Abstract
Symptom onset in amyotrophic lateral sclerosis (ALS) may occur in the muscles of the limbs (spinal onset) or those of the head and neck (bulbar onset). Most preclinical studies have focused on spinal symptoms, despite the prevalence of and increased morbidity and mortality associated with bulbar disease. We measured lick rhythm and tongue force to evaluate bulbar disease in the SOD1-G93A rat model of familial ALS. Body weight and grip strength were measured concomitantly. Testing spanned the early (maturation), middle (pre-symptomatic), and late (symptomatic and end-stage) phases of the disease. We measured a persistent tongue motility deficit that became apparent in the early phase of the disease, providing behavioral evidence of bulbar pathology. At end-stage, however, cytochrome oxidase (CO) activity was normal in the hypoglossal nucleus, and in the tongue, neuromuscular innervation, citrate synthase (CS) protein levels and activity, and uncoupling protein 3 (UCP3) protein levels remained unchanged. Interestingly, significant denervation and atrophy were evident in the end-stage sternomastoid muscle, providing peripheral anatomical evidence of bulbar pathology. Changes in body weight and grip strength occurred in the late phase of the disease. Extensive atrophy and denervation were observed in the end-stage gastrocnemius muscle. In contrast to our findings in the tongue, CS protein levels were decreased in the extensor digitorum longus (EDL) and soleus, although CS activity was maintained or increased. UCP3 protein was decreased also in the EDL. These data provide evidence of differential effects in muscles that were more or less affected by disease.
Keywords: bulbar, dysphagia, familial ALS, grip strength, operant, orolingual, oromotor, tongue
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive degenerative disease of upper and lower motor neurons that results in marked muscle weakness and atrophy, fasciculations, reflex abnormalities, and eventually death [56]. Approximately 90% of cases occur sporadically (sALS); in the remaining cases, the disease is inherited in an autosomal dominant manner (familial ALS, fALS) [13, 55]. The etiology of sALS remains unknown, but it is believed to result from the interaction of environmental and genetic factors [46]. Mutations in at least 6 different genes have been shown to cause or predispose to fALS [reviewed in 18, 36], and mutations in the Cu/Zn superoxide dismutase-1 (SOD1) gene are associated with approximately 20% of fALS cases [13, 55]. The SOD1 gene (located on chromosome 21q22) codes for a major antioxidant enzyme that plays a crucial role in the scavenging of superoxide and the mitigation of oxidative stress [38; reviewed in 45]. Both forms of ALS are clinically and pathologically similar, suggesting a common pathogenesis [19].
To study the pathogenesis and progression of ALS, transgenic rodent models harboring a variety of human SOD1 mutations have been developed. These models vary in disease onset, progression, and survival. The SOD1-G93A mouse is the most widely studied. In this model the SOD1 gene contains a glycine to alanine substitution at position 93, resulting in a toxic gain of SOD1 function [22, 77]. For reasons still unknown, these animals develop an ALS-like phenotype and pathology, including adult-onset muscle weakness and atrophy and motor neuron degeneration. The majority of studies have focused on spinal pathology despite the prevalence of and increased morbidity and mortality associated with bulbar disease [14, 37, 47, 54].
The purpose of this study was to examine bulbar deficits in the SOD1-G93A rat model of ALS. In a recent study, we documented the time course of orolingual motor deficits in SOD1-G93A mice [62]. These SOD1-mutant mice are believed to be a spinal (hindlimb) onset model, and our findings were largely consistent with that since limb muscle deficits preceded tongue motility and force deficits in these animals. SOD1-G93A rats, however, are believed to exhibit greater heterogeneity with regards to site of onset, with some rats exhibiting a hindlimb onset and others a forelimb onset [27, 42]. It has been suggested that the forelimb onset SOD1-mutant rats that exhibit very early weight loss and subsequent forelimb muscle weakness are actually suffering the effects of dysphagia resulting from bulbar onset disease [64]. To our knowledge, however, bulbar motor deficits have not been explored in the SOD1-G93A rat model of ALS until now.
Experimental procedures
A breeding colony was established with 4 Sprague-Dawley female and 2 hemizygous TgN(SOD1G93A)L26H male rats (Sprague-Dawley background) obtained from Taconic. Twelve TgN(SOD1G93A)L26H (SOD1-G93A; n=9 males, n=3 females) and 8 wild-type Sprague-Dawley (control; n=3 males, n=5 females) rats from the first 2 litters from this breeding colony served as subjects in this experiment. These litters were from 2 females bred to the same male and were born on consecutive days. Tail clips were sent to Transnetyx, Inc. for genotyping. Following weaning, rats were housed individually in an AAALAC-approved animal care facility with free access to food. Rats were placed on a gradual water restriction schedule that ultimately allowed access to water for approximately 15–30 minutes/day. Rats were maintained on a 12/12 hour light/dark cycle and all behavioral testing was performed during the light portion of this cycle. Body weight was monitored 3 times/week. For this study, disease end-stage was defined by the presence of overt paralysis in at least 1 limb or by complete failure to engage in the orolingual motor task (described below). All SOD1-G93A rats were euthanized within 1 week of reaching this end-point. Control rats were euthanized after all SOD1-G93A rats reached end-stage. All procedures were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee and were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
The estrus cycles of female rats were not monitored during this study. However, because estrogen levels are known to affect hypoglossal nucleus physiology [5, 58], we evaluated lick rhythm data during the stable middle phase for variations that may have occurred due to the estrus cycle. If estrus cycle was affecting licking rhythm, variability for this measure should have been greater for the female rats than for the males. We therefore determined the coefficient of variation (CV) for licking rhythm during the middle phase (33 time points) for every rat (male and female). The mean CV for males was 0.0300 ± 0.0030. The mean CV for females was 0.0325 ± 0.0040. A t-test revealed that these values were not significantly different (p=0.6375, t=0.2298, df=18). Therefore, we were satisfied that the female rats did not exhibit a significantly greater variation in lick rhythm when compared to the male rats.
Behavioral assessment
Orolingual motor function
Tongue motility and force were evaluated 3 times/week (84 measurements taken) using an operant apparatus described in depth by Fowler and Wang [16]. This apparatus has been used successfully to study both rats and mice, and was used in our recent study of orolingual motor deficits in SOD1-G93A mice [16, 62, 72]. The only adjustment made to our previous protocol was to increase the volume of the water reward from 0.015 ml to 0.05 ml. Rats were placed in the apparatus for 6 minute sessions for which the entire force-time record was stored and then subjected to Fourier-based rhythm analysis as described previously [16].
A minimum of 100 licks was required for analysis of orolingual motor variables. Rats readily engaged in the task, far exceeding this threshold during their first exposure to the task (mean number of licks ± s.e.m. during first test session = 442.4 ± 24.8), and continuing throughout the experiment. The effects of genotype and time on number of licks/session were evaluated in the early, middle, and late phases of the disease in control and SOD1-G93A rats. In the early and middle phases, analyses yielded significant effects of time (p<0.0001, F= 28.3046, df=21; and p<0.0001, F=5.2425, df=32, respectively). In the late phase, analysis yielded significant effects of genotype (p<0.0030, F=11.7578, df=1) and time (p<0.0001, F=3.0459, df=28), as well as a significant genotype × time interaction effect (p<0.0001, F=4.7911, df=28). These analyses revealed that control and SOD1-G93A rats engaged similarly in the task until the late stage of the disease when a decline in the SOD1-G93A rats’ participation became evident. Even then, however, SOD1-G93A rats participated at a level that far exceeded the threshold for statistical analysis (mean number of licks (± s.e.m.) during last session = 734.1 ± 123.7).
Grip force
Fore- and hindlimb grip force was evaluated 3 times/week (80 measurements taken) using an animal grip strength system (San Diego Instruments). Rats were passed over a metal mesh grid connected to a force transducer. Rats gripped the grid with either their fore- or hindlimbs and then were tugged gently away until the grip was released. The peak force in grams was recorded by the transducer. Three fore- and 3 hindlimb trials were performed during each test session. The highest of the 3 values for each was recorded for analysis.
Dissection
All rats were deeply anesthetized with sodium pentobarbital (Nembutal; 45 mg/kg) and the extensor digitorum longus (EDL) and soleus muscles were dissected bilaterally from the hindlimbs. The tongues were dissected at the point of insertion into the base of the oral cavity, and the intrinsic tongue muscles were used for these studies. These muscles were clamp-frozen in liquid nitrogen. From a subset of rats (SOD1-G93A n=4, control n=4), sternomastoid and gastrocnemius muscles were dissected unilaterally from the neck and hindlimb, respectively. These muscles were post-fixed in 4% paraformaldehyde for 1 hour at room temperature, cryoprotected in 20% sucrose in PBS, and frozen in OCT on dry ice. Following muscle dissections, rats were decapitated and the brains dissected. Brains were frozen in heptane cooled with dry ice. All tissues were stored at −80° C.
Hypoglossal nucleus cytochrome c oxidase (CO) activity
Methods for histochemical analysis of CO activity have been described previously [63]. Frozen brains were sectioned in the coronal plane at 16 µm on a cryostat. Every fourth section through the hypoglossal nucleus was thaw-mounted onto SuperFrost Plus microscope slides (Fisher Scientific). Sections were incubated for 30 minutes at 40° C in a solution containing 50 mg diaminobenzidine (Sigma), 20 mg cytochrome c (from horse heart) (Sigma), and 4 g sucrose per 100 ml of 1M phosphate buffered saline. Sections were then dehydrated in a graded series of ethanols and coverslipped with DPX (BDH Laboratory Supplies).
