Abstract
Although photoprotective properties of skin melanin have been well documented, a few studies on the effect of melanin on reactive oxygen species (ROS) generated by ultraviolet (UV) irradiation have been reported. To study the interaction of melanin with ROS, scavenging or quenching effect of melanin on O2•− and 1O2 was examined by electron spin resonance (ESR)-spin trapping methods and a spectrophotometric method, respectively. Melanin potently interacted with O2•− generated in a hypoxanthine (HPX)-xanthine oxidase (XOD) reaction, and with 1O2 generated from a peroxidase, H2O2, and halide system. In the HPX-XOD reaction, it was proved that melanin doses not interfere with the enzyme reaction. It is confirmed that one of the mechanisms by which melanin protects UV-induced skin damage is likely scavenging or quenching activity against ROS such as O2•− and 1O2.
Keywords: melanin, reactive oxygen species, antioxidant
Introduction
Exposure of ultraviolet (UV) irradiation to the skin causes acute and chronic detrimental cutaneous effects, which may result in photocarcinogenesis [1–7]. Native human melanin includes eumelanin and pheomelanin that contains sulfur, and eumelanin has been found in almost every type of human skin [8, 9]. The exact chemical structures of the two types of melanin have not been identified yet, probably because of the complication of polymerization and modifications of polymerization [10, 11]. Melanin in the skin is suggested to play an important role for protecting skin from the harmful effects of UV irradiation [7]. More in detail, melanin acts as a safeguard against the UV-mediated effects on skin through protecting membrane and DNA [12, 13]. Photoprotection for the skin by melanin is due to its role as a UV filter, and other properties of melanin are still remained to be clarified [14–16].
It has been reported that UV light energetic photons exerts biological effects through series of biological reactions [17, 18]. One is direct absorption of UV via cellular chromophores that results in excited states formation and subsequent chemical reactions. The other is photosensitization mechanism, where the UV light is absorbed by endogenous sensitizers that are excited to lead to formation of ROS. As for the effects of melanin on ROS as a photoprotection mechanism of melanin, a few studies have been reported. In a study having examined the reactivity of melanins with radicals from water radiolysis, •OH exhibited the strongest reactivity with melanins [19]. In the other study, O2•− was found to react to produce melanin free radicals in a reaction inhibited by superoxide dismutase (SOD) [20]. Furthermore, the authors also suggested that xanthine-xanthine oxidase (XOD) system is not suitable for studying the reaction of O2•− with melanin, since the enzyme activity of XOD is considerably inhibited by melanin as evidenced by the diminished production of uric acid and H2O2. Recently, we applied the kinetic analysis to study if a substance directly scavenge O2•− in hypoxanthine (HPX)-XOD system [21, 22]. In the present study, we examined scavenging activity of melanin against O2•− by using this kinetic analysis, and also quenching activity of melanin against singlet oxygen (1O2). 1O2 is known to be generated by physiological doses of UVA irradiation, and cause skin damage due to detrimental effects on lipids and proteins [23, 24].
Materials and Methods
Test materials and reagents
Reagents were purchased from the following sources: Melanin (eumelanin prepared by oxidation of tyrosine with hydrogen peroxide), HPX, SOD from bovine erythrocytes, allopurinol, 1,3-diphenyl-isobenzofuran (DPIBF), and astaxanthin from Sigma-Aldrich Corp. (St. Louis, MO); XOD (from cow milk) from Roche Diagnostics (Basel Switzerland); 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) from Labotec (Tokyo, Japan); (+)-catechin from Tokyo Kasei Kogyo (Tokyo, Japan). All other reagents used were of analytical grade.
