of deoxygenation of hemoglobin takes place during the passage of the blood through the cerebrovascular bed at high altitude and at sea level, reflecting a lower venous oxygen saturation and tension in view of the lower arterial oxygen saturation at high altitude (Møller et al, 2002).
Oxygenation at the Cellular Level
Detailed studies with high time resolution of the tissue oxygenation tension in rats has revealed an initial dip in oxygen tension during activation, lasting about 2 secs. This was followed by a marked increase of the oxygen tension with a delay of 1–2 secs as compared with the observed flow increase (Ances et al, 2001). Using visual stimulation in cats, and simultaneous recording of oxygen tension and single cell neural spike rate for 4 secs, revealed a decrease in oxygen tension accompanying the neural activity (Thompson et al, 2003). This could correspond to the initial dip in oxygen tension observed in the former study, but might also reflect a longer lasting decrease in oxygen tension in some highly active cells. In contrast to these studies, a recent preliminary report showed that the mitochondrial oxygen tension did not decrease during functional activation, but rather increased (Springett et al, 2007). These studies may give rise to some considerations regarding the spatial-temporal spread function. At the subcellular level, for example mitochondria (in which oxidative metabolism actually takes place), one could envisage that oxygen tension and the concentration of glucose and/or lactate was lower than in the surrounding cellular elements. In such subcellular fractions, oxygen tension might be low and decrease further during activation. However, this would also be the case for glucose and lactate. In fact, diffusion across cellular or subcellular lipid membranes is at least 10-fold larger for oxygen than for glucose and lactate. Thus, a very low oxygen tension in these subcellular compartments might very well be accompanied by similarly low concentrations of glucose and lactate. Considering the temporal relationship between metabolism and flow, a delay in the flow response might be anticipated. Thus, even with an instantaneous relaxation of the tone of the resistance vessels, there will be a delay before vasodilation occurs and new blood has flowed into the capillary bed. With an average capillary transit time of 1–2 secs, one would expect such a delay to be a few seconds in magnitude.
Kasischke et al, using a non-perfused brain slice preparation, elucidated the temporal and spatial dispersion of oxygenation and metabolic changes at the cellular level during activation. Using two-photon fluorescence imaging of NADH, evidence was found for a mitochondrial NADH oxidation related to neuronal dendrite activity during a 20 secs activation, maximal after 10 secs (Kasischke et al, 2004). This was followed by an increase in astrocytic response that gradually increased from 10 secs to a peak around 40 secs (Figure 1C). In a mathematical model of compartmentalized energy metabolism with complex NADH kinetics, Aubert et al (2007) found further evidence for distinct metabolic responses in two cell populations, probably representing neurons and astrocytes, also supporting the importance of lactate. The biphasic response, with an early increase in NADH oxidation (dendrites) and a late decrease (astrocytic), is completely compatible with earlier studies of an activation-induced resetting of the oxygen to glucose ratio in humans (Madsen et al, 1995) and rats (Madsen et al, 1998) and is also compatible with findings from a recent PET–FDG activation study (Vlassenko et al, 2006) discussed above.
rCBF and rCMRglc coupling: the astrocyte–arteriole connection
Signaling Factors
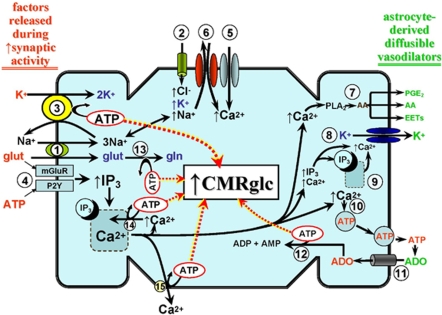
When attempting to understand the close coupling between rCBF and CMRglc, especially under conditions of increased neural activity, one cannot ignore the astrocyte. One reason is that astrocytes are major glucose-consuming cells. That is, in astrocytes, glycolytic conversion of glucose to lactate seems to be the preferred energy-generating pathway, as opposed to neurons, in which lactate and glucose oxidation may be more predominant (Berkich et al, 2007). Another consideration relates to the well-documented physical association between astrocytic processes (especially endfeet) and cerebral arterioles. Astrocytic endfeet extensively ensheath cerebral arterioles (Straub and Nelson, 2007). Astrocytes physically link neurons and their synapses with the vasculature, and are in a strategic position to convey neuronal signals to the blood vessels. Increased synaptic activity may lead to the release of factors (e.g., glutamate, ATP) that are ‘detected' by adjacent astrocytes (Figure 2). This initiates a signal that can be transmitted, even over multiple astrocytes, to perivascular glial endfeet, and elicits changes in arteriolar tone. During neural activation states, such astrocytic communication may help to ensure an adequate increase in substrate delivery through signaling both local and upstream arteriolar segments to dilate (Iadecola and Nedergaard, 2007; Xu et al, 2008). A key participant in the link between neural activation, inter-astrocytic communication, increased astrocytic glucose utilization, and vasodilation is Ca2+ (Takano et al, 2006; Scemes and Giaume, 2006; Wang et al, 2006). Ca2+ contribution is thought to arise from ATP and/or glutamate-mediated activation of astrocytic metabotropic purinergic and glutamatergic receptors (P2Y and mGluR, respectively) (Zonta et al, 2003; Nedergaard et al, 2003; Shi et al, 2008).