Sections containing the hypoglossal nucleus were identified under brightfield illumination with a 10x objective. Digital images were captured through the entire caudal-rostral axis of the nucleus and then analyzed using the image analysis package of Photoshop Extended CS4 (Adobe Systems Inc.). First, background correction/white balance was applied equally to each image. Then, the hypoglossal nucleus was outlined in each imaged section using the free-form selection tool and the integrated density was measured. Similar measurements were made in the reticular formation of each section. For each animal, bilateral measurements of hypoglossal nucleus and reticular formation integrated density were averaged and then the hypoglossal nucleus mean was divided by the reticular formation mean to correct for within and between animal differences in staining intensity. These corrected means were used for statistical analysis.
NMJ immunohistochemistry
Methods for immunohistochemical analysis have been described previously [51]. Frozen gastrocnemius, sternomastoid, and tongue muscles were cross-sectioned at 10 – 14 µm on a cryostat and sections were thaw-mounted onto SuperFrost Plus microscope slides. Sections were stained with primary antibodies against nerves (SMI-312R (neurofilament light, medium, and heavy chains), Covance, mouse IgG1, 1:1000; and SV2 (synaptic vesicles), DSHB, mouse IgG1, 1:20) followed by Alexa488-conjugated anti-mouse IgG1 secondary antibody (Molecular Probes, 1:1000), as well as Alexa594-alpha-bungarotoxin (Molecular Probes, 1:1000) to label the acetylcholine receptor. Control sections were incubated in the absence of primary antibodies. Stained sections were analyzed under epifluorescence with a 20x Plan-fluor objective lens.
A normal, adult motor nerve terminal shows perfect overlap with a cluster of acetylcholine receptors at the NMJ (i.e., it shows full innervation) [57]. Any non-occupied area of the acetylcholine receptor cluster, whether in part (partial innervation) or in full (full denervation), constitutes an innervation abnormality. Thirty to forty NMJs were analyzed in each muscle from each animal, and were classified as innervated or denervated. In our analysis, full and partially innervated NMJs were classified as innervated and fully denervated NMJs were classified as denervated. NMJ scoring was performed by two examiners who were blind to the muscle source and genotype. The inter-rater reliability was 83%. The percent denervation scores from each examiner were averaged and these mean percent denervation scores were used for statistical analysis.
When a NMJ was analyzed in the gastrocnemius and sternomastoid muscles, the cross-sectional area of the postsynaptic muscle fiber was also measured using Image J software (NIH). The mean cross-sectional area was calculated and used for statistical analysis.
Muscle protein expression
Methods for Western blotting have been described previously [21]. Frozen EDL, soleus, and tongue muscles were homogenized in a 12:1 (volume/weight) ratio of ice-cold buffer containing 10 mM Tris-HCl (pH 7.4); 100 mM NaCl; 1 mM each of EDTA, EGTA, NaF, PMSF, and 2 mM Na3VO4; 20 mM Na4P2O7; 1% Triton X-100; 10% glycerol; 0.1% SDS; 0.5% deoxycholate; and 250 µl/5 ml protease inhibitor cocktail. Homogenates were incubated on ice for 30 minutes to allow for protein extraction and centrifuged at 13,000 rpm for 20 minutes at 4° C. Supernatants were collected and protein concentrations were analyzed via Bradford assay (Bio-Rad). Samples were prepared in 2x Laemmli buffer containing 100 mM DTT and boiled for 5 minutes.
Seventy-five µg of protein were separated on 10% gels using traditional SDS-PAGE methods and wet-transferred onto nitrocellulose membranes (Whatman). Membranes were blocked for 1 hour in Tris-buffered saline, 0.1% Tween 20 (TBST)-5% non-fat dry milk at room temp and incubated overnight at 4° C in rabbit anti-citrate synthase (1:2,000; Alpha Diagnostics) or rabbit anti-UCP3 (1:500; Chemicon) primary antibody diluted in TBST-1% nonfat dry milk. Following a series of washes, membranes were incubated in HRP-conjugated goat anti-rabbit secondary antibody (1:5,000 or 1:10,000 in TBST-1% nonfat dry milk; Jackson) for 2 hours at room temperature. Bands were visualized by enhanced chemiluminescence (Pierce) and quantified via densitometry (Image J). All conditions were maintained across gels and membranes.
To ensure equal protein loading and transfer, membranes were stripped and probed for tubulin (mouse anti-tubulin primary; 1:1,000; Sigma; goat anti-mouse secondary; 1:5,000; Bio-Rad). However, tubulin expression was significantly increased in SOD1-G93A muscles, making it an inappropriate loading control. Others have documented similar changes in tubulin protein expression following denervation [8]. Therefore, to verify equal protein loading and transfer, membranes were stained with Ponceau S stain (Sigma) [20]. A band was chosen at random and quantified using densitometry. There was no significant difference in band intensity between SOD1-G93A and control muscles. Western blots were performed in duplicate and similar results were achieved each time.
Muscle citrate synthase (CS) activity
Methods for spectrophotometric analysis of enzyme activity have been described previously [21]. Muscle extracts (as prepared for Western blot) were diluted in 1M PBS to a concentration of 7 mg/ml and citrate synthase activity was measured spectrophotometrically according to the method of Smirnova et al. [71]. Briefly, a reaction mix consisting of 20 µl 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB), 5 mM in 1 M Tris-HCl, pH 8.1; 130 µl MilliQ H2O; 30 µl acetyl coenzyme A (acetyl CoA), 10 mM in H2O; 10 µl muscle extract; and 10 µl oxalacetic acid, 50 mM in 0.1 M Tris-HCl, pH 8.1 (for a total volume of 200 µl) was added to each well of a 96-well plate. The oxalacetic acid was added to the mix immediately before absorbance recordings, which were performed in each well at 405 nm wavelength every 20 seconds for 3 minutes using a MRXII Microplate Reader and Kinetic software package (Dynex Technologies, Chantilly, VA). The linear portion of the reaction curve was used to calculate the citrate synthase activity (A405/min). Extracts were tested in duplicate/assay and the assay was performed in triplicate. Similar results were achieved each time.
Statistical analysis
When lick rhythm and grip strength data were graphed, 3 stages of performance were readily apparent: a maturation phase related to animal growth and learning, a plateau phase where behavior was stable, and a symptomatic phase where a behavioral decline was apparent in SOD1-G93A rats. The cut-off for each stage varied slightly (± a few days) depending on the behavioral variable examined, so for the sake of consistency, all behavioral data were separated into early (39–95 days of age; 23 time points), middle (97–185 days of age; 34 time points), and late (187–252 days of age; 29 time points) stages based on the maturation, plateau, and symptomatic phases observed in the lick rhythm data. These phases were analyzed separately. Statistical analyses consisted of multiple 2-way repeated measures ANOVA with genotype (control and SOD1-G93A) as the between factors variables and time (age) as the within factors variable. Each testing session yielded one value for each measure (licking rhythm and mean force of licks) for each rat. In the few cases where data points were missing (n=10 out of 1720 cases), those points were filled in with the mean of the preceding and following test dates. Some SOD1-G93A rats reached end-stage earlier than others. The study continued until the last remaining SOD1-G93A rat reached this end-point. Therefore, in cases where a rat had to be euthanized before the end of the study, the values of the last measurements taken were filled in for the remaining test dates to prevent that animal from being excluded from the repeated measures analyses.
NMJ denervation and muscle fiber cross-sectional area data were analyzed using 2-way ANOVA with genotype and muscle type as the between factors variables. All other data were analyzed using 2-sample t-tests. All analyses were performed using Systat (SYSTAT Software, Inc., Richmond, CA), and for all analyses α=0.05.
Results
Body weight
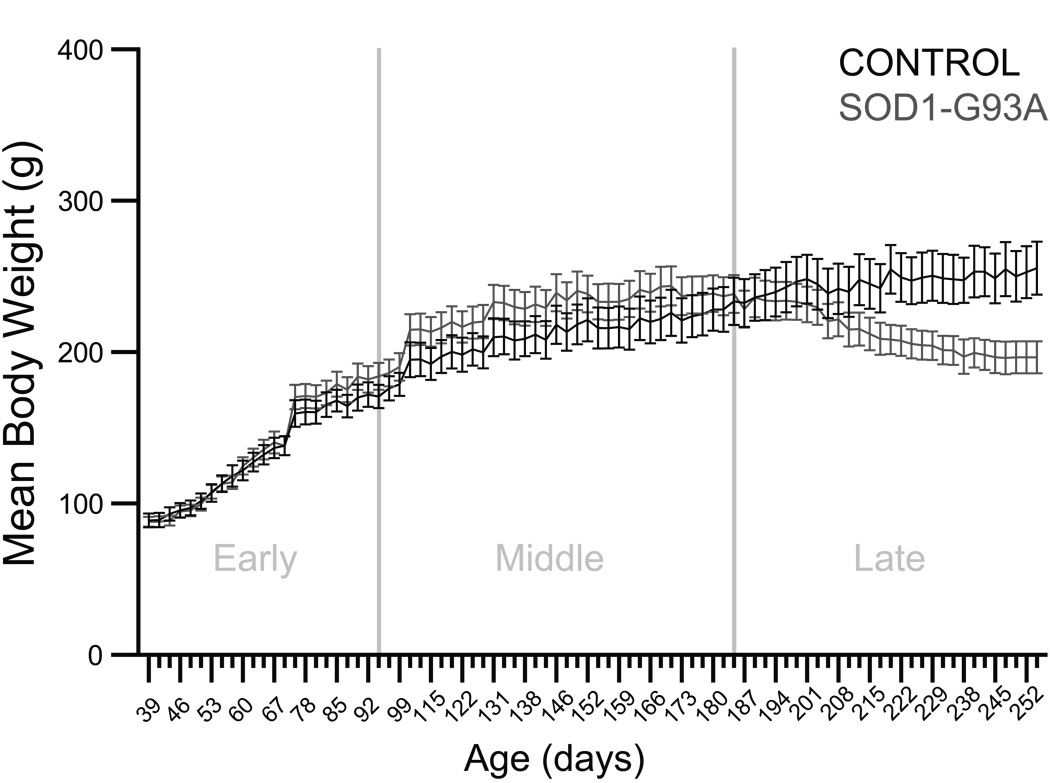
The effects of genotype and time on body weight were evaluated in the early, middle, and late phases of the disease in control and SOD1-G93A rats (Figure 1). In the early and middle phases, analyses yielded significant effects of time (p<0.0001, F=165.7469, df=22; and p<0.0001, F=42.3634, df=33, respectively). In the late phase, analysis yielded a nearly significant effect of genotype (p=0.0654, F=3.8514, df=1), a significant effect of time (p<0.0001, F=6.1877, df=28), and a significant genotype × time interaction effect (p<0.0001, F=19.9305, df=28). In summary, these analyses revealed no differences in body weight between control and SOD1-G93A rats until the late phase of the disease. Then, control rats continued to gain weight, whereas SOD1-G93A rats exhibited a plateau in body weight followed by a gradual weight loss as end-stage approached.
