Abstract
Key molecular players that link inflammation to carcinogenesis are prostaglandins, cytokines, nuclear factor-κB (NF-κB), chemokines, angiogenic growth factors, and free radicals, all of which lead to increased mutations and altered functions of important enzymes and proteins, for example, activation of oncogenic products and/or inhibition of tumor suppressor proteins, in inflamed tissues, thus contributing to multi-stage carcinogenesis process. Interpreted reversely, the identification of the molecular mechanisms by which chronic inflammation increases cancer risk or optimal intervention of targeted drugs or agents during the inflammation-associated carcinogenic process could be a necessary basis for developing new strategy of cancer prevention at many sites. In this review, we discuss the possibilities for cancer prevention by controlling inflammation process in Helicobacter pylori (H. pylori)-associated inflamed stomach with Korea red ginseng. Korea red ginseng is a good example of a natural herb that has ubiquitous properties that are conductive to stop inflammatory carcinogenesis that is un wanted outcome of H. pylori infection, rendering rejuvenation of chronic atrophic gastritis.
Keywords: Korea red ginseng, inflammation, carcinogenesis, rejuvenation, cancer prevention
Connection between Inflammation and Cancer
Carcinogenesis is a long and multi-step process that includes initiation, promotion and progression as a consequence of an imbalance between cell proliferation and cell death, during which complex interplay concerning inflammation between epithelial layer and mesenchymal tissues occurred, after which more genetic and epigenetic events are required to drive from initiated cells to malignant tumors, each conferring one and another type of growth advantage, and leads to the progressive conversion of normal cells into cancer cells [1–3]. In fact, a wide array of chronic inflammatory conditions predisposes susceptible cells to neoplastic transformations during this journey to carcinogenesis [4–5].
In general, the longer the inflammation persists, the higher the risk of cancer, suggesting that though inflammation is a step-by-step process that includes injury, repair and resolution, all inflammatory cells, for instance, neutrophils, monocytes, macrophages, eosinophils, dendritic cells, mast cells, and lymphocytes, are recruited after a damage or an infection, and may contribute to the onset and progression of cancer [6]. Moreover, once tumor develops, tumor cells educated macrophages or inflammatory cells to promote tumor progression. With the continuing medical education that inflammatory cells including macrophages are continuously educated by the tumor microenvironment, they adopt a trophic role that facilitates angiogenesis, matrix breakdown and tumor cell motility, all of which are elements of the metastatic process [7]. During an inflammatory response, these inflammatory cells also produce many compounds, ranging from mutagenic oxygen and nitrogen radicals to angiogenic factors that further contribute to cancer initiation and promotion [8]. Therefore, crudely saying, this is why inflammation represents an important drug target for cancer prevention and cure.
It has been reported that approximately 15–20% of all malignancies are initiated or exacerbated by inflammation. Furthermore, the recruitment and infiltration of inflammatory cells in the tumor microenvironment activates them to spurt the malignant progression of cancer cells. Almost 150 years, Rudolf Virchow first indicated the concept that lymphoreticular infiltration reflects the origin of cancer at sites of chronic inflammation, suggesting a close connection between inflammation and the development of cancer, of which theory was further extended to implicate the stromal interactions between malignant cells and inflammatory cells associated with angiogenesis. Inflammatory stimuli include chemicals and foreign bodies (e.g. asbestosis, fiber, silica particles, catheters, alcohol, bile acids, gastric acids, gall bladder stones, and ultraviolet light) and infectious organisms (e.g. Helicobacter pylori (H. pylori), hepatitis B and C viruses, Epstein-Barr virus, herpes virus, papilloma virus, HIV) [9–10].
Irrespecive of involved organs and etiologies of inflammations, the common features essential to inflammation-associated carcinogenesis were as follows; proinflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-8, interferon-γ, MDM2, p53 etc, prevail in inflamed sites, imbalance between reactive oxygen species (ROS)-generating enzymes and anti-oxidant defense mechanisms, so called, oxidative stress, activation of NF-κB, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), proteinase, and activation of oncogenes. Based on these implications, various chemopreventive agents targeted for attenuating inflammation have been studies, including curcumin, retinoids, Vitamin E, silimarin, aspirin, celecoxib, some plant polyphenols, proton pump inhibitor and others [11–13].