Figure 2.
A generalized depiction of the energy cost of neurovascular coupling and its impact on astrocytic glucose utilization (CMRglc). After enhanced synaptic activity, increases in extracellular levels of ATP, glutamate (glut), and K+ occur (shown in red on the left hand margin of the figure). It has been suggested that these factors, paricularly glutamate, arise from pre-synaptic neuronal elements, at least at low to moderate increases in synaptic activity. However, at higher levels of activity, post-synaptic contributions may come into play (Petzold et al, 2008). Glutamate enters the astrocyte through the glut-3Na+ cotransporter [1], whereas key K+ routes of entry include the Na+-K+-2Cl− cotransporter [2], and, in exchange for the increased Na+ from [1] and [2], the Na-K ATPase [3]. Glutamate and ATP can activate their respective metabotropic receptors [4] leading to IP3-mediated Ca2+ release from the ER. Depletion of ER Ca2+ promotes Ca2+ entry through store-operated Ca2+ channels [5]. In addition, the elevated intracellular Na+ may be exchanged for extracellular Ca2+, through the plasma membrane Na-Ca exchanger operating in reverse mode [6], contributing further to the elevation in intracellular Ca2+. The Ca2+ rise provides a proximal stimulus for a variety of vasodilating pathways. Some of the major paracrine vasodilating factors arising from these pathways are shown in green on the right hand margin of the figure. This includes arachidonic acid (AA) derived from Ca2+-activated phospholipase A2 (PLA2) [7]. The AA can be further metabolized, through cyclooxygenase-1 (COX1), to vasodilating prostanoids, primarily PGE2, and/or converted to epoxyeicosatrienoic acids (EETs) by a cytochrome P450 epoxygenase (Takano et al, 2006; Shi et al, 2008). Arachidonic acid itself can act as a direct vasodilator (Bryan et al, 2006). An increase in K+ levels (up to 15–20 mmol/L) in the extracellular milieu provides a potent vasodilating stimulus through acting on smooth muscle inward rectifier K+ channels (Straub and Nelson, 2007). A key conduit for astrocytic release of K+ is the large-conductance Ca2+-operated K+ (BKCa) channel [8]. Those channels are well expressed in astrocyte foot processes (Price et al, 2002), along with IP3-sensitive Ca2+ storage and release sites [9] (Straub et al, 2006; Straub and Nelson, 2007). The latter is thought to provide the localized elevations in [Ca2+] necessary for channel activation. Increased cytosolic Ca2+ may also promote ATP exocytosis (Blum et al, 2008) [10], although Ca2+-independent and plasma membrane hemichannel routes may also exist (Coco et al, 2003). The released ATP is rapidly hydrolyzed to adenosine (ADO) by astrocytic ecto-nucleotidases (Xu and Pelligrino, 2007). The ADO is returned to the cell through nucleoside transporters [11] to enter the adenosine kinase-mediated adenine nucleotide salvage pathway [12]. There are multiple sites of ATP consumption that, in turn, may trigger increased CMRglc. Some of the key sites that can be directly linked to astrocyte-derived vasodilating factors are shown. Principal among these is the plasma membrane Na-K ATPase [3]. Other ATP-consuming sites depicted in the figure are glutamine (gln) synthase-mediated conversion of glut to gln [13], the ER Ca-ATPase [14], the plasma membrane Ca-ATPase [15], and adenosine kinase-mediated formation of ADP and AMP [12].
As illustrated in Figure 2, activation of mGluR or P2Y receptors promotes IP3-evoked release of Ca2+ from intracellular storage sites in the endoplasmic reticulum (ER). This may promote a cascade of events resulting in further increases in intracellular Ca2+ levels through activation of plasma membrane store-operated Ca2+ channels and/or increased activity of the plasma membrane Na+/Ca2+ exchanger operating in the reverse mode (Blaustein et al, 2002; Rojas et al, 2007). Although the increased intracellular [Ca2+] may have an important function both in propagating astrocyte-to-astrocyte signaling, for example through Ca2+-stimulated ATP and glutamate exocytosis (Bezzi et al, 2004; Bowser and Khakh, 2007; Pangrsic et al, 2007) and Ca2+-dependent vasodilation (see Figure 2 and below), this added Ca2+ load is likely to impact on astrocytic energy demand through activation of ATP-dependent Ca2+ extrusion at the plasma membrane and ATP-dependent refilling of ER Ca2+ stores.