Figure 1. Body weight.
Graph depicts the mean body weight in grams (± S.E.M.) during the early, middle, and late phases of the disease in control and SOD1-G93A rats. In the early and middle phases, analyses yielded significant effects of time (p<0.0001 in both cases). In the late phase, analysis yielded a nearly significant effect of genotype (p=0.0654), a significant effect of time (p<0.0001), and a significant genotype × time interaction (p<0.0001).
Tongue motility and force
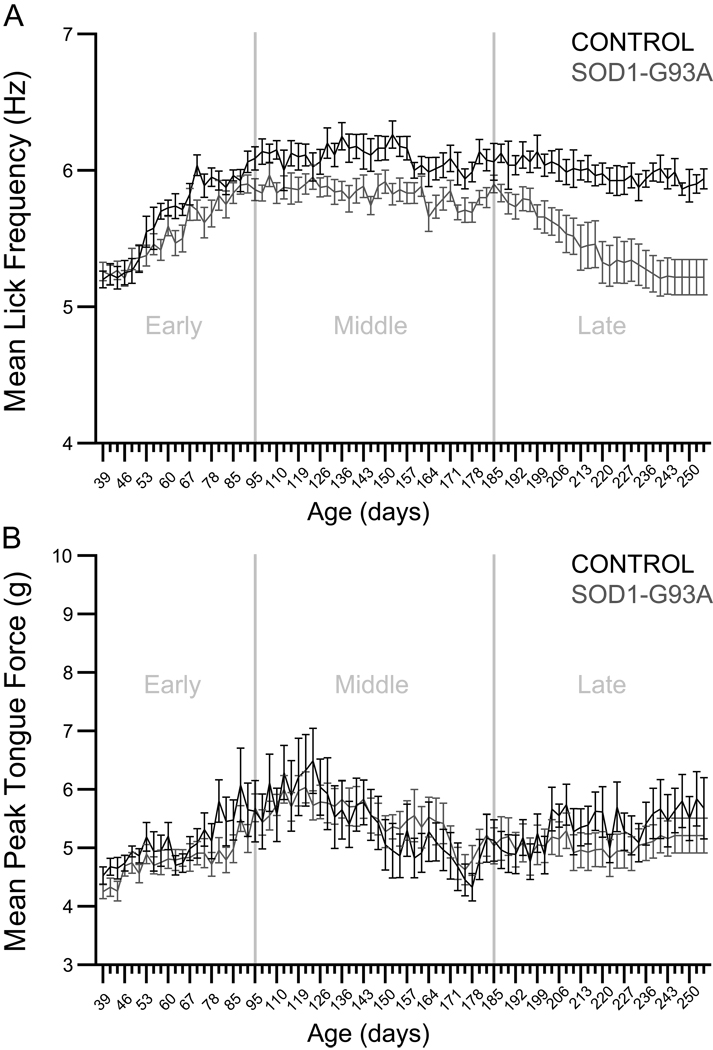
The effects of genotype and time on lick frequency were evaluated in the early, middle, and late phases of the disease in control and SOD1-G93A rats (Figure 2, panel A). In the early phase, analysis yielded a significant effect of time (p<0.0001, F=33.7474, df=21) and a significant genotype × time interaction effect (p=0.0176, F=1.7957, df=21). In the middle phase, analysis yielded significant effects of genotype (p=0.0061, F=9.6685, df=1) and time (p<0.0001, F=2.3924, df=32). In the late phase, analysis yielded significant effects of genotype (p=0.0011, F=15.1142, df=1) and time (p<0.0001, F=7.9384, df=28), as well as a significant genotype × time interaction effect (p<0.0001, F=2.5770, df=28). In summary, these analyses revealed an early-onset tongue motility deficit that was persistent in nature. In the early phase, SOD1-G93A rats’ lick rhythm increased, but more gradually than in controls. In the middle phase, SOD1-G93A rats’ lick rhythm plateaued at a level significantly lower than controls. In the late phase, lick rhythm progressively and significantly decreased in SOD1-G93A rats, while lick rhythm was maintained in control rats. Overall, these results demonstrate the presence of a long-standing tongue motility deficit in SOD1-G93A rats.
Figure 2.
Panel A: Lick rhythm. Graph depicts the mean lick frequency in Hertz (± S.E.M.) during the early, middle, and late phases of the disease in control and SOD1-G93A rats. In the early phase, analysis yielded a significant effect of time (p<0.0001) and a significant genotype × time interaction (p=0.0176). In the middle phase, analysis yielded significant effects of genotype (p=0.0061) and time (p<0.0001). In the late phase, analysis yielded significant effects of genotype (p=0.0011) and time (p<0.0001), as well as a significant genotype × time interaction (p=<0.0001). Panel B: Tongue force. Graph depicts the mean tongue force in grams (± S.E.M.) during the early, middle, and late phases of the disease in control and SOD1-G93A rats. In the early and middle phases, analyses yielded significant effects of time (p<0.0001 in both cases). In the late phase, analysis yielded a significant effect of time (p<0.0001) and a significant genotype × time interaction (p=0.0108).
The effects of genotype and time on tongue force were evaluated in the early, middle, and late phases of the disease in control and SOD1-G93A rats (Figure 2, panel B). In the early and middle phases, analyses yielded significant effects of time (p<0.0001, F=9.0499, df=21; and p<0.0001, F=9.5518, df=32, respectively). In the late phase, analysis yielded a significant effect of time (p<0.0001, F=3.6946, df=28) and a significant genotype × time interaction effect (p=0.0108, F=1.7512, df=28). In summary, these analyses revealed no differences in tongue force between control and SOD1-G93A rats, demonstrating that tongue muscle strength was maintained in SOD1-G93A rats.
Fore- and hindlimb grip force
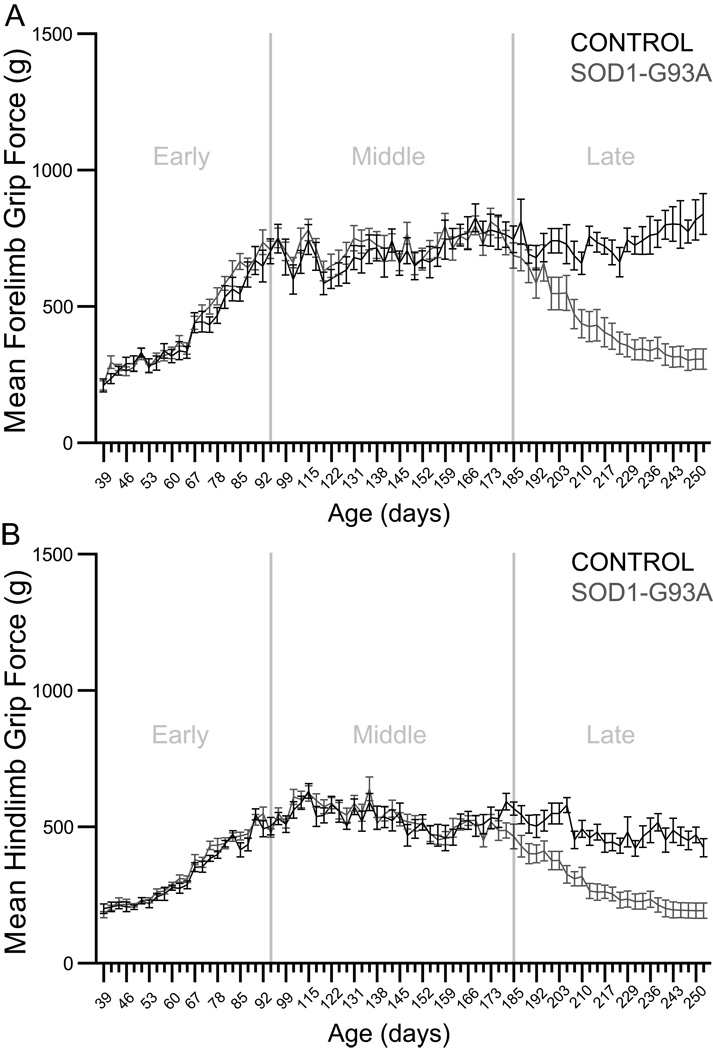
The effects of genotype and time on forelimb grip force were evaluated in the early, middle, and late phases of the disease in control and SOD1-G93A rats (Figure 3, panel A). In the early and middle phases, analyses yielded significant effects of time (p<0.0001, F=95.3666, df=22; and p<0.0001, F=6.2150, df=31, respectively). In the late phase, analysis yielded significant effects of genotype (p<0.0001, F=30.5041, df=1) and time (p<0.0001, F=7.0542, df=24), as well as a significant genotype × time interaction effect (p<0.0001, F=12.0655, df=24). The effects of genotype and time on hindlimb grip force were also evaluated in the early, middle, and late phases of the disease in control and SOD1-G93A rats (Figure 3, panel B). In the early and middle phases, analyses yielded significant effects of time (p<0.0001, F=163.2269, df=22; and p<0.0001, F=7.6411, df=31, respectively) and significant genotype × time interaction effects (p=0.0470, F=1.5819, df=22; and p=0.0154, F=1.6561, df=31, respectively). In the late phase, analysis yielded significant effects of genotype (p<0.0001, F=38.8808, df=1) and time (p<0.0001, F=13.5722, df=24), as well as a significant genotype × time interaction effect (p<0.0001, F=3.7257, df=24). In summary, these analyses revealed no differences in fore- or hindlimb grip strength between control and SOD1-G93A rats until the late phase of the disease. Then, control rats maintained grip strength, whereas grip strength progressively declined in SOD1-G93A rats. Together, these results demonstrate the presence of late-phase limb muscle weakness in SOD1-G93A rats.