Crosslink between H. pylori-Associated Chronic Atrophic Gastritis and Gastric Cancer
Though it shows the tendency of decrease in gastric cancer for last 80 years, it is still second highest cause of death following lung cancer and in Korea. 25–30 people out of 100,000 catch gastric cancer regardless of their gender and its motility rate is number one. However, the discovery of causative pathogen of gastric cancer, H. pylori, has shown the light to inhibit gastric cancer because International Agency for Research on Carcinogenesis (IARC, Lyon) in 1994 defined H. pylori as an imminent carcinogenic pathogen based on many studies, epidemiological research and animal studies. The definition has the historical background based on the theological evidence and several animal experiments results showing infection of H. pylori causes infiltration of inflammatory, oxidative damage and mutations. In a resected specimen due to gastric cancer, the intestinal metaplstic lesions were easily identified around cancer lesions, for which H. pylori infection is closely associated. The reason why gastric cancer is not controlled even after killing H. pylori, is that under the equation: “chronic atrophic gastritis–intestinal metaplasia-gastric cancer”, the patients who did not stepping into malignant process could be benefited from H. pylori-associated carcinogenesis [14–15]. In other words, even though H. pylori, defined as Class I carcinogen, are eradicated, the inflammation itself remains in the stomach, thus it seems that the control and the amelioration of gastric inflammation itself is far more essential in cancer prevention rather than the eradication of the pathogen itself. This inversely emphasizes that chronically continuation of gastritis has greater chance of onset of gastric cancer than infection of H. pylori itself. H. pylori-induced gastric cancer is based on chronic atrophic gastritis as well as intestinal metaplasia, and gone into the stage of dysplasia, resulting in intestinal gastric cancer and accompanied by genetic or environmental change synergistically to accelerate the formation of cancer. With this background, genetic factors, toxicity of the pathogen and environmental factors are orchestrated to develop H. pylori-induced gastric cancer from inflammation, thus with broad category, alleviation or treatment of inflammation would be the fundamentals to the prevention of gastric cancer.
Rejuvenation of Atrophic Gastritis, It is Possible?
Then, we have another question whether atrophic gastritis can be restored as before infection with either the removal of bacteria or clearing gastric inflammation. In spite two recent large-scale, prospective studies, both performed in high risk population, have reported that H. pylori infection as a definite risk factor for the development of gastric cancer, the opposite premise that eradication of H. pylori infection is an appropriate target for the prevention of gastric cancer is still uncertain. Tree randomized, placebo-controlled trials performed in China and Columbia demonstrated no significant protective effect by H. pylori eradication [16–18], whereas contradictory results have emerged out of three Japanese studies published recently [19–21], indicating that H. pylori eradication may prevent the development of gastric cancer significantly, even in patients with precancerous gastric lesions. These reciprocal results can be explained by the fact that, unlike the studies from China and Columbia, protective studies from Japan were neither randomized nor placebo-controlled. However, the common evidence of each Japanese study was that no gastric cancers developed after eradication treatment in patients without precancerous gastric lesions at entry. Stated the other way around, all gastric cancer cases appeared in patients who had chronic atrophic gastritis/intestinal metaplasia at trial entry before H. pylori eradication. This observation ascertains us that earlier eradication therapy or other kinds of efforts to lessen gastric inflammation could be beneficial in high-risk populations to completely abolish overall gastric cancer risk arising from chronic atrophic gastritis [22]. Another key issue regarding the influence of H. pylori eradication on gastric cancer prevention is the fact that atrophic gastritis is reversible lesion after H. pylori eradication, leading to hypothesis that H. pylori eradication could retard or reverse gastric carcinogenesis before it reaches the stage of H. pylori-associated chronic atrophic gastritis and/or dysplasia. As clear evidence of merit of H. pylori eradication, Fukase et al. [23] have published important results from a study where, following endoscopic resection of early gastric cancer, a group of patients in a randomized control trial were subjected to H. pylori eradication treatment and monitored at different time intervals. At 3 years, metachronous gastric cancer had developed in only 9 of 255 patients in the eradication group compared with 24 of 250 patients in the control group, a significant difference with indicates that prophylactic eradication of H. pylori in atrophic gastritis can substantially reduce gastric cancer rates.
However, similar to afore-mentioned implication of H. pylori eradication on gastric cancer prevention, studies examining the reversibility of precancerous lesions following eradication of H. pylori also have provided conflicting results. Among earlier studies, several reported that the severity of gastric atrophy and intestinal metaplasia does not change after treatment [24]. In contrast, another study has suggested that gastric atrophy and some of intestinal metaplasia can improve after H. pylori eradication [25]. The contradictory results can be explained by a fact that significant proportions among the eradicated patients have progression of premalignant lesions, already stepping to pass a “point-of-no-return”. Therefore, the eradication of H. pylori infection might not be beneficial if therapy is given passing the “point-of-no-return”. However, it is very difficult to discriminate whether the gastric premalignant lesion is passed such a “point-of-no-return” or not by conventional endoscopy or histopathology [26–27]. Many efforts had been made to find biomarkers or clinical findings that suggest reversibility of gastric atrophy or intestinal metaplasia; ideally, these might adopt high throughput analytical techniques, including microarray or proteomics [28]. Based on these observations, we hypothesize that supplementary or continuing suppressive way for gastric inflammation to mitigate chronic gastric gastritis will be the next step for gastric cancer prevention and we strongly send confidence that Korea red ginseng can play tackling in the progression of atrophic gastritis and tangle to revert to non-atrophic gastritis.
