Abstract
Natural CD4+CD25+Foxp3+ regulatory T cells (Treg) effectively prevent autoimmune disease development, but their role in maintaining physiological tolerance against self Ag of internal organs is not yet defined. Herein, we quantified disease-specific Treg (DS-Treg) as Treg that preferentially suppress one autoimmune disease over another in day 3-thymectomized recipients. A striking difference was found among individual lymph nodes (LN) of normal mice: Treg from draining-LN were 15-50 times more efficient than those of non-draining LN at suppressing autoimmune disease of ovary, prostate and lacrimal gland. The difference disappeared upon autoAg ablation and returned upon autoAg re-expression. In contrast, the CD4+CD25- effector T cells from different individual LN induced multi-organ inflammation with comparable organ distribution. We propose that peripheral tolerance for internal organs relies on the control of autoreactive effector T cells by strategic enrichment of Ag-specific Treg in the regional LN.
Keywords: Regulatory T cells, Tolerance, Lymph Nodes, Self-Antigen
INTRODUCTION
Self tolerance is the fundamental process that guards against autoimmune disease. Maintained by self antigens, tolerance is a physiological function that operates in the normal steady state. Although studies have implicated Treg in peripheral tolerance, many details are not yet clarified. This is especially true for the internal organs, including: endocrine organs, gonads, pancreatic islets, thyroid, central nervous system, and exocrine glands – all of which are major targets of human autoimmune disease. A current concept, the LN surveyance by Ag specific Treg, is supported by the finding that Ag specific Treg are continuously capacitated by responding to autoAg in normal regional LN5 (1). Accordingly, Treg with transgenic TCR proliferate in regional LN of normal or lymphopenic host (2,3), and regional LN are a location where Treg control effector autoimmune T cell responses (4). Recently, in mice with highly restricted TCR-VDJ expression, the Treg from individual LN showed distinctive TCR repertoires based on VDJ analysis (5). Collectively, these findings support LN-specific distribution of antigen-specific Treg; however, the functional capacity of the Treg in autoimmune disease suppression was not investigated in these studies, therefore the relevance of the data to self tolerance has remained undefined.
The requisite to investigate tolerance mechanisms, based on detection and functional analysis of antigen specific polyclonal T cells, is hindered by their rare occurrence. As an alternative approach, we have investigated antigen-specific polyclonal Treg as a T cell population that suppresses one organ-specific autoimmune disease over another in the day 3-thymectomized (d3tx) mice (disease-specific Treg [DS-Treg]) (6,7). Herein describe a functional assay for the DS-Treg, and document for the first time their regional and non-random distribution in the LN of normal mice.
MATERIALS AND METHODS
Mice
Mice and breeders, obtained from NCI (Frederick MD), or the Jackson Laboratory (Bar Harbor, ME), were kept in a pathogen-free facility. Thymectomy and orchiectomy were done under hypothermia (4,8). 5α-dihydrotestosterone pellets (20mg/60 day release; Innovative Research of America, Sarasota, FL) were implanted subcutaneously. Experiments were performed according to guidelines of the Animal Care and Use Committee of UVA.
Cell Transfer
Renal, cervical, and lumbar/sacral LN were identified and used as regional LN of ovary, lacrimal gland, and prostate, respectively. CD4+CD25+ Treg or CD4+ CD25- T cells were isolated to ~95% purity via magnetic beads (Auto-MACS system, Miltenyi Biotec). Cells were transferred by i.p. injection to 5 day-old d3tx mice, which were studied 8 weeks later, or by i.v. injection into syngeneic adult RAG knockout recipients that were studied 3-4 weeks later.
Histology and Immunofluorescence
Pathology was graded as blinded samples as follows (4). AOD: 1 (focal oophoritis), 4 (severe and diffuse inflammation), 2 and 3 (increasing monocytic inflammation). EAP: 1 (focal monocytic inflammation), 4 (severe EAP with diffuse inflammation and loss of glands), 2 and 3 (increasing severity between 1 and 4). Inflammation in the following organs was similarly graded as EAP: lacrimal gland, testis and epididymis, lung, liver, colon, skin, kidney, stomach, thyroid, eye, small intestine. Indirect immunofluorescence was used to detect serum prostate and ovarian antibodies (dilution 1:50). Pooled sera with prostate antibodies from d3tx mice were used to detect prostate antigen expression.