Figure 3.
Panel A: Forelimb grip strength. Graph depicts the mean forelimb grip force in grams (± S.E.M.) during the early, middle, and late phases of the disease in control and SOD1-G93A rats. In the early and middle phases, analyses yielded significant effects of time (p<0.0001 in both cases). In the late phase, analysis yielded significant effects of genotype (p<0.0001) and time (p<0.0001), as well as a significant genotype × time interaction (p<0.0001). Panel B: Hindlimb grip strength. Graph depicts the mean hindlimb grip force in grams (± S.E.M.) during the early, middle, and late phases of the disease in control and SOD1-G93A rats. In the early and middle phases, analyses yielded significant effects of time (p<0.0001 in both cases) and significant genotype × time interactions (early phase p=0.0470; middle phase p=0.0154). In the late phase, analysis yielded significant effects of genotype (p<0.0001) and time (p<0.0001), as well as a significant genotype × time interaction (p<0.0001).
Hypoglossal nucleus CO activity
The effect of genotype on mean CO integrated density was evaluated in end-stage hypoglossal nuclei (data not shown). This analysis did not reveal a significant effect, demonstrating that hypoglossal nucleus CO activity was maintained in end-stage SOD1-G93A rats.
NMJ denervation and muscle atrophy
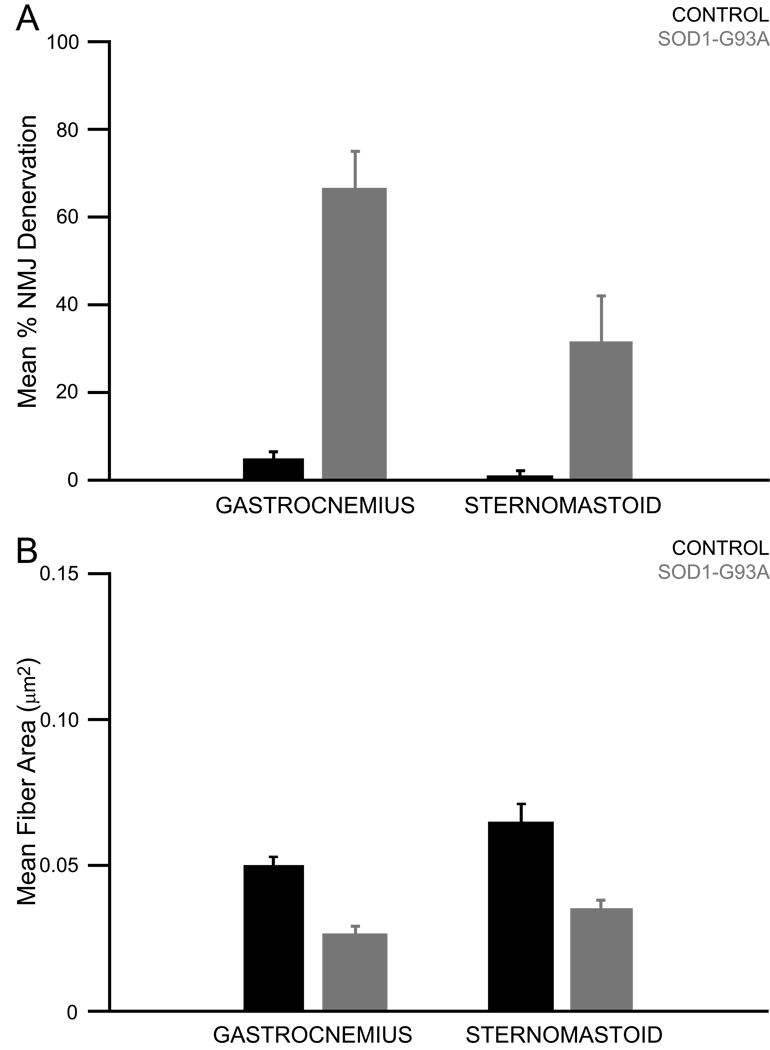
Qualitative evaluation of SOD1-G93A muscles revealed signs of denervation and atrophy in the SOD1-G93A gastrocnemius and sternomastoid relative to control muscles (Figure 4). Subsequently, the mean percent denervation and mean post-synaptic muscle fiber cross-sectional area were calculated and quantitative analyses were undertaken. No denervation was apparent qualitatively or quantitatively (data not shown) in the SOD1-G93A tongue muscles, so atrophy was not evaluated in the tongue. The effect of genotype and muscle type on mean percent denervation was evaluated in end-stage gastrocnemius and sternomastoid muscles (Figure 5, panel A). This analysis yielded significant effects of genotype (p<0.0001, F=69.7528, df=1) and muscle type (p=0.0042, F=12.3984, df=1), as well as a significant genotype × muscle type interaction effect (p=0.0155, F=7.9790, df=1). The effect of genotype and muscle type on mean muscle fiber cross-sectional area was evaluated in end-stage gastrocnemius and sternomastoid muscles (Figure 5, panel B). This analysis yielded significant effects of genotype (p<0.0001, F=54.2549, df=1) and muscle type (p=0.0012, F=10.6893, df=1). In summary, these analyses revealed that SOD1-G93A gastrocnemius and sternomastoid muscles exhibited significant denervation and atrophy by disease end-stage. In addition, significantly greater denervation occurred in SOD1-G93A gastrocnemius muscles when compared to SOD1-G93A sternomastoid muscles.
Figure 4. NMJ innervation in control gastrocnemius and SOD1-G93A gastrocnemius, sternomastoid, and tongue muscles.
Panel A: Nerve terminals were labeled with antibodies against neurofilament and SV2. Acetylcholine receptors were labeled with alpha-bunagarotoxin. In normal muscle there is perfect overlap of the terminals and receptors, as demonstrated in the control gastrocnemius. In denervated muscle, the receptors remain but the terminals are missing, as demonstrated in the SOD1-G93A gastrocnemius and sternomastoid muscles. Normal innervation was observed in the SOD1-G93A tongue muscle. White arrowheads indicate fully innervated NMJs. Yellow arrowheads indicate partially innervated NMJs. Red arrows indicate denervated NMJs. Dashed-line boxes indicate the NMJs represented in the high magnification images in Panel B. Panel B: High magnification images of fully innervated, tongue muscles. Analyses revealed a significant effect in the EDL (p=0.0004). Ponceau S stain was used to verify equal protein loading across lanes (see Methods).
Figure 5.
Muscle CS protein expression and activity
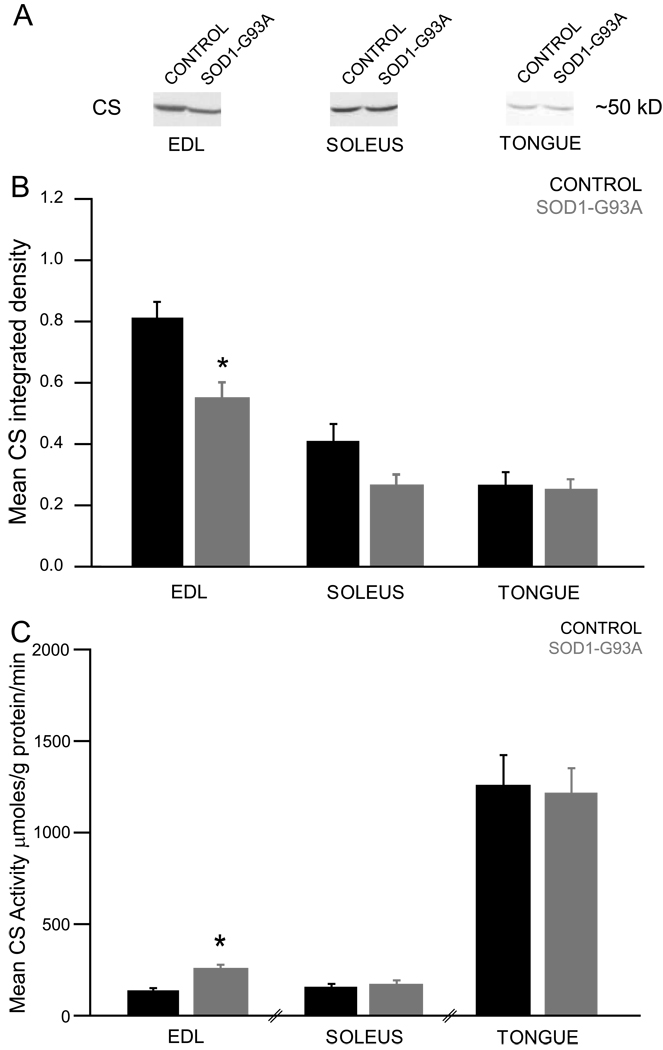
The effect of genotype on mean CS integrated density was evaluated in end-stage EDL, soleus, and tongue muscles (Figure 6, panel B). These analyses revealed a significant effect in the EDL (p=0.0039, t=−3.3708, df=16) and a nearly significant effect in the soleus (p=0.0507, t=−2.1240, df=15), demonstrating that CS protein levels were decreased in these end-stage SOD1-G93A rat muscles. Next, CS activity values were normalized to CS protein levels and the effect of genotype on mean CS activity was evaluated in end-stage EDL, soleus, and tongue muscles (Figure 6, panel C). These analyses revealed a significant effect in the EDL (p=0.0042, t=3.3365, df=16), demonstrating that CS activity was increased in this end-stage SOD1-G93A rat muscle.