Efficacy of Panax ginseng C.A. Meyer on “Cancer Prevention” Purpose; Tangle the Progression of Atrophic Gastritis
Before entering the description about the beneficial effects of Korea red ginseng on H. pylori-associated atrophic gastritis, we will briefly touch the cancer preventive action of Panax ginseng. The name ginseng comes from the Chinese words “Jen Sheng”, meaning “man-herb”, based on the humanoid shape of the root or rhizome of the plant, which is the part of the plant most commonly consumed. The name Panax means “all healing” (panacea), which describes the traditional belief that ginseng has properties to heal all aspects of the body. Even though there are several different species of ginseng, two of the most commonly used are P. ginseng (Chinese ginseng) and P. quinquefolius (American ginseng), among which P. ginseng C.A. Meyer (Araliaceae) has been used as a medicine by the people of Eastern Asia for at least 2,000 years [29–31]. Native to Korea, this red-berried plant, commonly called Korean ginseng, is now open employed for diabetes, cardiovascular diseases, neurodegenerative diseases, urological diseases including sexual improvement, several kinds of viral diseases, including various kinds of cancer.
A research of PubMed for “cancer” and “P. ginseng” yields over 400 articles, signaling that P. ginseng apparently mitigates cancer through their anti-inflammatory, antioxidant, and apoptotic mechanisms to influence carcinogenic process. Briefly stated, 1) inducing differentiation [32]; ginseng’s induction of repair or reverse transformation of cells into more differentiated cells has been noted in hepatoma, melanoma, and teratocarcinoma cells, 2) reduced effects from chemical carcinogens [33]; inhibitory effects on the development of rat mammary adenocarcinoma induced by methyl-N-nitrosourea and N-ethyl-N-nitrosourea administration, 3) mitigating inflammatory carcinogenesis [34]; ginseng reduced COX-2, iNOS, and NK-κB, 4) antioxidant chemoprevention [35]; ginseng extracts have been shown to scavenge reactive oxygen species (ROS) as well as lipid peroxidation. As shown in Fig. 1A, electron spin resonance (ESR) measurement showed direct antioxidative action of Korea red ginseng extracts on Fenton reaction-induced hydroxyl radicals, 5) induction of apoptosis [36]; active components of ginsenoside induced direct executions of apoptosis, 6) inhibition of proliferation [37]; inhibition of cell cycle progression has been implicated as a chemopreventive mechanisms of ginsenosidesm Rh2 Rg3, etc, halting the metastasis in addition to tumorigenesis except one study showing increased metastatic potential of P. ginseng, 7) immnuomodulation [38]; mostly studied postoperatively to encourage the recovery from surgery or illness, 8) the relief of multi-drug resistance (MDR) [39]; chemosensitizing effect and reduced efflux pump activity related to MDR mechanism, 9) anti-angiogenic or angiogenic effect [40]; dual effects on angiogenesis imposing anti-angiogenic action against carcinogenesis,but angiogenic effects for healing. As for effective gradients of ginseng, ginsenosides or ginseng saponins are the principal active ingredients in ginseng and more than 40 different ginsenosides have been identified. Based on their structural differences, they can be classified into three categories; the panaxdiol group (e.g. Rb1, Rb2, Rc, Rd, Rg3, Rh2), the panaxatriol group (e.g. Re, Rf, Rg1, Rg2, Rh1) and the oleanolic group (e.g. Ro).
Fig. 1.
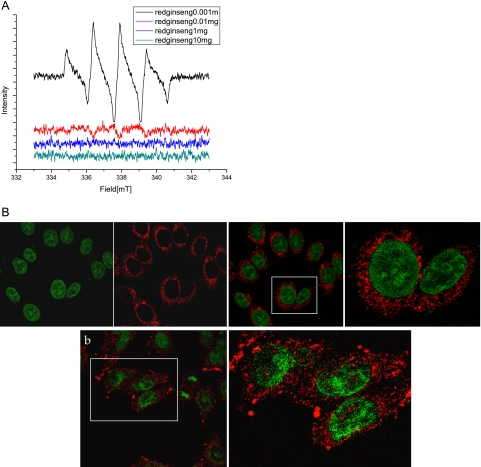
(A) ESR changes with red ginseng extracts. Electron spin resonance (JOEL, Tokyo, Japan) was performed using DMPO as adductor to visualize hydroxyl radicals in the presence of 0.01 mg Korea red ginseng extracts. As the doses of Korea red ginseng extracts were increased, scavenging action was observed more than 0.01 mg of red ginseng extracts, suggesting the antioxidative action of Korea red ginseng. (B) Confocal imaging of 8-OHdG in gastric mucosal cells. In non-stimulated state, 8-OHdG was mostly observed scattered in cytoplasm, whereas in the presence of H. pylori infection, the expressions of 8-OHdG were increased in either cytoplasm or nucleus, suggesting that 8-OHdG might reflect oxidative stress after H. pylori infection. Korea red ginseng was very effective in mitigating the 8-OHdG levels even after H. pylori infection.