RESULTS AND DISCUSSION
Normal ovarian-LN draining Treg have enhanced capacity to suppress AOD
D3tx B6AF1 mice develop dacryloadenitis (DA), concurrently with autoimmune ovarian disease (AOD) or experimental autoimmune prostatitis (EAP) (7), and autoAb against the respective tissue Ag (8-10). In each case, the disease and autoAb response are fully suppressed by 0.5×106 or more Treg pooled from all the LN of normal donors (8).
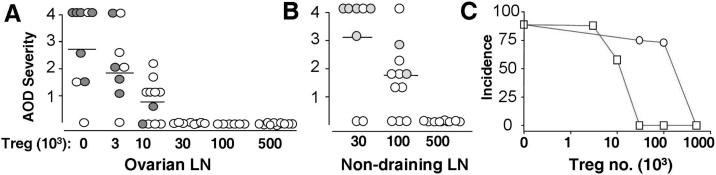
To compare the Treg from regional versus non-regional LN in AOD suppression, CD4+CD25+ Treg from ovarian LN, or pooled from non-draining LN of untreated B6AF1 female donors, were transferred to groups of d3tx female mice at doses of 0.003 to 0.5 × 106, and the ovarian pathology was determined 8 weeks later. AOD was suppressed only by 0.5×106 Treg from non-draining LN (Figure 1B and Supplemental Figure 1B). In stark contrast, AOD was completely suppressed by 0.03×106 Treg from the ovarian LN (Figure 1A and Supplemental Figure 1A). Thus a 15-fold difference in AOD suppression was detectable between the Treg from draining and non-draining LN (Figure 1C). Serum oocyte autoantibody was detected only in d3tx recipients of less than 0.01×106 Treg from ovarian LN, and in d3tx recipients of less than 0.1.×106 Treg from the non-draining LN (Figures 1A and B, Supplemental Figure 1C).
Figure 1.
Treg from normal ovarian LN suppress AOD more efficiently than Treg from non-ovarian LN. (A and B): Severity of AOD in d3tx recipients of ovarian LN Treg (A) or non-ovarian LN Treg (B). Filled circles represent positive serum oocyte antibody. (C): AOD incidences between recipients of ovarian LN Treg (squares) and recipients of non-ovarian LN Treg (circles) (n = 6-8).
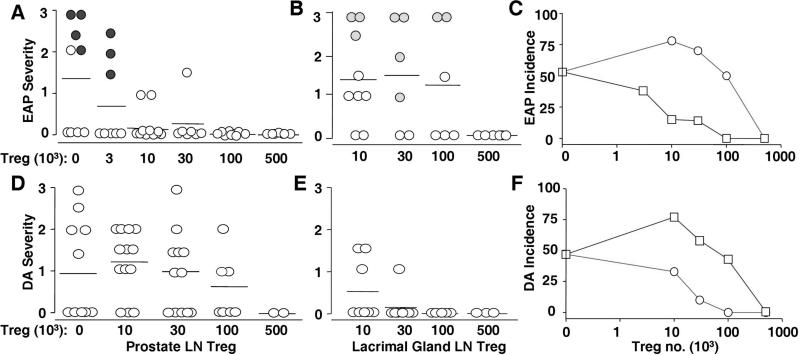
Prostate LN Treg and lacrimal gland LN Treg of normal mice preferentially suppress EAP and DA (respectively) in the same host
To determine whether the finding on AOD was a general phenomenon, we investigated EAP and DA. EAP was completely prevented by 0.01×106 Treg from prostate LN, whereas 0.5×106 Treg from the same LN was required to completely suppress DA (Figures 2A and D and Supplemental Figures 1D and H). In contrast, Treg from LN draining the lacrimal gland completely suppressed DA at a dose of 0.03×106 Treg, whereas 0.5×106 Treg were needed to prevent EAP (Figures 2B and E and Supplemental Figures 1E and G). Serum autoantibody to the prostate gland was detected only in recipients of less than 0.003×106 Treg from the prostate LN, and in recipients of less than 0.1×106 Treg from lacrimal gland LN (Figures 2A and B and Supplemental Figure 1F). Thus the Treg from prostate LN were 50 times more potent in suppressing EAP over DA, whereas Treg from the lacrimal gland LN were 15 times more potent in suppressing DA over EAP (Figures 2C and F).
Figure 2.