Figure 6.
Muscle UCP3 protein expression
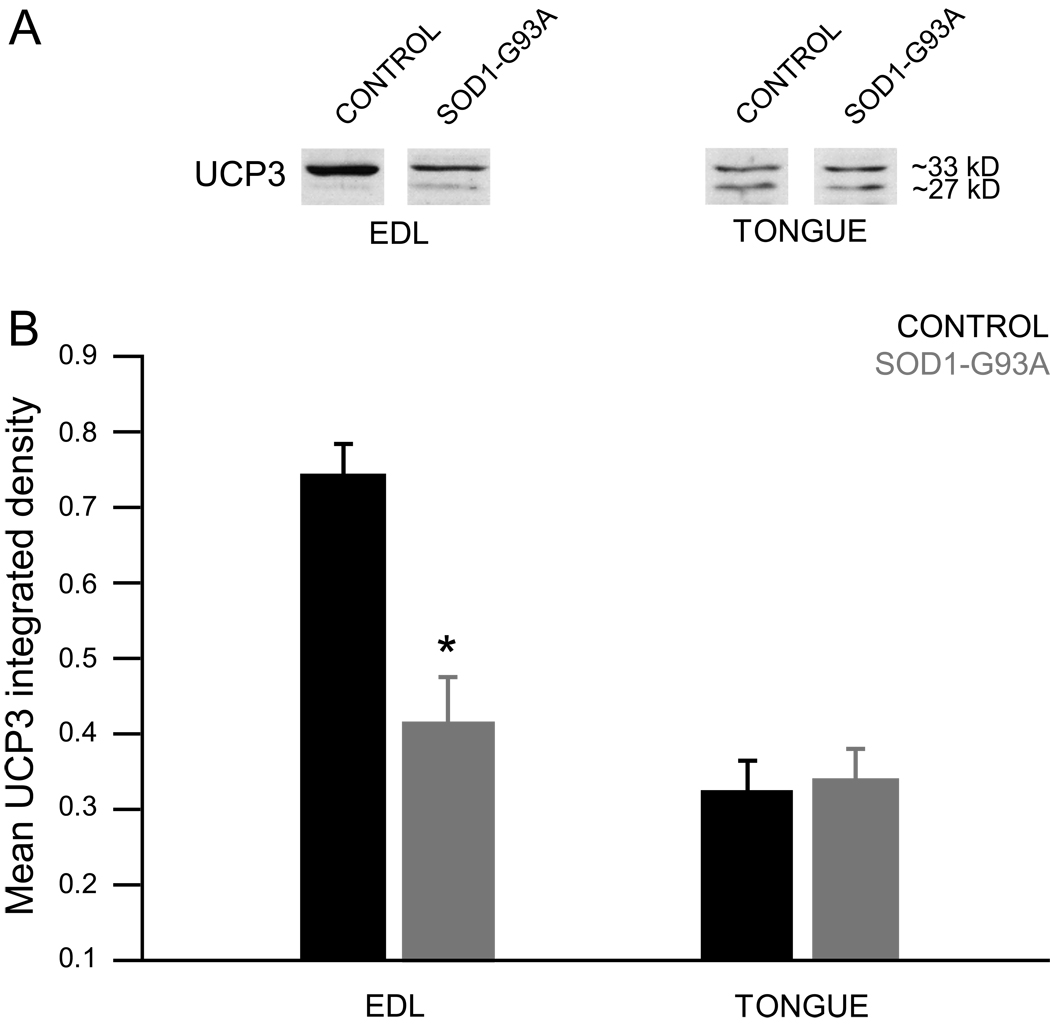
The effect of genotype on mean UCP3 integrated density was evaluated in EDL and tongue muscles (Figure 7, panel B). These analyses revealed a significant effect in the EDL (p=0.0004, t=−4.4102, df=16), demonstrating that UCP3 protein was decreased in this end-stage SOD1-G93A rat muscle.
Figure 7.
Discussion
Disease onset and survival
In this preclinical ALS model, disease onset is predicted to be ~115 days of age, followed by a swift progression to end-stage at ~126 days of age [26]. Our rats demonstrated delayed disease onset and an extended lifespan of 185–251 days, which was shortened somewhat by our use of an artificial end-point. Once overt paralysis was apparent, the disease progressed swiftly to end-stage, suggesting that although onset was delayed, progression remained relatively unchanged. We believe our rats were low expressors of the SOD1-G93A transgene. Although this hypothesis was not tested empirically, reduced transgene copy number increases survival in SOD1-mutant mice [2]. Such phenotypic drift has been documented through progressive generations of these animals by Taconic and several research groups [24, 26, 33, 66]. Unfortunately, such variability in animal life span makes comparisons with the literature somewhat difficult as the age at which any reported changes occur may differ substantially due to differences in transgene expression.
Body weight
Changes in body weight are characteristic of ALS in humans. Lean body mass decreases as motor neurons are lost early on in the course of the disease [49]. As the disease progresses, bulbar complications such as malnutrition and dehydration result in further weight loss [59]. Weight loss continues during the end stage of the disease, as respiratory failure increases resting energy expenditure [31].
The decrease in body weight is recapitulated in the SOD1-G93A rat model of ALS [27, 42, 66]. In general, these studies report late stage changes in body weight, indicating that weight loss is not an early marker of disease. In agreement with these findings, we found that body weight was similar between control and SOD1-G93A rats until the late phase of the disease. At that point, control rats continued to gain weight, while in SOD1-G93A rats, body weight was maintained until a gradual weight loss occurred as end-stage approached.
As in human ALS patients, muscle wasting likely contributes to the observed weight loss in SOD1-G93A rats [49]. Indeed, we found that by disease end-stage, significant muscle atrophy had occurred in SOD1-G93A rats (discussed below). In addition to muscle wasting, it is possible that changes in orolingual motor function also contribute to the weight loss observed in SOD1-G93A rats. As mentioned above, it has been suggested that the earlier weight loss observed in “forelimb onset” SOD1-mutant rats is actually an effect of dysphagia resulting from bulbar onset disease [64].
Bulbar pathology
In ALS patients, corticobulbar and bulbar motor neurons of the facial, trigeminal, and hypoglossal nuclei are affected, resulting in weakness and atrophy of the corresponding musculature. Typically, the tongue is affected more so than the muscles of the lips or jaw [14]. Orolingual motor deficits can manifest as a variety of symptoms, including slurred speech and difficulties with chewing and swallowing. Up to 35% of new ALS patients seek medical attention for dysarthria, suggesting that tongue motility is the prevalent orolingual motor deficit in ALS and an early sign of the disease [47]. However, clinical studies evaluating tongue force and motility have found that tongue weakness precedes dysarthria in bulbar onset ALS [14, 37]. Tongue weakness is also present in spinal onset patients not exhibiting dysarthria. The maximal temporal rate of normal speech places great demands on tongue motility but does not require maximal tongue force, allowing tongue weakness to go undetected in many cases [37]. Tongue weakness is particularly dangerous as it not only contributes to aspiration during swallowing, but may worsen respiratory failure by contributing to upper airway obstruction during sleep [52].
Studies agree that SOD1-mutant rat brainstem motor neurons are vulnerable to pathology, but report conflicting findings. One study reports facial nucleus motor neuron loss at disease end-stage, while another describes a loss of trigeminal, but not facial, nucleus motor neurons [40, 64]. There is no evidence of hypoglossal motor neuron loss, but vacuolar pathology is evident in the neuropil [40]. Here, we evaluated activity in the hypoglossal nucleus using CO as a marker of metabolism. No differences were revealed, suggesting that hypoglossal nucleus metabolic activity was maintained in end-stage SOD1-G93A rats. We did not observe evidence of vacuolation, but perhaps that was due to the type of stain used on our tissue, rather than a true lack of vacuolar pathology. Our results provide further evidence that the hypoglossal nucleus is relatively spared in this model. This sparing constitutes a difference between SOD1-G93A rat and mouse models of ALS. A variety of studies have documented motor neuron loss and degeneration in the facial, hypoglossal, and trigeminal nuclei in SOD1-G93A mice [3, 23, 50].
Changes in any of the brainstem nuclei mentioned above could affect orolingual motor output, which has not been evaluated in SOD1-mutant rats until now. We found that an orolingual motor deficit was indeed present in these animals. SOD1-G93A rats exhibited a significant tongue motility deficit that became apparent early on in the disease process. However, a tongue force deficit was not revealed in this study. Together, these results suggest that these animals exhibited a long-standing tongue motility deficit, despite the maintenance of tongue muscle strength. This chronic motility deficit is unlike the motility deficit we observed in SOD1-G93A mice, which appeared relatively late in the disease following the emergence of limb muscle deficits [62]. However, earlier tongue motility deficits have been identified in SOD1-G93A mice by a more recent study using a different licking test paradigm (animals lapped water from a sipper tube v. a flat surface) [39].