As representational study showing anti-carcinogenic properties of ginseng, Dr. T.K. Yun issued human epidemiology and well organized animal study in the Lancet Oncology (2001) [41]. He investigated the effects of ginseng consumption on the risk of cancers by interviewing 905 pairs of cases and controls matched by age, sex, and date of admission to the Korea Cancer center Hospital, Seoul. As results, a trend test showed a significantly decrease in the frequency of cancer cases among those with the highest ginseng intake for men (p<0.0001) and for women (p<0.05), strongly support the hypothesis that ginseng has cancer preventive effects.
Korea Red Ginseng for Mitigating Helicobacter pylori-Associated Gastric Diseases; Toggle Inflammatory Activities
Several studies in the filed of microbiology and immunology have demonstrated that ginseng exerts antioxidant, anti-bacterial growth, and anti-inflammatory effects. Ginsenoside Rb1 has been known to inhibit lipopolysaccharide (LPS)-induced expression of the proinflammatory cytokine, TNF-α, and acidic polysaccharide from ginseng have been reported to inhibit the adherence of H. pylori to human gastric epithelial cells. Also a growth inhibiting effect of the ginseng fraction, panaxdiol, on H. pylori was suggested in vitro with an MIC of 50 µg/ml [42–43]. In addition to these anti-microbial actions of ginseng ingredients, it has been documented that ginseng has distinctive actions on cell growth in various carcinoma cells, through growth inhibition or cytotoxic effects in some cell lines, such as human melanoma cells A375-S2, hepatoma cells SK-HEP-1, and prostate cells LNCaP, but growth stimulating or protective cells in other cell lines, such as neuroblastoma SHSY5Y and keratinocyte HaCaT, have been reported, suggesting that ginseng might provide diverse biological actions depending on cell context [44–45]. Then, how about ginseng on H. pylori-infected gastric mucosal cells?
First, we documented that Korea red ginseng could rescue H. pylori-induced cytotoxicity [46]. In addition to rescuing actions of Korea red ginseng, they mitigate the oxidative stress-induced DNA mutation. Red ginseng extracts significantly attenuated both H. pylori-induced DNA damage assessed by comet assay, apoptosis measured by DNA fragmentation, and expressions of 8-OH-dG (Fig. 1B) for which inactivation of ERK1/2 signal transduction and attenuation of caspase-3 activation were operated. Simutaneously, Korea red ginseng decreased the H. pylori-stimulated IL-8 expression, suggesting that based on these gastroprotective effects of ginseng against H. pylori-associated gastric mucosal damages, red ginseng could be used as a medicinal phytonutrient against H. pylori infection (Fig. 2A).
Fig. 2.
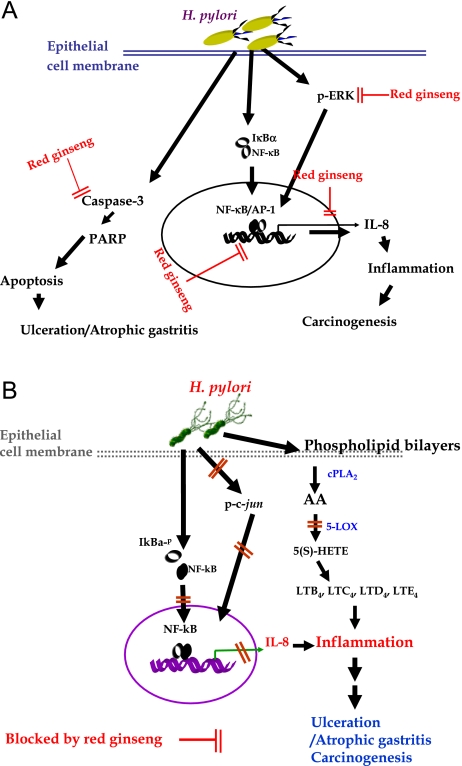
(A) Rescuing effects of Korea red ginseng against H. pylori-induced inflammation or cytotoxicity [46] Red ginseng could block several pathways to H. pylori-associated gastric damages including the connection between inflammation and carcinogenesis. They can block signal transduction pathway to inflammatory signals, attenuate apoptosis execution, and repress redox sensitive transcriptional activation. (B) Inhibitory activities and attenuated expression of 5-LOX with red ginseng in H. pylori-infected gastric epithelial cells [47]. Besides of COX-2 activation after H. pylori infection, H. pylori infection triggered 5-LOX activation, leading to increased production of 5(S)-HETE. Red ginseng showed efficient blockade to 5-LOX activation as equivalently efficient to 5-LOX inhibitors, geraniin.
Then, we extended our experiment to prove whether red ginseng extract influences 5-lipoxygenase (5-LOX) pathway, thereby suppressing the biosynthesis of 5(S)-HETE [47]. H. pylori infection increased exclusively 5-LOX enzyme activity, which is strongly associated with carcinogenic pathway. Red ginseng extracts inhibited these increments of H. pylori-stimulated 5-LOX through inactivation of c-jun phosphorylation and redox-sensitive transcriptional activation together, finally led to reduced levels of IL-8 and 5-LOX in gastric mucosal cells (Fig. 2B). The LOX inhibiting capacity of red ginseng was equivalent to 200 µM of geraniin or EGTA.