Selective enrichment of EAP-specific Treg and DA-specific Treg in prostate LN and lacrimal gland LN of normal mice. (A and B): EAP severity between d3tx recipients of Treg from prostate LN (A) and lacrimal gland LN (B). Incidences of EAP between these Treg recipients are shown in C (squares, prostate LN Treg recipients; circles, lacrimal gland LN Treg recipients). (D and E): DA severity between d3tx recipients of Treg from prostate LN (D) and lacrimal gland LN (E). (F): Incidence of DA between these Treg recipients (squares, prostate LN Treg recipients; circles: lacrimal gland LN Treg recipients). Filled circles denote positive prostate autoantibody.
We wish to emphasize that the 50-fold enrichment of EAP-specific Treg in the prostate-draining LN is 17 times greater than the 3-fold enrichment of the global Treg present in the pooled LN cells of prostate-bearing male mice over female mice determined earlier (8). Notwithstanding this marked difference in functional capacity, the Treg from LN draining the prostate, ovary, and lacrimal gland have similar expression of Foxp3 and comparable ability to suppress in vitro pan-T cell proliferation (Supplemental Figure 2).
The new bioassay described herein is useful for semi-quantitation of functional Ag-specific Treg. Notably, the assay is not dependent on T cell recognition of a specific T cell epitope that may or may not be relevant to, or be exclusive, for a given autoimmune disease. Instead, it detects Treg that recognize all organ-derived autopeptides that are relevant to suppression of disease in that organ. Based on the assay, we have documented for the first time that Treg from normal regional LN are highly enriched in functional Treg with the capacity to suppress autoimmune disease of its draining organ. In our earlier reports, we studied DS-Treg from individual LN, but from d3tx mice with ongoing autoimmunity (4, 11), or DS-Treg from pooled LN of normal mice (8), therefore they are not pertinent to self tolerance.
Prostate-specific antigen expression influences LN enrichment of EAP-specific Treg
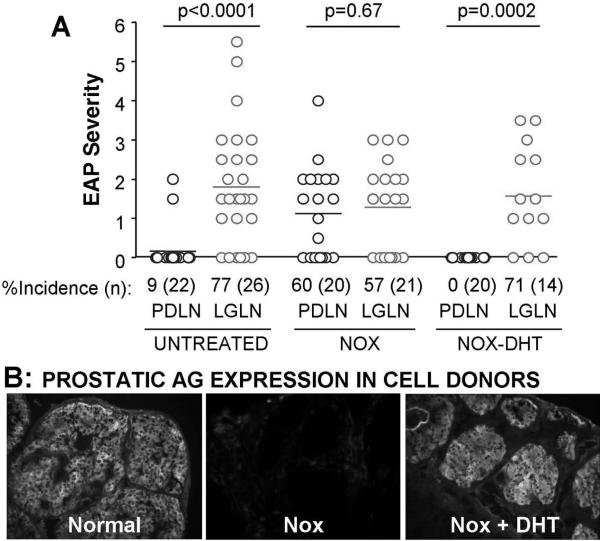
The DS-Treg enrichment in regional LN could result from an increase in Treg number and/or an enhancement of intrinsic Treg activity; and this may depend on the response of Treg to autoAg, their preferential homing to the regional LN, or both. To address the requirement of autoAg, we determined whether prostate antigen expression influences regional LN enrichment of EAP-specific Treg. When prostate Ag expression was eliminated in mice with neonatal orchiectomy (NOX) (8), the primacy of Treg from the prostate LN to suppress EAP was lost. When prostate Ag expression resumed in Treg donors that underwent NOX and 5α- dihydrotestosterone (DHT) treatment (8), the EAP-specific Treg activity in prostate LN was restored (Figure 3). Thus in the steady state, presentation of organ-derived tissue Ag to Treg is required for regional LN-specific enrichment of DS-Treg. A caveat of this study is that it has not ruled out a potential sex hormone effect on Treg function, inherent in the experimental design.
Figure 3.
Loss and gain in prostate Ag expression influences EAP-specific Treg activity in the normal prostate LN. (A): EAP severity and incidence in mice that received Treg (0.03 – 0.05×106) from prostate LN (PDLN) or lacrimal gland LN (LGLN), obtained from: normal donors, NOX donors, or donors with NOX and DHT treatment (NOX+DHT). (B): Immunohistochemical detection of prostate antigens in normal mice, NOX mice, and NOX+DHT. P-values determined by Mann-Whitney test.