Electron spin resonance (ESR)-spin trapping determinations of O2•− generated by HPX-XOD reaction
The assay used in this study was essentially identical to that described in our previous papers [21, 22]. In brief, 50 µL of 2 mM HPX, 50 µl of 0.1 M phosphate buffer (pH 7.4), 20 µl of 4.45 M DMPO, 25 µl of dimethyl sulfoxide (DMSO), and 5 µl of different concentrations of melanin dissolved in DMSO, and 50 µl of 0.4 U/ml of XOD were placed in a test tube and mixed. In the reaction mixture, to eliminate the effect of •OH, DMSO was added as a •OH-scavenger. The mixture was transferred to an ESR spectrometry cell, and the DMPO-OOH spin adduct was quantified 97 s after the addition of XOD. The signal intensities of DMPO-OOH were determined from the peak height of the first signal as described in our previous papers [23, 24]. The measurement conditions of ESR (JES-FA-100, JEOL, Tokyo, Japan) were as follows: field sweep, 330.80–340.80 mT; field modulation frequency, 100 kHz; filed modulation width, 0.07 mT; amplitude, 400; sweep time, 1 min; time constant, 0.1 s; microwave frequency, 9.430 GHz; microwave power, 5 mW. Signal intensities were evaluated from the peak height of the first signal of DMPO-OOH spin adduct. In an experiment for kinetic analyses with melanin by double reciprocal plots, different concentrations of DMPO were added to the system. Instead of different concentrations of melanin, different concentrations of SOD as a superoxide scavenger or of allopurinol as an XOD inhibitor were added to the system as described in our previous paper [21, 22]. To confirm the results of kinetic analyses, the production of uric acid from HPX was determined by ε = 1.1 × 104 M−1cm−1 [25].
Spectrophotometric determination of 1O2 generated by a peroxidase, H2O2, and halide system
1O2 was generated by a peroxidase, H2O2, and halide system [26], and 1O2 quenching activity was measured by the oxidation of DPBIF at 420 nm [26, 27]. In brief, the reaction mixture was prepared in 50 mM acetate buffer (pH 4.5) to contain 1 unit of lactoperoxidase, 0.25 mM potassium bromide, 0.25 mM H2O2, 0.1 mM DPIBF solubilized in 0.05% Triton X-100, and the test substances in DMSO. The reaction was initiated by addition of H2O2 into a cuvette containing the reaction mixture at room temperature, and the decrease in absorbance at 420 nm was read at 10 s intervals for 1 min. The quenching percent was calculated from the reduction in the presence and absence of test substances.
Results and Discussion
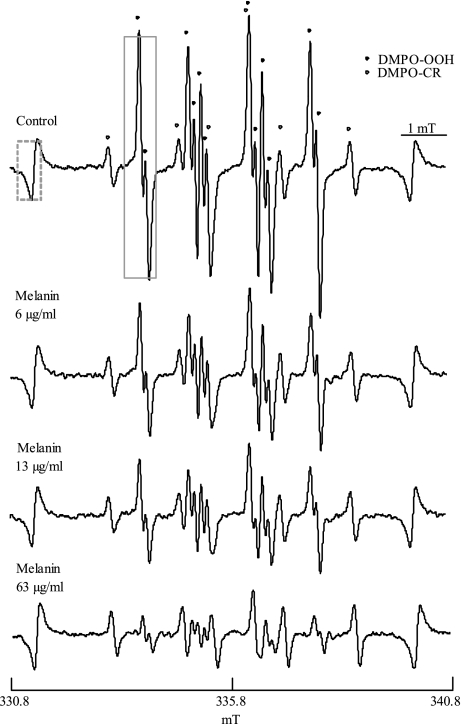
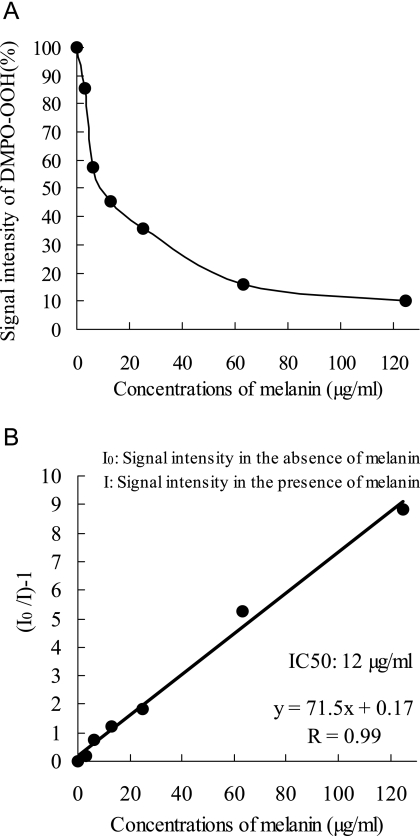
Representative ESR spectra of DMPO-OOH (an adduct formed by DMPO and O2•−) obtained by the addition of solvent alone or different concentration of melanin are shown in Fig. 1, which indicates that the amount of O2•− was reduced by melanin in a concentration dependent manner. DMPO-CR (an adduct of carbon-center radical derived from DMSO and hydroxyl radical), which has six-line spectrum with the hyperfine coupling constant of aN = 1.64, aH = 2.24, was observed as in the previous study [28], and signal intensities of DMPO-CR were not changed by the addition of melanin. Fig. 2 shows an inhibition curb against DMPO-OOH formation in the ESR-spin trapping method with the HPX-XOD system obtained by the addition of different concentrations of melanin, and its linear transformation. The IC50 (concentration that inhibited the formation of the spin adduct by 50%) for melanin was 0.012 mg/ml, which is equivalent to 0.9 U/ml of SOD, and to 0.008 mg/ml of (+)-catechin (data not shown).