All of our study described above for the first time, revealed the H. pylori infection induced significant levels of 5-LOX activity rather than its expression and documented the apparent biosynthesis of its metabolite, 5-HETE, which eventually contributes to inflammatory activities through LTB4 formation. Red ginseng treatment was efficient in decreasing H. pylori-induced 5-LOX activities and attenuating 5-LOX expression through inactivating c-jun pathway. Several studies showed NSAID played significant chemopreventive effects against H. pylori-induced gastric carcinogenesis through attenuating COX activities, but our study opens the possibility that LOX activity as much as COX activity might influence the inflammatory or carcinogenic activities related to its disasters associated with H. pylori infection, suggesting that modulation of activities of both COX and LOX will be quite beneficial in avoiding the deleterious outcomes of chronic H. pylori infection.
Tackle the Progression of Chronic Atrophic Gastritis with Korea Red Ginseng
As Korea red ginseng has been reported to show a significant protective effect against H. pylori-induced cytotoxicity and DNA damage in vitro, we designed a clinical study to assess the efficacy of red ginseng treatment in patients with H. pylori-associated chronic gastritis [48]. A total of 84 patients with H. pylori-associated chronic gastritis were recruited and randomly divided into two groups. During the trial, 34 patients out of 42 patients in the placebo control group and 36 patients out of 42 patients in the red ginseng group completed the protocol. The patients received a one week triple therapy for the eradication of H. pylori and then received either placebo capsules that were composed of flour for the placebo group or red ginseng capsules for the treatment group, which were administered for 10 weeks. An endoscopic examination of gastritis with a visual analogue scale, a test for detection of H. pylori, immunohistochemistry of 8-OHdG, the 8-OHdG immunohistochemical staining for assessing oxidative DNA damage and TUNEL staining for apoptosis were performed, respectively. As results, H. pylori eradication rates were augmented in the red ginseng group as compared to the placebo group (91.7% in the red ginseng group and 79.4% in the placebo group), but there was no statistical significance (p = 0.147). For an analysis of gastritis based on Updated Sydney System (Fig. 3A), the red ginseng group showed significant improvement in neutrophil infiltrations (p = 0.008), chronic atrophic gastritis (Fig. 3B, p<0.05), and even intestinal metaplasia (p = 0.005). An attenuation of 8-OHdG immunohistostaining after treatment was seen more frequently in the red ginseng group (p<0.001) (Fig. 4). An attenuation of DNA damage and apoptosis was seen for the red ginseng group as compared to the placebo group (p<0.001). Therefore, supplementary administration of red ginseng augmented eradication rates of H. pylori, attenuated gastric inflammation, and reduced oxidative DNA damage and apoptosis, suggesting the clinical usefulness of red ginseng.
Fig. 3.
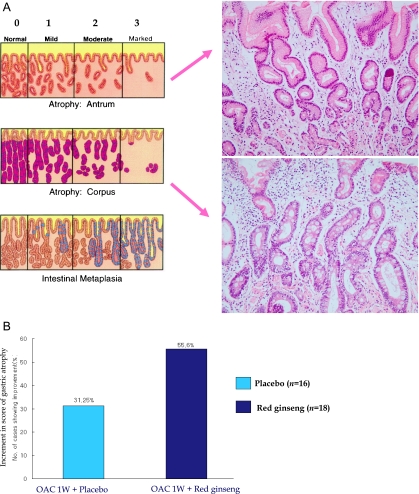
Rejuvenation of atrophic gastritis with Korea red ginseng (A) Updated Sydney system to score the degree of gastric atrophy. Two examples of atrophic gastritis and intestinal metaplasia (B) Changes of gastric atrophy. OAC 1W stands for 1 week of triple therapy including omeprazole, amoxicillin and clarithromycin [48]. Simply successful eradication of H. pylori alone could led to improvement of gastric atrophy in around 30% of cases, but OAC 1W plus 10 weeks of Korea red ginseng supplementation resulted in statistically significantly increment in improvement of gastric atrophy (p<0.05), signifying that rejuvenation of gastric atrophy can be accelerated with Korea red ginseng supplementation after eradication regimen.
Fig. 4.