Another entity linked to inter-astrocytic communication and regulation of arteriolar tone is K+. Thus, it has been suggested that, during brain activation, a spatial redistribution of K+ may occur, whereby the increases in extracellular [K+] that take place at the sites of increased neuronal activity would be removed by nearby astrocytes, through a variety of influx mechanisms (e.g., inward rectifier K+ [Kir] channels; Na-K-2Cl cotransporters; and Na-K ATPase), and transferred (possibly through multiple astrocytes, through gap junctions) to astrocytic endfeet in which it could be released to the extracellular environment of adjacent cerebral arterioles, through a Ca2+-dependent mechanism primarily involving large-conductance Ca2+-operated K+ (BKCa) channels (Filosa et al, 2006; Straub and Nelson, 2007). Evidence indicates that BKCa channels are particularly concentrated in astrocyte endfoot plasma membranes facing cerebral microvessels (Price et al, 2002). Also expressed in astrocyte endfoot processes are ER IP3 receptors which, when activated, seem to provide the necessary (albeit spatially restricted) Ca2+ increases for BKCa channel opening (Straub et al, 2006). The subsequent interaction of K+, released through endfoot BKCa channels, with Kir channels on arteriolar smooth muscle represents a potentially powerful stimulus for vasodilation (Paulson and Newman, 1987; Filosa et al, 2006).
In addition, as summarized in Figure 2, one must consider the energy cost arising from the increase in extracellular [K+], and its uptake into astrocytes, during periods of enhanced neural activity. The increased extracellular [K+], together with the associated enhanced entry of Na+ through the Na+-K+-2Cl− cotransporter plus the Na/glutamate cotransporter, will stimulate Na-K ATPase activity. This represents a major, if not the principal, demand on glycolytic energy production. It should also be noted that some of the glutamate taken up by the astrocyte will be transferred to neurons in the form of glutamine. The intra-astrocytic conversion of glutamate to glutamine consumes ATP. Finally, the ATP released in the process of inter-astrocytic communication is lost as an energy source. That is, it cannot return to the cell as ATP, as it is rapidly hydrolyzed by ecto-nucleotidases, which are well expressed on astrocytes (Wink et al, 2006; Zimmermann, 2006), ultimately forming adenosine. To limit depletion of intracellular adenine nucleotide levels, adenosine re-enters the astrocyte [through a cell membrane nucleoside transport system (Peng et al, 2005)] to enter adenine nucleotide salvage pathways. The key enzyme of the salvage pathway is adenosine kinase, whose expression is largely confined to astrocytes (Boison, 2008). The energy cost of salvage arises from the ATP consumed in the adenosine kinase-mediated formation of ADP and AMP.
In a compelling study using primary cultures of murine cortical astrocytes, Bernardinelli et al (2004) examined the implications of the appearance of an astrocytic Ca2+ wave, as it impacts on glucose utilization and, by extension, energy production. These authors reported that an electrically evoked induction of a Ca2+ wave was accompanied both by a parallel Na+ wave and a wave of increased glucose uptake as well. Furthermore, the Na+ wave was associated with an increased glutamate uptake, presumably through the Na/glutamate cotransporter. Thus, not only may propagated inter-astrocytic cationic waves occur in response to neural activation, but also parallel ‘waves' of increased glucose metabolism may occur as well.
Besides its postulated function in BKCa channel-mediated vasodilations, the arrival of the Ca2+ wave at astrocytic endfoot points of contact with arterioles can elicit smooth muscle relaxation through other mechanisms. This includes stimulation of astrocytic Ca2+-activated phospholipase A2 and the release of arachidonic acid (AA). A function for astrocyte-derived AA metabolites, and AA itself, as paracrine factors in neural activation-evoked cerebral vasodilation has been indicated in studies showing neurovascular coupling to be attenuated in the presence of phospholipase A2, cyclooxygenase-1, and/or epoxygenase inhibition (Takano et al, 2006; Wang et al, 2006; Shi et al, 2008). Finally, one cannot ignore the potential vasodilating actions associated with the adenosine that is formed from extracellular ATP. That adenosine may influence brain arterioles indirectly by virtue of interactions with astrocytic adenosine receptors (A2B), perhaps contributing to inter-astrocytic (Ca2+) signaling (Shi et al, 2008) or can act as a paracrine factor, directly influencing vascular smooth muscle adenosine receptors (e.g., A2A, see Meno et al, 2001), leading to vasodilation.