As mentioned above, tongue force deficits occur earlier and are more prevalent than motility deficits in ALS patients [14, 37, 47]. In contrast, we observed largely normal tongue force in SOD1-G93A rats and a very late-appearing tongue force deficit in SOD1-G93A mice [62]. The difference between what is observed in ALS patients and preclinical models may be explained by the lack of a force challenge in our experiments. Clinical tests of muscle integrity require patients to exert maximal tongue force. Here, we observed that these rats naturally exerted at least ~4 g equivalent force when only 1 g equivalent force was required. Perhaps demanding a greater exertion of tongue force to elicit the water reward would have revealed force deficits in SOD1-G93A rats. Alternatively, this model may not recapitulate the tongue force deficits observed in ALS patients and SOD1-mutant mice [14, 37, 62]. The onset of hindlimb muscle weakness corresponds to the loss of 50% of spinal motor neurons [42]. No loss of hypoglossal motor neurons is detected even at disease end-stage in SOD1-mutant rats [40]. Therefore, it may be reasonable to assume that these animals should not exhibit tongue weakness.
This study did not reveal changes in end-stage SOD1-G93A rat tongue muscle integrity that might underlie the observed tongue motility deficit. Upon dissection, no qualitative differences were observed in the tongues of control and SOD1-G93A rats. Quantification of NMJ integrity revealed no denervation in SOD1-G93A tongues. Following this discovery, the SOD1-G93A rat exhibiting the greatest tongue motility deficit was singled out for further study. The tongue from this rat showed a modest denervation (19% of NMJs were affected), but no atrophy. Significant denervation and atrophy were noted in the end-stage sternomastoid muscle, suggesting that bulbar pathology was evident in other muscles of the head and neck. However, because animals were not overtly debilitated by this change in sternomastoid integrity (ie, rats could still hold up their heads and were still engaging in the lick task), we do not believe this played a role in the emergence of the tongue motility deficit.
Perhaps bulbar deficits had not progressed enough in the majority of these rats to detect significant hypoglossal nucleus or tongue muscle pathology using the methods employed in this study. It is also possible that the deficits in tongue motility arose from functional changes in the hypoglossal nucleus and nerve, in which case future measures of nerve conduction may be warranted [68]. Alternatively, changes in the trigeminal nucleus and/or facial nucleus could account for the tongue motility deficits. Neither of these nuclei was evaluated in this study, but have been shown by other groups to be affected [40, 64]. Lastly, perhaps a spinal motor deficit contributed to the observed decrease in lick rhythm. Ingestive fluid licking requires coordination of licking, swallowing, and breathing [67]. Motor neuron loss in the cervical spinal cord, phrenic nerve degeneration and dysfunction, and diaphragm atrophy—all indicative of respiratory dysfunction—have been documented in SOD1-G93A rats [40]. Further study is required to determine whether respiratory changes give rise to altered lick rhythm in these animals.
Spinal pathology
In ALS patients, corticospinal and spinal motor neurons are affected, resulting in weakness, atrophy, fasciculations, and abnormal reflex responses in the muscles of the arms and legs [56]. Thus far, most preclinical ALS studies have focused on degeneration of spinal motor neurons and related behavioral changes.
Spinal motor neuron pathology and loss have been evaluated in SOD1-G93A rats. In the cervical and lumbar spinal cord, studies have documented early vacuolar changes in the neuropil and subsequent α-motor neuron loss in the ventral horn at end-stage, as well as ventral root atrophy [26, 40, 48]. Muscle pathology has also been documented in SOD1-G93A rats. Electrophysiological recordings demonstrate reduced compound motor action potential amplitudes, positive sharp waves, and spontaneous fibrillation potentials (physiological indications of motor neuron loss and muscle denervation) in SOD1-G93A rat diaphragm, intrinsic foot, and hindlimb muscles at end-stage [26, 40]. These studies also reported extensive atrophy in end-stage SOD1-G93A rat diaphragm and hindlimb muscles. A decrease in NMJ innervation coincides with the onset of paralysis and progresses until most end-plates are denervated at disease end-stage [64, 65].
This motor neuron and skeletal muscle pathology result in a variety of motor deficits. Deficits have been documented in SOD1-G93A rats late in the disease using the inclined plane test (a test of muscle strength) [42]. In agreement with these findings, we found that SOD1-G93A rats exhibit grip strength deficits that became apparent in the late phase of the disease. In contrast to these findings in rats, many studies have documented chronic limb muscle weakness in SOD1-G93A mice using similar methods [10, 62, 73]. This may constitute a true difference in models, or represent these tests’ lack of sensitivity to ALS-related pathology in larger rodents. Interestingly, earlier deficits have been detected in SOD1-G93A rats through evaluation of open-field locomotor behaviors [27, 30, 42].
In agreement with the behavioral findings mentioned above, this study revealed changes in end-stage SOD1-G93A hindlimb muscle integrity that likely underlie the observed grip strength deficit. Upon dissection, marked muscle atrophy was apparent qualitatively in the hindlimbs of SOD1-G93A rats. Quantification of NMJ and muscle fiber integrity revealed significant denervation and atrophy in the SOD1-G93A rat gastrocnemius. The innervation changes were more pronounced in the gastrocnemius when compared to the sternomastoid, suggesting that muscles of the hindlimbs were affected earlier/more severely than those of the neck. This is consistent with disease progression in ALS patients with spinal onset disease.
Oxidative metabolic dysfunction
Mitochondrial dysfunction in the CNS has been implicated in ALS disease pathogenesis. Studies indicate that a reduction occurs in oxidative metabolism, perhaps due to a decrease in mitochondrial function and/or density. CO (complex IV of the electron transport chain) and CS (involved in the citric acid cycle) are mitochondrial enzymes that can be used as markers for oxidative metabolic activity. Several groups report reduced activity of electron transport chain complexes I-IV, as well as reduced CS activity, in spinal motor neurons of sporadic ALS patients [6, 17, 74, 75]. SOD1-mutant models of familial ALS recapitulate these findings of CNS electron transport chain deficits. Mutant SOD1-expressing NSC34 cells exhibit mitochondrial abnormalities and reduced activity of complexes II and IV [44]. Similarly, reduced activity of complexes I-IV is observed in the SOD1-mutant mouse and rat spinal cord and astrocytes [11, 15, 32, 34, 43, 76]. Studies employing a method similar to ours report decreased activity of complexes I, II, and IV in the SOD1-mutant mouse ventral horn prior to the onset of muscle weakness [28, 29].
Interestingly, while CS activity is decreased in spinal motor neurons of sporadic ALS patients, it is maintained or increased in skeletal muscle of ALS patients and patients exhibiting other types of neurogenic muscle atrophy [35, 69, 70, 74]. Similarly, CS activity is maintained in late pre-symptomatic phase SOD1-G93A mouse gastrocnemius muscles [41]. These findings suggest that differences exist in the mitochondrial functions affected in the CNS and muscle.
Measures of oxidative metabolic activity were performed in the hypoglossal nucleus and tongue muscle. We did not observe a difference in the SOD1-G93A rat hypoglossal nucleus CO activity, suggesting that complex IV activity remained normal even at disease end-stage. Likewise, no differences in CS protein expression and activity were noted in SOD1-G93A rat tongue muscle. These findings are consistent with our anatomical evaluation of the tongue, as well as with others’ findings that the hypoglossal nucleus is relatively unaffected in these animals [40].
We also evaluated CS protein levels and activity in the EDL and soleus muscles, and found that protein levels were decreased in both types of SOD1-G93A rat muscle. However, CS activity was maintained or increased in end-stage SOD1-G93A rat muscle. Our results are consistent with others’ findings that CS activity is maintained or increased in skeletal muscles of humans with ALS and SOD1-mutant mice [35, 41, 69, 70]. Perhaps the maintenance of activity despite reduced protein levels is indicative of a compensatory mechanism at work in denervated muscles.
UCP3
UCP3 is an isoform of UCP1, a mitochondrial uncoupling protein that is expressed in brown adipose tissue and plays a role in thermogenesis [9, 53]. UCP3 is predominantly expressed in skeletal muscle [7, 71]. The proposed mechanisms and functions of UCP3 are many, and include a role in the mitigation of reactive oxygen species (ROS) in muscle [reviewed in 12]. This putative role in ROS mitigation may be important in diseases characterized by oxidative damage, such as ALS. Indeed, UCP3 mRNA and protein expression is increased in skeletal muscle of sporadic ALS patients and an increase in UCP3 mRNA expression is found also in presymptomatic and end-stage SOD1-G86R mouse gastrocnemius [15]. The functional significance of this change in expression remains unknown.
UCP3 has been shown to exhibit a fiber-type specific expression pattern, with preferential expression in type II muscle fibers [25]. In accordance with Hesselink et al. [25], as well as with previous findings from our lab [21], UCP3 protein was not present at detectable levels in SOD1-G93A or control rat soleus muscle, which is composed largely of type I fibers [4]. UCP3 protein was detected in the EDL and tongue muscles of SOD1-G93A and control rats, both of which are composed mostly of type II muscle fibers [1, 4, 61]. In contrast to the findings in human and mouse described above, we found that UCP3 protein expression was decreased in the end-stage SOD1-G93A rat EDL. Differences in SOD1 mutation, muscle type, or definition of disease end-stage may underlie our differing results. No difference was noted in the tongue muscle of SOD1-G93A rats, which is consistent with our histological and metabolic findings. Our results suggest that in SOD1-G93A rats, UCP3 protein expression is dependent on the status of the muscle: changes in expression are evident in muscles exhibiting ALS-related pathology (denervation/atrophy), whereas normal expression is observed in muscles that remain largely unaffected. Muscle-specific dysregulation of UCP3 expression may prove important in future studies.
Conclusion & Future Direction
Here, for the first time, we present evidence of a persistent tongue motility deficit in SOD1-G93A rats that became apparent in the early phase of the disease, long before changes were observed in body weight or limb strength. At first glance, our behavioral measures suggest that SOD1-G93A rats present first with an orolingual deficit and second with limb muscle deficits. While we observed a motility, rather than a force, deficit (the opposite of what is observed in ALS patients), the presence of an early orolingual deficit still represents a disease course in agreement with bulbar, and even some cases of spinal, onset ALS in humans [14, 37]. However, none of the measures of hypoglossal nucleus metabolism and tongue muscle innervation and metabolism were indicative of disease. Only the denervation and atrophy observed in the sternomastoid were suggestive of bulbar pathology, and for the reasons described above, we do not believe these changes contributed to the tongue motility deficit.