Normal regional LN have enriched DS-Treg but not enriched DS-pathogenic T cells, correlation with reported TCR repertoire data
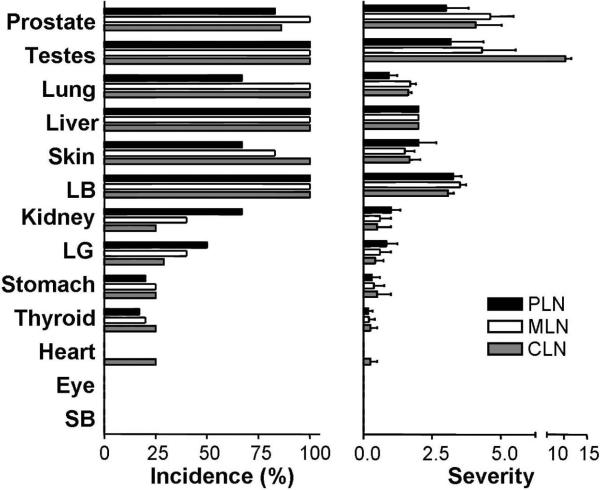
The antigen dependency or Ag specificity of polyclonal autoimmune T cell response can also be extrapolated from TCR repertoire analysis of LN T cells. Lathrop et. al. (5) recently documented the repertoire of Treg versus non-Treg based on thousands of individual T cells per LN in mice expressing a highly restricted TCR repertoire. Consistent with our finding of LN-specific DS-Treg, they also reported a distinctive repertoire among the Treg from individual LN. This was a finding unique to Treg since comparable TCR repertoires were detected among the naïve CD4+Foxp3- T cells of individual LN. In order to establish an additional correlate between the two studies, and add functional support to the TCR repertoire data, we next determined the distribution of autoimmune inflammation in recipients of CD4+CD25- T cells from individual LN of normal donors (12,13,14). As shown in Figure 4 and Supplemental Figure 3, when CD4+CD25- T cells from renal LN, mesenteric LN or cervical LN of normal mice were transferred to syngeneic RAG knockout recipients, they elicited autoimmune inflammation with very similar organ distribution and severity. Monocytic inflammation and tissue destruction were noted maximally in the prostate, testis and epididymis, lung, liver, skin and colon; less so in the kidney, lacrimal gland, stomach, and thyroid; and spared the pancreatic islets, heart, eye and small bowels (Supplemental Figure 3). The finding of such a striking correlation between TCR repertoire and T cell function for both LN-specific Treg and effector T cells provides additional support for the conclusion that the normal LN enrichment of DS-Treg is Ag-dependent.
Figure 4.
Organ distribution of tissue inflammation in B6 RAG-/- recipients of CD4+CD25- T cells from individual LN of wild type B6 donors. Data represent the incidence (%) and severity (mean ± SD) of tissue inflammation and destruction. Comparisons are made between pathology induced by lacrimal gland-draining cervical LN (CLN; gray bars), mesenteric LN (MLN; white bars) or prostate LN (PLN; black bars). Each recipient received 0.2×106 CD4+CD25- T cells i.v., and was studied 3-4 weeks later. Data represents the summation of three independent experiments, with two mice per experiment. LB, large bowel; LG, lacrimal gland; SB, small bowel.
The mechanism of LN specific Ag-specific Treg enrichment might depend on factors regulating T cell homing to LN, encounter with self Ag, and their retention in the LN. Homing of naïve T cells and Treg to normal LN are known to involve CD62L, CCR7 and the chemokines CCL19 and CCL21 (15). Autoimmune diseases occur in mice deficient in CD62L or CCR7 (16,17), for which we can now add a potential explanation: the loss of DS-Treg enrichment in regional LN. Treg retention may result from up-regulation of CD69 on Ag-specific Treg that temporarily sequesters sphingosine 1-phosphate receptor type 1 (S1P1), which is required for T cell egress from LN (18). Additional mechanisms may involve Treg response to anti-apoptotic and/or cellular proliferation signals (19). Constrained by T cell homeostatic mechanisms (20), the number or activity of DS-Treg in the regional LN would be maintained at a threshold 15-50 folds greater than those in the non-draining LN.
Additional mechanisms participate in Ag-specific Treg homing to regional LN and maintenance of abundant resident Ag-specific Treg in the normal skin, lung and mucosal sites (15,21). It is conceivable that they also participate in Treg homing to internal organs and their regional LN. However, unlike mucosa such as intestinal lamina propria, very few Foxp3+ cells are detected in normal internal organs; for example, only 1% of normal prostate CD4+ T cells are Foxp3+ (unpublished data). Moreover, TCR repertoire and functional capacity of T cells in single LN are distinctive among the Treg, but are shared among the T effectors; and this finding is not consistent with the prevailing concept that sharing of chemokine receptor expression allows for co-localization of Treg and T effectors with shared TCR specificity (15,22,23). Nonetheless, this is clearly an important area that requires further exploration.