Metabolic Consequences of Signaling
Taken together, the signaling factors given above can place substantial demands on astrocytic energy sources and probably provide a potent stimulus for increasing CMRglc during neural activation. There is strong evidence that the most-used procedures for measuring CMRglc in vivo (i.e., PET–FDG; autoradiographic 2-DG) largely reflect glucose uptake and use in astrocytes rather than neurons, both under resting conditions and during neural activation (Magistretti and Pellerin, 1996; Barros et al, 2005; Hyder et al, 2006). Thus, the decrease in the CMRO2/CMRglc ratio and the earlier noted close proportionality between increases in CMRglc and CBF during brain activation is likely to reflect metabolic activity in astrocytes supporting signaling factors that may have direct influence on brain arterioles. Moreover, additional consideration should be given to the postulate that the signaling components, and the energetic machinery supporting them, may be concentrated within subcompartments of the astrocyte in which oxidative metabolism is less active—that is, the peripheral astrocytic processes that ‘sample' synaptic activity, as opposed to the cell bodies (see review by Hertz et al, 2007). Put in another way, the lactate formed through glycolysis may be oxidized at sites separate from those triggering glucose metabolism. This could occur through lactate diffusion to the cell body in the astrocyte in which it was formed, transfer of lactate to other astrocytes (through e.g., gap junctions), and monocarboxylic transporter-linked transfer of lactate into neurons (Dienel and Cruz, 2008; Pellerin et al, 2007) or blood. Another relevant manifestation of compartmentation relates to the presence of glycogen granules in astrocytic endfeet (Dienel and Cruz, 2003). Glycogen, which is preferentially found in astrocytes, has been reported to provide a meaningful and rapid source of metabolic fuel during increased brain activity. However, if that energy is derived from metabolism of preformed glycogen, it will not directly affect measurements of CMRglc. However, enhanced ‘glucose–glycogen shunting' (increased glycogen turnover) after activation (Shulman et al, 2001) will influence the measured CMRglc value and contribute to reductions in the CMRO2/CMRglc ratio.
Signaling and Metabolism: Summary
There is abundant evidence that astrocytes have a central position in neurovascular coupling (Figure 2). Increases in synaptic activity are accompanied by an elevated presence of various signaling entities in the extracellular fluid bathing adjacent astrocytes. These include ATP, K+, and glutamate. ATP and glutamate can interact with receptors on astrocytes, which may give rise to a wave of increased Ca2+ across multiple astrocytes that eventually reaches astrocytic endfeet in contact with cerebral arterioles. The increased extracellular [K+] can enter astrocytes through several routes, in which it may be spatially redistributed and released from endfeet through a Ca2+-dependent process involving BKCa channels, subsequently eliciting arteriolar smooth muscle relaxation mediated by Kir channels. In addition, the extracellular ATP can be rapidly converted to adenosine, another potent signaling molecule and vasodilator. The astrocytic uptake of K+ and glutamate is associated with a rise in intracellular [Na+]. Taken together, the above processes, many of which can be linked directly to arteriolar dilation, place a substantial demand on astrocytic energy sources. In astrocytes, a significant portion of that energy may be derived from non-oxidative glucose consumption. Moreover, the model proposed in Figure 2 could be consistent with a later activation of glycolysis in astrocytes, after neuronal activation, as proposed by Kasischke et al (2004). That is, the astrocyte ATP utilization and subsequent energy replenishment through increased glycolysis might be expected to occur in the immediate post-stimulus recovery phase. Although this may contribute to the closer association between increased CBF and increased CMRglc (as opposed to CMRO2) observed during increased brain activity, it is probably not entirely accurate, as the Kasischke et al study was performed in non-perfused brain slices. Moreover, attempts to explain the CBF and CMRglc proportionality, or the lack thereof when considering CMRO2, can be confounded by non-linearities, threshold effects, variations in the influences arising from pre- versus post-synaptic neuronal elements (Petzold et al, 2008), and compartmentation of energy production within and among brain cells (Charles, 2005; Hertz et al, 2007).