In direct contrast to our behavioral results, measures of muscle innervation, fiber integrity, and metabolism suggest instead that muscles of the hindlimb were affected more so than those of the head and neck, a disease course in agreement with spinal onset ALS in humans. This apparent inconsistency is due most likely to the relative insensitivity of body weight and grip strength as measures of ALS-related pathology. We believe that lick rhythm is a sensitive marker for pathology, yet the site of pathology (bulbar v. spinal) remains unknown at this point. Overall, it seems most likely that SOD1-G93A rats model more closely spinal onset ALS. Future studies evaluating hypoglossal nerve function, and the role of other brainstem nuclei, the diaphragm, and the phrenic nerve in the development of tongue motility deficits will provide conclusive evidence.
Acknowledgements
This work was supported by NIH grants AG023549, AG026491 to J.A.S., P20 RR024214 to H.N., and by the Kansas Intellectual and Developmental Disabilities Research Center (HD02528). The authors wish to thank Stephen Fowler, Ph.D. for his assistance with MATLAB programming, Don Warn, Ph.D. for his assistance with image analysis, and Greg Bomhoff, M.S. for Western blot quantification.
List of abbreviations
- ALS
amyotrophic lateral sclerosis
- CS
citrate synthase
- CO
cytochrome oxidase
- EDL
extensor digitorum longus
- fALS
familial amyotrophic lateral sclerosis
- NMJ
neuromuscular junction
- RM ANOVA
repeated measures analysis of variance
- sALS
sporadic amyotrophic lateral sclerosis
- SOD1
superoxide-dismutase 1
- UCP3
uncoupling protein 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Abe S, Maejima M, Watanabe H, Shibahara T, Agematsu H, Doi T, Sakiyama K, Usami A, Gojyo K, Hashimoto M, Yoshinari M, Ide Y. Muscle-fiber characteristics in adult mouse-tongue muscles. Anat Sci Int. 2002;77(2):145–148. doi: 10.1046/j.0022-7722.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GM, Erwin KL, Byers N, Deitch JS, Augelli BJ, Blankenhorn EP, Heiman-Patterson TD. Effect of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain Res Mol Brain Res. 2004;130(1–2):7–15. doi: 10.1016/j.molbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Angenstein F, Niessen HG, Goldschmidt J, Vielhaber S, Ludolph AC, Scheich H. Age dependent changes in MRI of motor brain stem nuclei in a mouse model of ALS. Neuroreport. 2004;15:2271–2274. doi: 10.1097/00001756-200410050-00026. [DOI] [PubMed] [Google Scholar]
- 4.Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136(2–3):249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 6.Borthwick GM, Johnson MA, Ince PG, Shaw PJ, Turnbull DM. Mitochondrial enzyme activity in amyotrophic lateral sclerosis: implications for the role of mitochondria in neuronal cell death. Ann Neurol. 1999;46(5):787–790. doi: 10.1002/1531-8249(199911)46:5<787::aid-ana17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997;408(1):39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- 8.Boudriau S, Côté CH, Vincent M, Houle P, Tremblay RR, Rogers PA. Remodeling of the cytoskeletal lattice in denervated skeletal muscle. Muscle Nerve. 1996;19(11):1383–1390. doi: 10.1002/(SICI)1097-4598(199611)19:11<1383::AID-MUS2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Bouillaud F, Ricquier D, Mory G, Thibault J. Increased level of mRNA for the uncoupling protein in brown adipose tissue of rats during thermogenesis induced by cold exposure or norepinephrine infusion. J Biol Chem. 1984;259(18):11583–11586. [PubMed] [Google Scholar]
- 10.Canton T, Pratt J, Stutzmann J-M, Imperato A, Boireau A. Glutamate uptake is decreased tardively in the spinal cord of FALS mice. Neuroreport. 1998;9:775–778. doi: 10.1097/00001756-199803300-00001. [DOI] [PubMed] [Google Scholar]
- 11.Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de León A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28(16):4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costford SR, Seifert EL, Bézaire V, F Gerrits M, Bevilacqua L, Gowing A, Harper ME. The energetic implications of uncoupling protein-3 in skeletal muscle. Appl Physiol Nutr Metab. 2007;32(5):884–894. doi: 10.1139/H07-063. [DOI] [PubMed] [Google Scholar]
- 13.Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 14.DePaul R, Abbs JH, Caligiuri M, Gracco VL, Brooks BR. Hypoglossal, trigeminal, and facial motoneuron involvement in amyotrophic lateral sclerosis. Neurology. 1988;38:281–283. doi: 10.1212/wnl.38.2.281. [DOI] [PubMed] [Google Scholar]
- 15.Dupuis L, di Scala F, Rene F, de Tapia M, Oudart H, Pradat PF, Meininger V, Loeffler JP. Up-regulation of mitochondrial uncoupling protein 3 reveals an early muscular metabolic defect in amyotrophic lateral sclerosis. FASEB J. 2003;17(14):2091–2093. doi: 10.1096/fj.02-1182fje. [DOI] [PubMed] [Google Scholar]
- 16.Fowler SC, Wang G. Chronic haloperidol produces a time- and dose-related slowing of lick rhythm in rats: implications for rodent models of tardive dyskinesia and neuroleptic-induced parkinsonism. Psychopharmacology (Berl) 1998;137:50–60. doi: 10.1007/s002130050592. [DOI] [PubMed] [Google Scholar]
- 17.Fujita K, Yamauchi M, Shibayama K, Ando M, Honda M, Nagata Y. Decreased cytochrome c oxidase activity but unchanged superoxide dismutase and glutathione peroxidase activities in the spinal cords of patients with amyotrophic lateral sclerosis. J Neurosci Res. 1996;45:276–281. doi: 10.1002/(SICI)1097-4547(19960801)45:3<276::AID-JNR9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez de Aguilar JL, Echaniz-Laguna A, Fergani A, René F, Meininger V, Loeffler JP, Dupuis L. Amyotrophic lateral sclerosis: all roads lead to Rome. J Neurochem. 2007;101(5):1153–1160. doi: 10.1111/j.1471-4159.2006.04408.x. [DOI] [PubMed] [Google Scholar]
- 19.Gruzman A, Wood WL, Apert E, Prasad MD, Miller RG, Rothstein JD, Bowser R, Hamilton R, Wood TD, Cleveland DW, Lingappa VR, Liu J. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Nat Acad Sci USA. 2007;104:12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupte AA, Bomhoff GL, Morris JK, Gorres BK, Geiger PC. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J Appl Physiol. 2009a;106(4):1425–1434. doi: 10.1152/japplphysiol.91210.2008. [DOI] [PubMed] [Google Scholar]
- 21.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009b;58(3):567–578. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 23.Haenggeli C, Kato AC. Differential vulnerability of cranial motoneurons in mouse models with motor neuron degeneration. Neurosci Lett. 2002;335:39–43. doi: 10.1016/s0304-3940(02)01140-0. [DOI] [PubMed] [Google Scholar]
- 24.Herbik MA, Stanislaw JC, Kowalczyk A, Grieb P. Maintenance of the rat transgenic model of familial amyotrophic lateral sclerosis expressing human SOD1G93A mutation. Folia Neuropathol. 2006;44(3):149–153. [PubMed] [Google Scholar]
- 25.Hesselink MK, Keizer HA, Borghouts LB, Schaart G, Kornips CF, Slieker LJ, Sloop KW, Saris WH, Schrauwen P. Protein expression of UCP3 differs between human type 1, type 2a, and type 2b fibers. FASEB J. 2001;15(6):1071–1073. [PubMed] [Google Scholar]
- 26.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci USA. 2002;99(3):1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jokic N, Ling YY, Ward RE, Michael-Titus AT, Priestley JV, Malaspina A. Retinoid receptors in chronic degeneration of the spinal cord: observations in a rat model of amyotrophic lateral sclerosis. J Neurochem. 2007;103(5):1821–1833. doi: 10.1111/j.1471-4159.2007.04893.x. [DOI] [PubMed] [Google Scholar]
- 28.Jung C, Higgins CM, Xu Z. A quantitative histochemical assay for activities of mitochondrial electron transport chain complexes in mouse spinal cord sections. J Neurosci Methods. 2002a;114(2):165–172. doi: 10.1016/s0165-0270(01)00524-6. [DOI] [PubMed] [Google Scholar]
- 29.Jung C, Higgins CM, Xu Z. Mitochondrial electron transport chain complex dysfunction in a transgenic mouse model for amyotrophic lateral sclerosis. J Neurochem. 2002b;83(3):535–545. doi: 10.1046/j.1471-4159.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 30.Kafkafi N, Yekutieli D, Yarowsky P, Elmer GI. Data mining in a behavioral test detects early symptoms in a model of amyotrophic lateral sclerosis. Behav Neurosci. 2008;122(4):777–787. doi: 10.1037/0735-7044.122.4.777. [DOI] [PubMed] [Google Scholar]
- 31.Kasarkis EJ, Berryman S, Vanderleest JG, Schneider AR, McClain CJ. Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am J Clin Nutr. 1996;63:130–137. doi: 10.1093/ajcn/63.1.130. [DOI] [PubMed] [Google Scholar]
- 32.Kirkinezos IG, Bacman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, Bradley WG, Moraes CT. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 2005;25(1):164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16(4):509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 34.Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krasnianski A, Deschauer M, Neudecker S, Gellerich FN, Müller T, Schoser BG, Krasnianski M, Zierz S. Mitochondrial changes in skeletal muscle in amyotrophic lateral sclerosis and other neurogenic atrophies. Brain. 2005;128(Pt 8):1870–1876. doi: 10.1093/brain/awh540. [DOI] [PubMed] [Google Scholar]