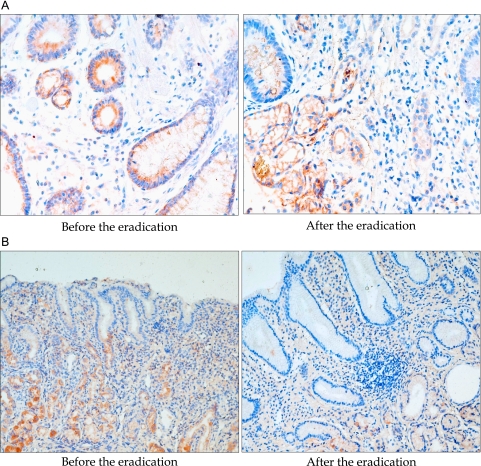
Changes of 8-OHdG expressions in the biopsied gastric tissue (A) Change of 8-OHdG in placebo control group [48]. Though H. pylori was successfully eradication with triple therapy, biopsied tissue obtained after 10weeks of H. pylori eradication showed the remaining of gastric inflammatory activities. Therefore, 8-OHdG expressions were decreased in a scant level compared to the levels before eradication. This finding suggest that simple removal of bug was not so efficient in attenuating 8-OHdG levels, biomarker of oxidative stress related to H. pylori infection (B) Change of 8-OHdG in Korea red ginseng treated group. In contrast to placebo group, the levels of 8-OHdG expression were significantly decreased in case, who were administered with supplementary red ginseng after eradication regimen, in spite of failure in eradication. These finding necessitates the supplementation of Korea red ginseng to tackle the oxidative stress imposed by H. pylori infection.
Recently we could add more evidence that Korea red ginseng was very efficient in eliminating troublesome halitosis. Since halitosis is closely associated with erosive changes in the stomach [49], the halitosis improving actions of Korea red ginseng were dependent on either direct suppressive action of Korea red ginseng on enzyme responsible for generating volatile sulfure compounds, that is, cystathionine γ-lyase and cystathionine β-synthase, or cyto-restorative actions [50].
Perspective
Ginseng has been reported to reduce the human cancer risk of lip, oral cavity, pharynx, larynx, esophagus, lung, liver, pancreas, ovary, colon, rectum, and stomach documented by clinical and epidemiological studies, rendering Panax ginseng as “non-organ specific cancer preventive” [41]. However, still many people have questioned the use of traditional medicinal herbs and this has been a prime issue in complementary and alternative medicine. Even though we issued several in vitro and in vivo results of feasibility of Korea red ginseng administration for mitigating H. pylori-associated atrophic changes and carcinogenesis, science and evidence based medicine must develop through verification of observations, necessitating that the need for clinical studies should be strongly recommended, especially more to put Korea red ginseng as “H. pylori-associated gastric cancer preventive” based on our publications [46–48, 51]. Scientific clinical trials of Korea red ginseng are now warranted to reach to the conclusion that rejuvenation of atrophic gastritis could be coming true with supplementation of Korea red ginseng after the eradication of H. pylori infection. Overall, ginseng is a good example of a natural herb that has ubiquitous properties that are conductive to stopping inflammatory carcinogenesis.
Acknowledgment
The study was granted from the Ministry of Education, Science and technology, Korea and The Korea Society of Ginseng funded by Korea Ginseng Cooperation.
References
- 1.Federico A., Morgillo F., Tuccillo C., Ciardiello F., Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal B.B., Shishodia S., Sandur S.K., Pandey M.K., Sethi G. Inflammation and cancer; how hot is the link? Biochem. Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Lu H., Ouyang W., Huang C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 4.Moss S.F., Blasé M.J. Mechanism of disease: inflammation and the origin of cancer. Nature Clin. Prat. Oncol. 2005;2:90–97. doi: 10.1038/ncponc0081. [DOI] [PubMed] [Google Scholar]
- 5.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99:1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oshima H., Tazawa H., Sylla B.S., Sawa T. Prevention of human cancer by modulation of chronic inflammatory process. Mutat. Res. 2005;591:110–122. doi: 10.1016/j.mrfmmm.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Pollard J.W. Tumor-educated macrophages promote tumor progression and metastasis. Nature Rev. Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 8.Hussain S.P., Hofseth L.J., Harris C.C. Radical causes of cancer. Nature Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 9.Coussens L., Werb Z. Inflammation and Cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balkwill F., mantovani A. Inflammation and cancer. Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 11.Bode A.M., Dong Z. Cancer prevention research—then and now. Nature Rev. Cancer. 