Concluding remarks
In the above consideration of substrate delivery to the brain during functional activation, we endeavored to take all relevant physiologic players into consideration: diffusibility of oxygen and glucose; lack of classic capillary recruitment, but presence of functional recruitment as a consequence of reduced capillary heterogeneity with increased CBF; as well as the very tight coupling between increases in CBF and CMRglc in contrast to a lower relative increase in oxygen consumption. Further, recent studies of oxidative and non-oxidative glucose metabolism during activation as well as studies on the astrocytic regulation of rCBF response support the view that the disproportional rCBF increase during functional activation relates to the need for sufficient glucose supply. Consequently, the increased oxygen content in the venous blood from the brain observed in the BOLD response is a secondary event to the CBF-glucose coupling. Future research may be directed toward the understanding of the basis for the CBF-glucose coupling—especially why the brain needs an excess glucose uptake during activation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Ances BM, Buerk DG, Greenberg JH, Detre JA. Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats. Neurosci Lett. 2001;306:106–110. doi: 10.1016/s0304-3940(01)01868-7. [DOI] [PubMed] [Google Scholar]
- Anderson AW, Heptulla RA, Driesen N, Flanagan D, Goldberg PA, Jones TW, Rife F, Sarofin H, Tamborlane W, Sherwin R, Gore JC. Effects of hypoglycemia on human brain activation measured with fMRI. Magn Reson Imaging. 2006;24:693–697. doi: 10.1016/j.mri.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Aubert A, Pellerin L, Magistretti PJ, Costalat R. A coherent neurobiological framework for functional neuroimaging provided by a model integrating compartmentalized energy metabolism. Proc Natl Acad Sci USA. 2007;104:4188–4193. doi: 10.1073/pnas.0605864104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Barros LF, Porras OH, Bittner CX. Why glucose transport in the brain matters for PET. Trends Neurosci. 2005;28:117–119. doi: 10.1016/j.tins.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Berkich DA, Ola MS, Cole J, Sweatt AJ, Hutson SM, LaNoue KF. Mitochondrial transport proteins of the brain. J Neurosci Res. 2007;85:3367–3377. doi: 10.1002/jnr.21500. [DOI] [PubMed] [Google Scholar]
- Bernardinelli Y, Magistretti PJ, Chatton JY. Astrocytes generate Na+-mediated metabolic waves. Proc Natl Acad Sci USA. 2004;101:14937–14942. doi: 10.1073/pnas.0405315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment thatis competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bie-Olsen LG, Kjaer TW, Pedersen-Bjergaard U, Lonsdale MN, Holst JJ, Law I, Thorsteinsson B. Changes of cognition and regional cerebral activity during acute hypoglycemia in normalsubjects: A H2 15O positron emission tomographic study. J Neurosci Res. 2009;87:1922–1928. doi: 10.1002/jnr.22002. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci. 2002;976:356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Blum AE, Joseph SM, Przybylski RJ, Dubyak GR. Rho-family GTPases modulate Ca2+-dependent ATP release from astrocytes. Am J Physiol Cell Physiol. 2008;295:C231–C241. doi: 10.1152/ajpcell.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. The adenosine kinase hypothesisof epileptogenesis. Prog Neurobiol. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. Vesicular ATP is the predominant causeof intercellular calcium waves in astrocytes. J Gen Physiol. 2007;129:485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Paulson OB, Bolwig TG, Rogon ZE, Rafaelsen OJ, Lassen NA. Cerebral hyperemia in electrically induced epileptic seizures. Arch Neurol. 1973;28:334–338. doi: 10.1001/archneur.1973.00490230070010. [DOI] [PubMed] [Google Scholar]
- Bryan RM., Jr, You J, Phillips SC, Andresen JJ, Lloyd EE, Rogers PA, Dryer SE, Marrelli SP. Evidence for two-pore domain potassium channels in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2006;291:H770–H780. doi: 10.1152/ajpheart.01377.2005. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Introduction to Functional Magnetic Resonance Imaging Principles and Techniques. Cambridge University Press: Cambridge; 2002. pp. 56–57. [Google Scholar]
- Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Charles A. Reaching out beyond the synapse: glial intercellular waves coordinate metabolism. Sci STKE. 2005;2005:e6. doi: 10.1126/stke.2702005pe6. [DOI] [PubMed] [Google Scholar]
- Chen SF, Richards HK, Smielewski P, Johnstrom P, Salvador R, Pickard JD, Harris NG. Relationship between flow-metabolism uncoupling and evolving axonal injury after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2004;24:1025–1036. doi: 10.1097/01.WCB.0000129415.34520.47. [DOI] [PubMed] [Google Scholar]