- 36.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136(6):1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langmore SE, Lehman ME. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. J Speech Hear Res. 1994;37:28–37. doi: 10.1044/jshr.3701.28. [DOI] [PubMed] [Google Scholar]
- 38.Levanon D, Lieman-Hurwitz J, Dafni N, Wigderson M, Sherman L, Bernstein Y, Laver-Rudich Z, Danciger E, Stein O, Groner Y. Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the Cu/Zn superoxide dismutase. EMBO J. 1985;4:77–84. doi: 10.1002/j.1460-2075.1985.tb02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lever TE, Gorsek A, Cox KT, O'Brien KF, Capra NF, Hough MS, Murashov AK. An animal model of oral dysphagia in amyotrophic lateral sclerosis. Dysphagia. 2009;24(2):180–195. doi: 10.1007/s00455-008-9190-z. [DOI] [PubMed] [Google Scholar]
- 40.Lladó J, Haenggeli C, Pardo A, Wong V, Benson L, Coccia C, Rothstein JD, Shefner JM, Maragakis NJ. Degeneration of respiratory motor neurons in the SOD1 G93A transgenic rat model of ALS. Neurobiol Dis. 2006;21(1):110–118. doi: 10.1016/j.nbd.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Mahoney DJ, Kaczor JJ, Bourgeois J, Yasuda N, Tarnopolsky MA. Oxidative stress and antioxidant enzyme upregulation in SOD1-G93A mouse skeletal muscle. Muscle Nerve. 2006;33(6):809–816. doi: 10.1002/mus.20542. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto A, Okada Y, Nakamichi M, Nakamura M, Toyama Y, Sobue G, Nagai M, Aoki M, Itoyama Y, Okano H. Disease progression of human SOD1 (G93A) transgenic ALS model rats. J Neurosci Res. 2006;83(1):119–133. doi: 10.1002/jnr.20708. [DOI] [PubMed] [Google Scholar]
- 43.Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277(33):29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 44.Menzies FM, Cookson MR, Taylor RW, Turnbull DM, Chrzanowska-Lightowlers ZM, Dong L, Figlewicz DA, Shaw PJ. Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis. Brain. 2002;125(Pt 7):1522–1533. doi: 10.1093/brain/awf167. [DOI] [PubMed] [Google Scholar]
- 45.Miao L, St. Clair D. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47(4):344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Migliore L, Coppedè F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res. 2009;667(1–2):82–97. doi: 10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Mitsumoto H, Chad DA, Pioro EP. Amyotrophic lateral sclerosis. Philadelphia, PA: F.A. Davis Company; 1998. [Google Scholar]
- 48.Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH, Jr, Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21(23):9246–9254. doi: 10.1523/JNEUROSCI.21-23-09246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nau KL, Bromberg MB, Forshew DA, Katch VL. Individuals with amyotrophic lateral sclerosis are in caloric balance despite losses in mass. J Neurol Sci. 1995;129:47–49. doi: 10.1016/0022-510x(95)00061-6. [DOI] [PubMed] [Google Scholar]
- 50.Niessen HG, Angenstein F, Sander K, Kunz WS, Teuchert M, Ludolph AC, Heinze H-J, Scheich H, Vielhaber S. In vivo quantification of spinal and bulbar motor neuron degeneration in the G93A-SOD1 transgenic mouse model of ALS by T2 relaxation time and apparent diffusion coefficient. Exp Neurol. 2006;201:293–300. doi: 10.1016/j.expneurol.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432(7017):580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- 52.Orlikowski D, Terzi N, Blumen M, Sharshar T, Raphael JC, Annane D, Lofaso F. Tongue weakness is associated with respiratory failure in patients with severe Guillain-Barre´ syndrome. Acta Neurol Scand. 2009;119:364–370. doi: 10.1111/j.1600-0404.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 53.Ricquier D, Thibault J, Bouillaud F, Kuster Y. Molecular approach to thermogenesis in brown adipose tissue. Cell-free translation of mRNA and characterization of the mitochondrial uncoupling protein. J Biol Chem. 1983;258(11):6675–6677. [PubMed] [Google Scholar]
- 54.Rosen AD. Amyotrophic lateral sclerosis: clinical features and prognosis. Arch Neurol. 1978;35:638–642. doi: 10.1001/archneur.1978.00500340014003. [DOI] [PubMed] [Google Scholar]
- 55.Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- 56.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. The New England Journal of Medicine. 2001;344:1688–1701. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 57.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 58.Seebart BR, Stoffel RT, Behan M. Age-related changes in the serotonin 2A receptor in the hypoglossal nucleus of male and female rats. Respir Physiol Neurobiol. 2007;158(1):14–21. doi: 10.1016/j.resp.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silani V, Kasarskis EJ, Yanagisawa N. Nutritional management in amyotrophic lateral sclerosis: a world-wide perspective. J Neurol. 1998;245:S13–S19. doi: 10.1007/pl00014805. [DOI] [PubMed] [Google Scholar]
- 60.Smirnova IV, Kibiryeva N, Vidoni E, Bunag R, Stehno-Bittel L. Abnormal EKG stress test in rats with type 1 diabetes is deterred with low-intensity exercise programme. Acta Diabetol. 2006;43:66–74. doi: 10.1007/s00592-006-0215-5. [DOI] [PubMed] [Google Scholar]
- 61.Smith JC, Goldberg SJ, Shall MS. Phenotype and contractile properties of mammalian tongue muscles innervated by the hypoglossal nerve. Respir Physiol Neurobiol. 2005;147(2–3):253–262. doi: 10.1016/j.resp.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 62.Smittkamp SE, Brown JW, Stanford JA. Time-course and characterization of orolingual motor deficits in B6SJL-Tg(SOD1-G93A)1Gur/J mice. Neuroscience. 2008;151(2):613–621. doi: 10.1016/j.neuroscience.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smittkamp SE, Park DL, Girod DA, Durham D. Effects of aging and cochlear damage on the metabolic activity of the avian cochlear nucleus. Hearing Research. 2003;175:101–111. doi: 10.1016/s0378-5955(02)00714-1. [DOI] [PubMed] [Google Scholar]
- 64.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, Man WY, De Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robberecht W, Conway EM, Collen D, Moons L, Carmeliet P. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8(1):85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE. 2007a;2(1):e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki M, Tork C, Shelley B, McHugh J, Wallace K, Klein SM, Lindstrom MJ, Svendsen CN. Sexual dimorphism in disease onset and progression of a rat model of ALS. Amyotroph Lateral Scler. 2007b;8(1):20–25. doi: 10.1080/17482960600982447. [DOI] [PubMed] [Google Scholar]
- 67.Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev. 1997;21:631–647. doi: 10.1016/s0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- 68.Urban PP, Wicht S, Hopf HC. Sensitivity of transcranial magnetic stimulation of cortico-bulbar vs. cortico-spinal tract involvement in Amyotrophic Lateral Sclerosis (ALS) J Neurol. 2001;248(10):850–855. doi: 10.1007/s004150170068. [DOI] [PubMed] [Google Scholar]
- 69.Vielhaber S, Kunz D, Winkler K, Wiedemann FR, Kirches E, Feistner H, Heinze HJ, Elger CE, Schubert W, Kunz WS. Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis. Brain. 2000;123(Pt 7):1339–1348. doi: 10.1093/brain/123.7.1339. [DOI] [PubMed] [Google Scholar]
- 70.Vielhaber S, Kudin A, Winkler K, Wiedemann F, Schröder R, Feistner H, et al. Is there evidence for mitochondrial dysfunction in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis? Ann Neurol. 2003;53:686–687. doi: 10.1002/ana.10564. [DOI] [PubMed] [Google Scholar]
- 71.Vidal-Puig A, Solanes G, Grujic D, Flier JS, Lowell BB. UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem Biophys Res Commun. 1997;235(1):79–82. doi: 10.1006/bbrc.1997.6740. [DOI] [PubMed] [Google Scholar]
- 72.Wang G, Fowler SC. Effects of haloperidol and clozapine on tongue dynamics during licking in CD-1, BALB/c and C57BL/6 mice. Psychopharmacology. 1999;147:38–45. doi: 10.1007/s002130051140. [DOI] [PubMed] [Google Scholar]
- 73.Weydt P, Hong SY, Kliot M, Moller T. Assessing disease onset and progression in the SOD1 mouse model of ALS. Neuroreport. 2003;14:1051–1054. doi: 10.1097/01.wnr.0000073685.00308.89. [DOI] [PubMed] [Google Scholar]
- 74.Wiedemann FR, Manfredi G, Mawrin C, Beal MF, Schon EA. Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients. J Neurochem. 2002;80(4):616–625. doi: 10.1046/j.0022-3042.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 75.Wiedemann FR, Winkler K, Kuznetsov AV, Bartels C, Vielhaber S, Feistner H, Kunz WS. Impairment of mitochondrial function in skeletal muscle of patients with amyotrophic lateral sclerosis. J Neurol Sci. 1998;156(1):65–72. doi: 10.1016/s0022-510x(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 76.Xu Z, Jung C, Higgins C, Levine J, Kong J. Mitochondrial degeneration in amyotrophic lateral sclerosis. J Bioenerg Biomembr. 2004;36(4):395–399. doi: 10.1023/B:JOBB.0000041774.12654.e1. [DOI] [PubMed] [Google Scholar]
- 77.Yim MB, Kang J-H, Yim H-S, Kwak H-S, Chock PB, Stadtman ER. A gain-of-function of an amyotrophic lateral sclerosis-associated Cu, Zn-superoxide dismutase mutant: an enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc Natl Acad Sci. 1996;93:5709–5714. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]