2009;9:508–516. doi: 10.1038/nrc2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki M., Suzuki H., Hibi H. Proton pump inhibitors and gastritis. J. Clin. Biochem. Nutr. 2008;42:71–75. doi: 10.3164/jcbn.2008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima N., Ito Y., Yokoyama K., Uno A., Kinukawa N., Nemoto N., Moriyama M. The Expression of murine double minute 2 (MDM2) on Helicobacter pylori-infected intestinal metaplasia and gastric cancer. J. Clin. Biochem. Nutr. 2009;44:196–202. doi: 10.3164/jcbn.08-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uemura N., Okamoto S., Yamamoto S., Matsumura N., Yamaguchi S., Yamakido M., Taniyama K., Sasaki N., Schlemper R.J. Helicobacter pylori infection and the development of gastric cancer. New Engl. J. Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 15.You W.C., Zhang L., Gail M.H., Chang Y.S., Liu W.D., Ma J.L., Li J.Y., Jin M.L., Hu Y.R., Yang C.S., Blaser M.J., Correa P., Biot W.J., Fraumeni J.F., Xu G.W. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J. Natl. Cancer Inst. 2000;92:1607–1612. doi: 10.1093/jnci/92.19.1607. [DOI] [PubMed] [Google Scholar]
- 16.Wong B.C., Lam S.K., Wong W.M., Chen J.S., Zheng T.T., Feng R.F., Lai K.C., Hu W.H., Yuen S.T., Leung S.Y., Fong D.Y., Ho J., Ching C.K., Chen J.S., China Gasrtric cancer Study Group Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. J.A.M.A. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 17.Leung W.K., Lin S.R., Ching J.Y., To F.T., Ng E.K., Chan F.K., Lau J.Y., Sung J.J. Factors predicting progression of gastric intestinal metaplasia: results of a randomized trial on Helicobacter pylori eradication. Gut. 2004;53:1244–1249. doi: 10.1136/gut.2003.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mera R., Fontham E.T., Bravo L.E., Bravo J.C., Piazuelo M.B., Camago M.C., Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–1540. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Take S., Mizuno M., Ishiki K., Nagahara Y., Yoshida T., Yokota K., Oguma K., Okada H., Shiratori Y. The effect of eradicating Helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am. J. Gastroenterol. 2005;100:1037–1042. doi: 10.1111/j.1572-0241.2005.41384.x. [DOI] [PubMed] [Google Scholar]
- 20.Kato M., Asaka M., Nakamura T., Azuma T., Tomita E., Kamoshida T. Helicobacter pylori eradication prevents the development of gastric cancer- results of a long-term retrospective study in Japan. Aliment. Pharmacol. Ther. 2006;24:203–206. [Google Scholar]
- 21.Takenaka R., Okada H., Kato J., Makidono C., Hori S., Kawahara Y., Miyoshi M., Yumoto E., Imagawa A., Toyokawa Y., Sakaguchi K., Shiratori Y. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Aliment. Pharmacol. Ther. 2007;25:805–812. doi: 10.1111/j.1365-2036.2007.03268.x. [DOI] [PubMed] [Google Scholar]
- 22.Kabir S. Effect of Helicobacter pylori eradication on incidence of gastric cancer in human and animal models: underlying biochemical and molecular events. Helicobacter. 2009;14:159–171. doi: 10.1111/j.1523-5378.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 23.Fukase K., Kato M., Kikuchi S., Inoue K., Uemura N., Okamoto S., Terao S., Amagai K., Hayashi S., Asaka M., Japan Gast Study Group Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomized controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 24.Forbes G.M., Warren J.R., Glaser M.E., Cullen D.J., Marshall B.J., Collins B.J. Long-term follow-up of gastric histology after Helicobacter pylori eradication. J. Gastroenterol. Hepatol. 1996;11:670–763. doi: 10.1111/j.1440-1746.1996.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 25.Sung J.J., Lin S.R., Ching J.Y., Zhou L.Y., To K.F., Wang R.T., Leung W.K., Ng E.K., Lau J.Y., Lee Y.T., Yeung C.K., Chao W., Chung S.C. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. 2000;119:7–14. doi: 10.1053/gast.2000.8550. [DOI] [PubMed] [Google Scholar]
- 26.Park S., Kim W.S., Choi U.J., Han S.U., Kim Y.S., Kim Y.B., Chung M.H., Nam K.T., Kim D.Y., Cho S.W., Hahm K.B. Amelioration of oxidative stress with ensuing inflammation contributes to chemoprevention of H. pylori-associated gastric carcinogenesis. Antioxd. Redox. Signal. 2004;6:549–560. doi: 10.1089/152308604773934305. [DOI] [PubMed] [Google Scholar]
- 27.Lee D.H., Hahm K.B. Inflammatory cytokine gene polymorphisms and gastric cancer. J. Gastroenterol. Hepatol. 2008;23:1465–1472. doi: 10.1111/j.1440-1746.2008.05563.x. [DOI] [PubMed] [Google Scholar]
- 28.Chung J.W., Mahm K.B. Rejuvenation of atrophic gastritis in the elderly. J. Gastroenterol. Hepatol. 2010;25:434–435. doi: 10.1111/j.1440-1746.2010.06251.x. [DOI] [PubMed] [Google Scholar]