- Chen W, Novotny EJ, Zhu XH, Rothman DL, Shulman RG. Localized 1H NMR measurement of glucose consumption in the human brain during visual stimulation. Proc Natl Acad Sci USA. 1993;90:9896–9900. doi: 10.1073/pnas.90.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Cooper R, Crow HJ, Walter WG, Winter AL. Regional control of cerebral vascular reactivity and oxygen supply in man. Brain Res. 1966;3:174–191. doi: 10.1016/0006-8993(66)90075-8. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Nguyen EV, Landaw EM. Acute upregulation of blood-brain barrier glucose transporter activity in seizures. Am J Physiol Heart Circ Physiol. 2000;279:H1346–H1354. doi: 10.1152/ajpheart.2000.279.3.H1346. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Ball KK, Dienel GA. Functional imaging of focal brain activation in conscious rats: impact of [(14)C]glucose metabolite spreading and release. J Neurosci Res. 2007;85:3254–3266. doi: 10.1002/jnr.21193. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Quistorff B, Danielsen ER, Selmer C, Vogelsang T, Secher NH. A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J Physiol. 2004;554:571–578. doi: 10.1113/jphysiol.2003.055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Neighborly interactions of metabolically-activated astrocytes in vivo. Neurochem Int. 2003;43:339–354. doi: 10.1016/s0197-0186(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Imaging brain activation: simple pictures of complex biology. Ann NY Acad Sci. 2008;1147:139–70. doi: 10.1196/annals.1427.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Frahm J, Krüger G, Merboldt KD, Kleinschmidt A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med. 1996;35:143–148. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- Gjedde A.1991Is oxygen diffusion limiting for blood-brain transfer of oxygen Brain Work and Mental ActivityLassen NA, Ingvar DH, Raichle MA, Friberg L. (eds).Copenhagen: Munksgaard; 177–184. [Google Scholar]
- Grieb P, Forster RE, Pape PC. Oxygen indicator dilution curves of the canine cerebral circulation. Adv Exp Med Biol. 1983;159:103–117. doi: 10.1007/978-1-4684-7790-0_10. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, Paulson OB. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am J Physiol. 1996;270:E746–E751. doi: 10.1152/ajpendo.1996.270.5.E746. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hertz MM, Paulson OB. Heterogeneity of cerebral capillary flow in manand its consequences for estimation of blood-brain barrier permeability. J Clin Invest. 1980;65:1145–1151. doi: 10.1172/JCI109769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YC, Vidyasagar R, Shen Y, Balanos GM, Golay X, Kauppinen RA. The BOLD response and vascular reactivity during visual stimulation in the presence of hypoxic hypoxia. Neuroimage. 2008;41:179–188. doi: 10.1016/j.neuroimage.2008.02.048. [DOI] [PubMed] [Google Scholar]
- Hosokawa C, Ochi H, Yamagami S, Yamada R. Regional cerebral blood flow and glucose utilization in spontaneously epileptic EL mice. J Nucl Med. 1997;38:613–616. [PubMed] [Google Scholar]
- Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Hyder F, Shulman RG, Rothman DL. A model for the regulation of cerebral oxygen delivery. J Appl Physiol. 1998;85:554–564. doi: 10.1152/jappl.1998.85.2.554. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Kassissia IG, Goresky CA, Rose CP, Schwab AJ, Simard A, Huet PM, Bach GG. Tracer oxygen distribution is barrier-limited in the cerebral microcirculation. Circ Res. 1995;77:1201–1211. doi: 10.1161/01.res.77.6.1201. [DOI] [PubMed] [Google Scholar]
- Kennan RP, Takahashi K, Pan C, Shamoon H, Pan JW. Human cerebral blood flow and metabolism in acute insulin-induced hypoglycemia. J Cereb Blood Flow Metab. 2005;25:527–534. doi: 10.1038/sj.jcbfm.9600045. [DOI] [PubMed] [Google Scholar]
- Kim SG, Rostrup E, Larsson HB, Ogawa S, Paulson OB. Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med. 1999;41:1152–1161. doi: 10.1002/(sici)1522-2594(199906)41:6<1152::aid-mrm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Knudsen GM, Pettigrew KD, Paulson OB, Hertz MM, Patlak CS. Kinetic analysis of blood-brain barrier transport of D-glucose in man: quantitative evaluation in the presence of tracer backflux and capillary heterogeneity. Microvasc Res. 1990;39:28–49. doi: 10.1016/0026-2862(90)90057-x. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W, Paulson OB. Capillary circulation in the brain. Cerebrovasc Brain Metab Rev. 1992;4:261–286. [PubMed] [Google Scholar]
- Kuwabara H, Ohta S, Brust P, Meyer E, Gjedde A. Density of perfused capillaries in living human brain during functional activation. Prog Brain Res. 1992;91:209–215. doi: 10.1016/s0079-6123(08)62337-7. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Ingvar DH, Skinhøj E. Brain function and blood flow. Sci Am. 1978;239:62–71. doi: 10.1038/scientificamerican1078-62. [DOI] [PubMed] [Google Scholar]