In summary, we have documented major differences in the LN distribution of autoimmune disease-relevant effector T cells and Treg in normal mice. In the normal steady state, each regional LN is highly enriched for Ag specific Treg with capacity to inhibit autoimmune attack to the specific organ that drains to it, but contains very few Treg that control the autoimmune disease of other non-draining organs. The data provide strong and biologically relevant information for a critical and anticipatory role of antigen-specific Treg in peripheral tolerance. Positioned in this strategic location, maintained by self Ag, we propose DS-Treg can best control organ-specific tolerance by negating response to self peptide in the event of infection, tissue necrosis and other forms of local danger. Our finding also emphasizes that investigation based on pooled LN Treg may not correctly inform the physiological status of Treg function in vivo.
Supplementary Material
Acknowledgements
We thank Yuefang Sun, Joshua Sparks and Virginia Rubianes for expert technical assistance.
Footnotes
Abbreviations: lymph nodes (LN), regulatory T cells (Treg), dacryoadenitis (DA), autoimmune ovarian disease (AOD), experimental autoimmune prostatitis (EAP), disease-specific Treg (DS-Treg), antigen-presenting cell (APC), neonatal orchiectomy (NOX), 5α- dihydrotestosterone (DHT).
The authors have no conflicting financial interests.
References
- 1.Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 2002;196:1079–1090. doi: 10.1084/jem.20020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp. Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samy ET, Parker LA, Sharp CP, Tung KSK. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J. Exp. Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J. Exp. Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi O, Nishizuka Y, Sakakura T, Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin. Exp. Immunol. 1980;40:540–553. [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 8.Setiady YY, Ohno K, Samy ET, Bagavant H, Qiao H, Sharp C, She JX, Tung KSK. Physiologic self antigens rapidly capacitate autoimmune disease-specific polyclonal CD4+CD25+ regulatory T cells. Blood. 2006;107:1056–1062. doi: 10.1182/blood-2005-08-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haneji N, Hamano H, Yanagi K, Hayashi Y. A new animal model for primary Sjogren's syndrome in NFS/sld mutant mice. J. Immunol. 1994;153:2769–2677. [PubMed] [Google Scholar]
- 10.Tong ZB, Nelson LM. A mouse gene encoding an oocyte antigen associated with autoimmune premature ovarian failure. Endocrinology. 1999;140:3720–3726. doi: 10.1210/endo.140.8.6911. [DOI] [PubMed] [Google Scholar]
- 11.Samy ET, Wheeler KM, Roper RJ, Teuscher C, Tung KSK. Cutting Edge: Autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease-specific regulatory T cells. J. Immunol. 2008;180:4366–70. doi: 10.4049/jimmunol.180.7.4366. [DOI] [PubMed] [Google Scholar]
- 12.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith H, Lou YH, Lacy P, Tung KS. Tolerance mechanism in experimental ovarian and gastric autoimmune diseases. J. Immunol. 1992;149:2212–2218. [PubMed] [Google Scholar]
- 14.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 15.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 16.Worbs T, Forster R. A key role for CCR7 in establishing central and peripheral tolerance. Trends Immunol. 2007;28:274–280. doi: 10.1016/j.it.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Bai Y, Liu J, Wang Y, Honig S, Qin L, Boros P, Bromberg JS. L-selectin-dependent lymphoid occupancy is required to induce alloantigen-specific tolerance. J. Immunol. 2002;168:1579–1589. doi: 10.4049/jimmunol.168.4.1579. [DOI] [PubMed] [Google Scholar]
- 18.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 19.Chappert P, Leboeuf M, Rameau P, Stockholm D, Liblau R, Danos O, Davoust JM, Gross DA. Antigen-driven interactions with dendritic cells and expansion of Foxp3+ regulatory T cells occur in the absence of inflammatory signals. J. Immunol. 2008;180:327–334. doi: 10.4049/jimmunol.180.1.327. [DOI] [PubMed] [Google Scholar]
- 20.Sprent J, Cho JH, Boyman O, Surh CD. T cell homeostasis. Immunol. Cell Biol. 2008;86:312–319. doi: 10.1038/icb.2008.12. [DOI] [PubMed] [Google Scholar]
- 21.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Kang SG, Kim CH. FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J. Immunol. 2007;178:301–311. doi: 10.4049/jimmunol.178.1.301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.