Fig. 1.
Representative ESR spectra of DMPO-OOH obtained from the HPX-XOD reaction, with different concentrations of melanin. Each value represents the mean of duplicate determinations.
Fig. 2.
Inhibition curb against DMPO-OOH formation obtained from the HPX-XOD reaction with different concentrations of melanin (A), and its linear transformation (B). Each value represents the mean of duplicate determinations.
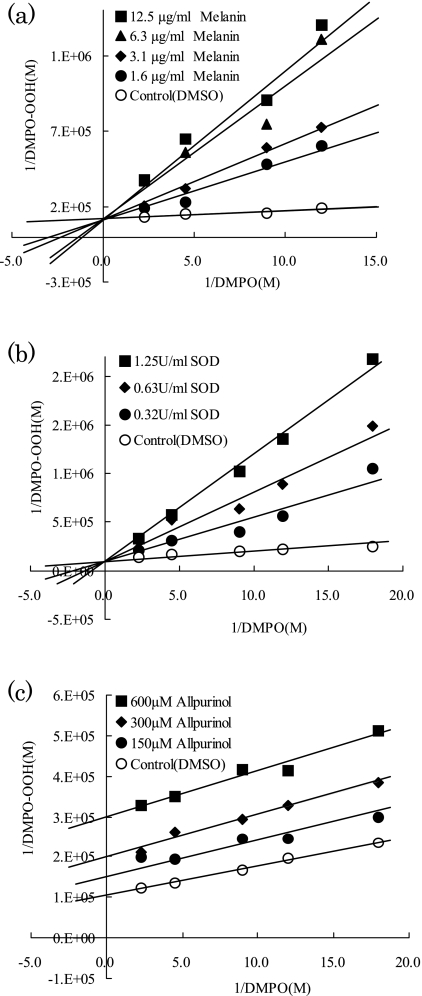
To determine whether melanin would interfere with the enzyme reaction of HPX-XOD, the ESR-spin trapping method was applied to evaluate the competitive reaction between DMPO and melanin or reference agents. Fig. 3 shows double reciprocal plots for melanin, SOD, an authentic superoxide scavenger, and for allopurinol, a XOD inhibitor [29]. The linear and intersecting patterns of the double reciprocal plots indicate that SOD acted as a competitive inhibitor of DMPO. On the other hand, the double reciprocal plots show that the inhibition of DMPO-OOH formation by allopurinol was uncompetitive with DMPO. As is the case with SOD, the double reciprocal plots indicate that melanin acted as a competitive inhibitor of DMPO. In other words, melanin scavenges directly O2•− without interference with HPX-XOD reaction. In a previous study, however, it was suggested tat melanin synthesized from dopa by autoxidation likely inhibited the activity of XOD [20]. To confirm if melanin inhibits the activity of XOD, we further examined the production of uric acid that is an end product of HPX-XOD reaction. As shown in Table 1, uric acid levels were not changed by addition of melanin, and a XOD inhibitor allopurinol diminished the level of uric acid. Thus we conclude that tyrosine-derived synthetic melanin used in this study dose not inhibit XOD activity, and has an ability to scavenge directly O2•−.