- Law I, Roach RC, Olsen NV, Holm S, Nowak M, Hornbein TF, Paulson OB.2002Oxygen delivery to the brain during behavioral activation at acute normobaric hypoxemia International Congress SeriesTomita M, Kanno I, Hamel E (eds).Amsterdam: Elsevier Science; 87–97. [Google Scholar]
- Lubow JM, Pinon IG, Avogaro A, Cobelli C, Treeson DM, Mandeville KA, Toffolo G, Boyle PJ. Brain oxygen utilization is unchanged by hypoglycemia in normalhumans: lactate, alanine, and leucine uptake are not sufficient to offset energy deficit. Am J Physiol Endocrinol Metab. 2006;290:E149–E153. doi: 10.1152/ajpendo.00049.2005. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normalduring recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19:393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bülow J, Holm S, Wildschiødtz G, Paulson OB, Lassen NA. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1995;15:485–491. doi: 10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Linde R, Hasselbalch SG, Paulson OB, Lassen NA. Activation-induced resetting of cerebral oxygen and glucose uptake in the rat. J Cereb Blood Flow Metab. 1998;18:742–748. doi: 10.1097/00004647-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular bases of brain energy metabolism and their relevance to functional brain imaging: evidence for a prominent role of astrocytes. Cereb Cortex. 1996;6:50–61. doi: 10.1093/cercor/6.1.50. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Tkác I, Gruetter R, Van de Moortele PF, Maraviglia B, Uğurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2018–H2027. doi: 10.1152/ajpheart.2001.281.5.H2018. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci USA. 2001;98:6859–6864. doi: 10.1073/pnas.111164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Vlassenko AG, Rundle MM, Raichle ME. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc Natl Acad Sci USA. 2004;101:659–664. doi: 10.1073/pnas.0307457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller K, Paulson OB, Hornbein TF, Colier WN, Paulson AS, Roach RC, Holm S, Knudsen GM. Unchanged cerebral blood flow and oxidative metabolism after acclimatization to high altitude. J Cereb Blood Flow Metab. 2002;22:118–126. doi: 10.1097/00004647-200201000-00014. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Wittendorp-Rechenmann E, Lam CD. Selective uptake of [14C]2-deoxyglucose by neurons and astrocytes: high-resolution microautoradiographic imaging by cellular 14C-trajectography combined with immunohistochemistry. J Cereb Blood Flow Metab. 2004;24:1004–1014. doi: 10.1097/01.WCB.0000128533.84196.D8. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Wang J, Rao H, Swanson RL, Wintering N, Karp JS, Alavi A, Greenberg JH, Detre JA. Concurrent CBF and CMRGlc changes during human brain activation by combined fMRI-PET scanning. Neuroimage. 2005;28:500–506. doi: 10.1016/j.neuroimage.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow. Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik G, Rackl A, Bing RJ. Quantitative capillary topography and blood flow in the cerebral cortex of cats: an in vivo microscopic study. Brain Res. 1981;208:35–58. doi: 10.1016/0006-8993(81)90619-3. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroenergetics: calling uponastrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- Peng L, Huang R, Yu AC, Fung KY, Rathbone MP, Hertz L. Nucleoside transporter expression and function in cultured mouse astrocytes. Glia. 2005;52:25–35. doi: 10.1002/glia.20216. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Hirsch IB, Cryer PE. Effect of stepped hypoglycemia on regional cerebral blood flow response to physiological brain activation. Am J Physiol. 1996;270:H554–H559. doi: 10.1152/ajpheart.1996.270.2.H554. [DOI] [PubMed] [Google Scholar]
- Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 2002;956:183–193. doi: 10.1016/s0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci USA. 1991;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas H, Colina C, Ramos M, Benaim G, Jaffe EH, Caputo C, DiPolo R. Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers Ca(i)2+-induced Ca2+ release in rat cerebellar Type-1 astrocytes. J Neurochem. 2007;100:1188–1202. doi: 10.1111/j.1471-4159.2006.04303.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal JM, Amiel SA, Yágüez L, Bullmore E, Hopkins D, Evans M, Pernet A, Reid H, Giampietro V, Andrew CM, Suckling J, Simmons A, Williams SC. The effect of acute hypoglycemia on brain function and activation: a functional magnetic resonance imaging study. Diabetes. 2001;50:1618–1626. doi: 10.2337/diabetes.50.7.1618. [DOI] [PubMed] [Google Scholar]
