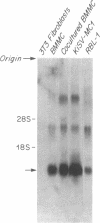
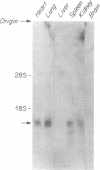
Abstract
A cDNA that encodes a mouse secretory granule proteoglycan peptide core was isolated from a cDNA library prepared from nontransformed mouse bone marrow-derived mast cells (BMMC) using as a probe a 280-base-pair fragment of a rat cDNA that encodes the proteoglycan peptide core of rat basophilic leukemia (RBL)-1 cells. Based on the consensus nucleotide sequence and deduced amino acid sequence of the cDNA, the mouse BMMC proteoglycan peptide core is 16.7 kDa and contains a 21-amino acid glycosaminoglycan attachment region consisting of alternating serine and glycine residues. When the predicted amino acid sequence of the mouse BMMC proteoglycan peptide core was compared with the predicted amino acid sequences of the homologous molecules expressed in RBL-1 cells and in human promyelocytic leukemia HL-60 cells, the mouse-derived sequence was more closely homologous to the rat sequence than the human sequence except for the length of the serine-glycine repeat region. The N terminus was found to be a highly conserved region of the molecule in the three species, suggesting that this region is important for the structure, function, and/or metabolism of this family of proteoglycans. Nucleotide sequences within the 5' and 3' untranslated regions of the mouse, rat, and human proteoglycan cDNA were conserved. That similar sequences were also present in the corresponding regions of a cDNA that encodes a rat mast cell protease suggests that particular nucleotide sequences may be important for regulation of expression of those proteins that are destined to reside in secretory granules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham S., Stevens R. L., Gartner M. C., Austen K. F., Lalley P. A., Weis J. H. Isolation of a cDNA that encodes the peptide core of the secretory granule proteoglycan of rat basophilic leukemia-1 cells and assessment of its homology to the human analogue. J Biol Chem. 1988 May 25;263(15):7292–7296. [PubMed] [Google Scholar]
- Benfey P. N., Yin F. H., Leder P. Cloning of the mast cell protease, RMCP II. Evidence for cell-specific expression and a multi-gene family. J Biol Chem. 1987 Apr 15;262(11):5377–5384. [PubMed] [Google Scholar]
- Bourdon M. A., Oldberg A., Pierschbacher M., Ruoslahti E. Molecular cloning and sequence analysis of a chondroitin sulfate proteoglycan cDNA. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1321–1325. doi: 10.1073/pnas.82.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M. A., Shiga M., Ruoslahti E. Gene expression of the chondroitin sulfate proteoglycan core protein PG19. Mol Cell Biol. 1987 Jan;7(1):33–40. doi: 10.1128/mcb.7.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M. A., Shiga M., Ruoslahti E. Identification from cDNA of the precursor form of a chondroitin sulfate proteoglycan core protein. J Biol Chem. 1986 Sep 25;261(27):12534–12537. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dayton E. T., Pharr P., Ogawa M., Serafin W. E., Austen K. F., Levi-Schaffer F., Stevens R. L. 3T3 fibroblasts induce cloned interleukin 3-dependent mouse mast cells to resemble connective tissue mast cells in granular constituency. Proc Natl Acad Sci U S A. 1988 Jan;85(2):569–572. doi: 10.1073/pnas.85.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Schaffer F., Austen K. F., Gravallese P. M., Stevens R. L. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6485–6488. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Hayman E. G., Ruoslahti E. Isolation of a chondroitin sulfate proteoglycan from a rat yolk sac tumor and immunochemical demonstration of its cell surface localization. J Biol Chem. 1981 Nov 10;256(21):10847–10852. [PubMed] [Google Scholar]
- Razin E., Cordon-Cardo C., Good R. A. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2559–2561. doi: 10.1073/pnas.78.4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Ihle J. N., Seldin D., Mencia-Huerta J. M., Katz H. R., LeBlanc P. A., Hein A., Caulfield J. P., Austen K. F., Stevens R. L. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984 Mar;132(3):1479–1486. [PubMed] [Google Scholar]
- Razin E., Mencia-Huerta J. M., Stevens R. L., Lewis R. A., Liu F. T., Corey E., Austen K. F. IgE-mediated release of leukotriene C4, chondroitin sulfate E proteoglycan, beta-hexosaminidase, and histamine from cultured bone marrow-derived mouse mast cells. J Exp Med. 1983 Jan 1;157(1):189–201. doi: 10.1084/jem.157.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Reynolds D. S., Serafin W. E., Faller D. V., Wall D. A., Abbas A. K., Dvorak A. M., Austen K. F., Stevens R. L. Immortalization of murine connective tissue-type mast cells at multiple stages of their differentiation by coculture of splenocytes with fibroblasts that produce Kirsten sarcoma virus. J Biol Chem. 1988 Sep 5;263(25):12783–12791. [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Serafin W. E., Dayton E. T., Gravallese P. M., Austen K. F., Stevens R. L. Carboxypeptidase A in mouse mast cells. Identification, characterization, and use as a differentiation marker. J Immunol. 1987 Dec 1;139(11):3771–3776. [PubMed] [Google Scholar]
- Serafin W. E., Katz H. R., Austen K. F., Stevens R. L. Complexes of heparin proteoglycans, chondroitin sulfate E proteoglycans, and [3H]diisopropyl fluorophosphate-binding proteins are exocytosed from activated mouse bone marrow-derived mast cells. J Biol Chem. 1986 Nov 15;261(32):15017–15021. [PubMed] [Google Scholar]
- Stevens R. L., Avraham S., Gartner M. C., Bruns G. A., Austen K. F., Weis J. H. Isolation and characterization of a cDNA that encodes the peptide core of the secretory granule proteoglycan of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1988 May 25;263(15):7287–7291. [PubMed] [Google Scholar]
- Stevens R. L., Otsu K., Austen K. F. Purification and analysis of the core protein of the protease-resistant intracellular chondroitin sulfate E proteoglycan from the interleukin 3-dependent mouse mast cell. J Biol Chem. 1985 Nov 15;260(26):14194–14200. [PubMed] [Google Scholar]
- Stevens R. L., Otsu K., Weis J. H., Tantravahi R. V., Austen K. F., Henkart P. A., Galli M. C., Reynolds C. W. Co-sedimentation of chondroitin sulfate A glycosaminoglycans and proteoglycans with the cytolytic secretory granules of rat large granular lymphocyte (LGL) tumor cells, and identification of a mRNA in normal and transformed LGL that encodes proteoglycans. J Immunol. 1987 Aug 1;139(3):863–868. [PubMed] [Google Scholar]
- Stevens R. L., Razin E., Austen K. F., Hein A., Caulfield J. P., Seno N., Schmid K., Akiyama F. Synthesis of chondroitin sulfate E glycosaminoglycan onto p-nitrophenyl-beta-D-xyloside and its localization to the secretory granules of rat serosal mast cells and mouse bone marrow-derived mast cells. J Biol Chem. 1983 May 10;258(9):5977–5984. [PubMed] [Google Scholar]
- Tantravahi R. V., Stevens R. L., Austen K. F., Weis J. H. A single gene in mast cells encodes the core peptides of heparin and chondroitin sulfate proteoglycans. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9207–9210. doi: 10.1073/pnas.83.23.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]