Fig. 3.
Double reciprocal plots of formation of DMPO-OOH versus DMPO concentrations at different fixed concentrations of (a) melanin, (b) SOD, and (c) allopurinol.
Table 1.
Effects of melanin, SOD, and allopurinol on the production of uric acid in HPX-XOD reaction
| Treatment | Concentration of uric acid (µM) |
|---|---|
| Control | 75.7 |
| 12.5 µg/ml of melanin | 76.5 |
| 1.25 U/ml of SOD | 71.4 |
| 600 µM allopurinol | 6 |
Each value represents the mean of duplicate determinations.
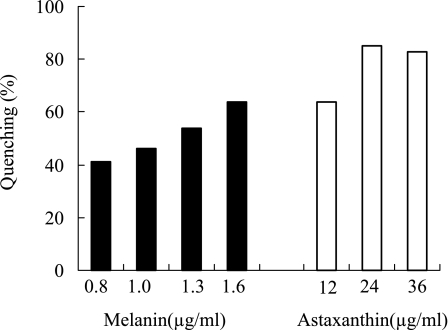
As shown in Fig. 4, the 1O2 quenching activity of melanin was compared to that of astaxanthin, an authentic quencher of 1O2 [30]. Melanin showed concentration dependent quenching activity against 1O2, and 1.6 µg/ml of melanin quenched 64% of 1O2. Astaxanthin also showed potent quenching activity, and almost 85% of 1O2 was quenched at a concentration of 24 µg/ml (=40 µM).
Fig. 4.
Singlet oxygen quenching activity of melanin and astaxanthin. Each value represents the mean of duplicate determinations.
UV light is absorbed by endogenous sensitizers that are excited to generate ROS, which can interact with DNA, proteins, and fatty acids to cause oxidative damage [17, 18]. Our study showed that melanin has an ability to scavenge representative ROS, especially O2•− and 1O2. As for photoprotective effect of melanin, two underlying mechanisms are proposed [17], these are an efficient UV filter, and removal of UV-damaged cells. In addition, interaction with ROS such as O2•− is suggested to be involved in the photoprotection mechanism of melanin [19, 20]. Our study confirmed the idea that scavenging or quenching activity of melanin against O2•− and 1O2. is one of the pivotal photoprotection mechanisms of melanin. In contrast to photoprotective function, however, it was recently reported that melanin, especially phoemlanin, also acts as a potent UVB photosensitizer that generates ROS upon UV irradiation [31]. In other words, we can say that melanin is either beneficial or deleterious in terms of photobiological end point. Thus, it is of our further interest how the balance between scavenging or quenching effect on ROS and ROS-generating photosensitization effect affects the sunlight sensitivity of individuals.