- Rostrup E, Larsson HB, Born AP, Knudsen GM, Paulson OB. Changes in BOLD and ADC weighted imaging in acute hypoxia during sea-level and altitude adapted states. Neuroimage. 2005;28:947–955. doi: 10.1016/j.neuroimage.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Sibson NR, Hyder F, Shen J, Behar KL, Shulman RG. In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate-glutamine neurotransmitter cycle and functional neuroenergetics. Philos Trans R Soc Lond B Biol Sci. 1999;354:1165–1177. doi: 10.1098/rstb.1999.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol. 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992;12:584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab. 2008;28:111–125. doi: 10.1038/sj.jcbfm.9600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley RP, LaManna JC. Determination of rat cerebral cortical blood volume changes by capillary mean transit time analysis during hypoxia, hypercapnia and hyperventilation. Brain Res. 1988;454:170–178. doi: 10.1016/0006-8993(88)90816-5. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci USA. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha AK, Klein J, Schultze P, Weiss J, Weiss HR. Cerebral regional capillary perfusion and blood flow after carbon monoxide exposure. J Appl Physiol. 1991;71:1196–1200. doi: 10.1152/jappl.1991.71.4.1196. [DOI] [PubMed] [Google Scholar]
- Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab. 2003;23:658–664. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- Springett R, Abajian M, Ripple M. Mitochondrial oxygenation in the rat cerebral cortex. J Cereb Blood Flow Metab. 2007;27 (S1:BP03–BP02W. [Google Scholar]
- Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol. 2006;128:659–669. doi: 10.1085/jgp.200609650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub SV, Nelson MT. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc Med. 2007;17:183–190. doi: 10.1016/j.tcm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Thompson JK, Peterson MR, Freeman RD. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science. 2003;299:1070–1072. doi: 10.1126/science.1079220. [DOI] [PubMed] [Google Scholar]
- Tuunanen PI, Kauppinen RA. Effects of oxygen saturation on BOLD and arterial spin labelling perfusion fMRI signals studied in a motor activation task. Neuroimage. 2006;30:102–109. doi: 10.1016/j.neuroimage.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Uludağ K, Dubowitz DJ, Yoder EJ, Restom K, Liu TT, Buxton RB. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage. 2004;23:148–155. doi: 10.1016/j.neuroimage.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Urrila AS, Hakkarainen A, Heikkinen S, Vuori K, Stenberg D, Häkkinen AM, Lundbom N, Porkka-Heiskanen T. Metabolic imaging of human cognition: an fMRI/1H-MRS study of brain lactate response to silent word generation. J Cereb Blood Flow Metab. 2003;23:942–948. doi: 10.1097/01.WCB.0000080652.64357.1D. [DOI] [PubMed] [Google Scholar]
- Vafaee MS, Gjedde A. Model of blood-brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex. J Cereb Blood Flow Metab. 2000;20:747–754. doi: 10.1097/00004647-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Valabrègue R, Aubert A, Burger J, Bittoun J, Costalat R. Relation between cerebral blood flow and metabolism explained by a model of oxygen exchange. J Cereb Blood Flow Metab. 2003;23:536–545. doi: 10.1097/01.WCB.0000055178.31872.38. [DOI] [PubMed] [Google Scholar]
- Vazquez AL, Cohen ER, Gulani V, Hernandez-Garcia L, Zheng Y, Lee GR, Kim SG, Grotberg JB, Noll DC. Vascular dynamics and BOLD fMRI: CBF level effects and analysis considerations. Neuroimage. 2006;32:1642–1655. doi: 10.1016/j.neuroimage.2006.04.195. [DOI] [PubMed] [Google Scholar]
- Vetterlein F, Demmerle B, Bardosi A, Göbel U, Schmidt G. Determination of capillary perfusion pattern in rat brain by timed plasma labeling. Am J Physiol. 1990;258:H80–H84. doi: 10.1152/ajpheart.1990.258.1.H80. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Rundle MM, Mintun MA. Human brain glucose metabolism mayevolve during activation: findings from a modified FDG PET paradigm. Neuroimage. 2006;33:1036–1041. doi: 10.1016/j.neuroimage.2006.06.065. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Wink MR, Braganhol E, Tamajusuku AS, Lenz G, Zerbini LF, Libermann TA, Sevigny J, Battastini AM, Robson SC. Nucleoside triphosphate diphosphohydrolase-2 (NTPDase2/CD39L1) is the dominant ectonucleotidase expressed by rat astrocytes. Neuroscience. 2006;138:421–432. doi: 10.1016/j.neuroscience.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol. 2008;294:H622–H632. doi: 10.1152/ajpheart.00530.2007. [DOI] [PubMed] [Google Scholar]
- Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol. 2007;92:647–651. doi: 10.1113/expphysiol.2006.036863. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Martindale J, Johnston D, Jones M, Berwick J, Mayhew J. A model of the hemodynamic response and oxygen delivery to brain. Neuroimage. 2002;16:617–637. doi: 10.1006/nimg.2002.1078. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp. 2006;276:113–128. [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]