- 29.Helms S. Cancer prevention and therapeutics: Panax Ginseng. Alternative Med. Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 30.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology Multiple constituents and multiple actions. Biochem. Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 31.Yun T.K., Lee Y.S., Lee Y.H., Kim S.I., Yun H.Y. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J. Korean Med. Sci. 2001;16S:S6–S18. doi: 10.3346/jkms.2001.16.S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odashima S., Ohta T., Kohno H., Matsuda T., Kitagawa I., Abe H., Arichi S. Control of phenotypic expression of cultured B16 melanoma cells by plant glycosides. Cancer Res. 1985;45:2781–2784. [PubMed] [Google Scholar]
- 33.Yun T.K., Yun Y.S., Han I.W. Anticarcinogenic effect of long-term oral administration of red ginseng on newborn mice exposed to various chemical carcinogens. Cancer Detect. Prev. 1983;6:515–525. [PubMed] [Google Scholar]
- 34.Surh Y.J., Na H.K., Lee J.Y., Keum Y.S. Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer. J. Korean Med. Sci. 2001;16:S38–S41. doi: 10.3346/jkms.2001.16.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keum Y.S., Park K.K., Lee J.M., Chun K.S., Park J.H., Lee S.K., Kwon H., Surh Y.J. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 36.Lee S.J., Ko W.G., Kim J.H., Sung J.H., Moon C.K., Lee B.H. Induction of apoptosis by a novel intestinal metabolite of ginseng saponin via cytochrome c-mediated activation of caspase-3 protease. Biochem. Pharmacol. 2000;60:677–685. doi: 10.1016/s0006-2952(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 37.Sato K., Mochizuki M., Saiki I., Yoo Y.C., Samukawa K., Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol. Pharm. Bull. 1994;17:635–639. doi: 10.1248/bpb.17.635. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y.S., Chung I.S., Lee I.R., Kim K.H., Hong W.S., Yun Y.S. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulating acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 1997;17:323–331. [PubMed] [Google Scholar]
- 39.Kim E.H., Park J.D., Pyo S.N., Rhee D.K. Effects of non-saponin red ginseng compounds on multi-drug resistance. J. Ginseng Res. 2007;31:74–78. [Google Scholar]
- 40.Yue P.Y., Mak N.K., Cheng Y.K., Leung K.W., Ng T.B., Ping Fan D.T., Yeung H.W., Wong R.N. Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin. Med. 2007;2:1–21. doi: 10.1186/1749-8546-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yun T.K. Panax ginseng—a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 42.Hofseth L.J., Wargovich M.J. Inflammation, cancer, and target of ginseng. J. Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 43.Lee W.M., Kim S.D., Kim K.S., Song Y.B., Kwak Y.S., Cho J.Y., Park H.J., Oh J.W., Rhee M.H. Protopanaxadiol modulates LPS-induced inflammatory activity in murine macrophage RAW264.7 cells. J. Ginseng Res. 2006;30:181–187. [Google Scholar]
- 44.Lee Y.K., Im Y.J., Kim Y.L., Sackcet S.J., Lim S.M., Kim K., Kim H.L., Ko S.R., Im D.S. Increase of membrane potential by ginsenosides in prostate cancer and glioma cells. J. Ginseng Res. 2006;30:70–77. [Google Scholar]
- 45.Joo H.K., Lee S.K., Kim H.S., Song Y.J., Kang G., Park J.B., Lee K.H., Cho E.J., Lee J.H., Seong I.W., Kim S.H., Cho C.H., Jeon B.H. Korean red ginseng extract inhibits tumor necrosis factor-alpha-induced monocyte adhesion in the human endothelial cells. J. Ginseng Res. 2008;32:244–249. [Google Scholar]
- 46.Park S., Yeo M., Jin J.H., Lee K.M., Jung J.Y., Choue R., Cho S.W., Hahm K.B. Rescue of Helicobacter pylori-induced cytotoxicity by red ginseng. Dig. Dis. Sci. 2005;50:1218–1227. doi: 10.1007/s10620-005-2763-x. [DOI] [PubMed] [Google Scholar]
- 47.Park S., Yeo M., Jin J.H., Lee K.M., Kim S.S., Choi S.Y., Hahm K.B. Inhibitory activities and attenuated expressions of 5-LOX with red ginseng in Helicobacter pylori-infected gastric epithelial cells. Dig. Dis. Sci. 2007;52:973–982. doi: 10.1007/s10620-006-9440-6. [DOI] [PubMed] [Google Scholar]
- 48.Kim D.K., Lee J.A., Kim Y.B., Lee K.M., Hahm K.B. A randomized controlled trial assessing Korean red ginseng treatment of Helicobacter pylori-associated chronic gastritis. Kor. J Med. 2007;72:20–28. [Google Scholar]
- 49.Yoo S.H., Jung H.S., Sohn W.S., Kim B.H., Ku B.H., Kim Y.S., Park S.W., Hahm K.B. Volatile sulfure compounds as a predictor for esophagogastroduodenal mucosal injury. Gut Liver. 2008;2:113–118. doi: 10.5009/gnl.2008.2.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J.S., Kwon K.A., Jung H.S., Kim J.H., Hahm K.B. Korea red ginseng on Helicobacter pylori-induced halitosis; newer therapeutic strategy and a plausible mechanism. Digestion. 2009;80:192–199. doi: 10.1159/000229997. [DOI] [PubMed] [Google Scholar]
- 51.Lee S.Y., Shin Y.W., Hahm K.B. Phytoceuticals: mighty but ignored weapons against Helicobacter pylori infection. J. Dig. Dis. 2008;9:129–139. doi: 10.1111/j.1751-2980.2008.00334.x. [DOI] [PubMed] [Google Scholar]