Abbreviations
- ESR
electron spin resonance
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- TEMPOL
4-hydroxyl-2,2,6,6-tetrametylpiperidin-1-oxyl
- DMPO-OH
DMPO spin adducts of •OH
- DMPO-OOH
DMPO spin adducts of O2•−
- DMPO-CR
DMPO spin adduct of carbon-center radical
References
- 1.Brash D.E., Ziegler A., Jonason A.S., Simon J.A., Kunala S., Leffell D.J. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J. Invest. Dermatol. Symp. Proc. 1996;1:136–142. [PubMed] [Google Scholar]
- 2.Wikonkal N.M., Brash D.E. Ultraviolet radiation signature mutations in photocarcinogenseis. J. Invest. Dermatol. Symp. Proc. 1996;4:6–10. [Google Scholar]
- 3.Mckay B.C., Stubbert L.J., Fowler C.C., Smith J.M., Cardamore R.A., Spronck J.C. Regulation of ultraviolet light-induced gene expression by gene size. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6582–6586. doi: 10.1073/pnas.0308181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agar N.S., Halliday G.M., Barnetson R.S., Ananthaswamy H.N., Wheeler M., Jones A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner D.J., Doll R., Goodhead D.T., Hall E.J., Land C.E., Little J.B., Lubin J.H., Preston D.L., Preston R.J., Puskin J.S., Ron E., Sachs R.K., Samet J. M., Setlow R.B., Zaider M. Cancer risks attributable to low does of ionizing radiation: assessing what really know. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setlow R.B. Spectral regions contributing to melanoma: a personal view. J. Invest. Dermatol. Symp. Proc. 1999;4:46–49. doi: 10.1038/sj.jidsp.5640180. [DOI] [PubMed] [Google Scholar]
- 7.Moan J., Dahlback A., Setlow R.B. Epidemiological support for a hypothesis for melanoma induction indicating a role for UVA radiation. Photochem. Photobiol. 1999;70:243–247. [PubMed] [Google Scholar]
- 8.Hunt G., Kyne S., Ito S., Wakamatsu K., Todd C., Thody A. Eumelanin and pheomelanin contents of human epidermis and cultured melanocytes. Pigment Cell Res. 1995;8:202–208. doi: 10.1111/j.1600-0749.1995.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 9.Vincensi M.R., d’Ischia M., Napolitano A., Rocaccini E.M., Riccio G., Monfrecola G., Santoianni P., Prota G. Pheomelanin versus eumelanin as a chemical indicator of ultraviolet sensitivity in fair-skinned subjects at high risk for melanoma: A pilot study. Melanoma. Res. 1998;8:53–58. doi: 10.1097/00008390-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Ito S. Reexamination of the structure of eumelanin. Biochem. Biophys. Acta. 1986;883:155–161. doi: 10.1016/0304-4165(86)90146-7. [DOI] [PubMed] [Google Scholar]
- 11.Kollias N., Sayre R.M., Zeise L., Chedekel M.R. Photoprotection by melanin. J. Photochem. Photobiol. B Biol. 1991;9:135–160. doi: 10.1016/1011-1344(91)80147-a. [DOI] [PubMed] [Google Scholar]
- 12.Kvam E., Dahle J. Pigmented melanocytes are protected against ultraviolet-A-induced membrane damage. J. Invest. Dermatol. 2003;121:564–569. doi: 10.1046/j.1523-1747.2003.12418.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamazaki F., Okamoto H., Miyauchi-Hashimoto H., Matsumura Y., Itoh Taketo., Kunusada T., Horio T. XPA gene-deficient, SCF-transgenic mice with epidermal melanin are resistant to UV-induced carcinogenesis. J. Invest. Dermatol. 2004;123:220–228. doi: 10.1111/j.0022-202X.2004.22710.x. [DOI] [PubMed] [Google Scholar]
- 14.Wagner J.K., Parra E.J., Norton H.L., Jovel C., Shriver M.D. Skin responses to ultraviolet radiation: effects of constitutive pigmentation, sex, and ancestry. Pigment Cell Res. 2002;15:385–390. doi: 10.1034/j.1600-0749.2002.02046.x. [DOI] [PubMed] [Google Scholar]
- 15.Ortonne J.P. Photoprotective properties of skin melanin. Brit. J. Dermatol. 2002;146:7–10. doi: 10.1046/j.1365-2133.146.s61.3.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi Y., Takahashi K., Zmudzka B.Z., Kornhauser A., Miller S.A., Tadokoro T., Berens W., Beer J.Z., Hearing V.J. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB. J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- 17.Svobodova A., Walterova D., Vostalova J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Olomouc. Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 18.Cadet J., Sage E., Douki T. Ultravolet radiation-mediated damage to cellular DNA. Mutat. Res. 2005;571:3–17. doi: 10.1016/j.mrfmmm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Sarna T., Pilas B., Land E.J., Truscott T.G. Interaction of radicals from water radiolysis with melanin. Biochim. Biophys. Acta. 1986;883:162–167. doi: 10.1016/0304-4165(86)90147-9. [DOI] [PubMed] [Google Scholar]
- 20.Korytowski W., Kalyanaraman B., Menon I.A., Sarna T., Sealy R.C. Reaction of superoxide anions with melanins: electron spin resonance and spin trapping studies. Biochim. Biophys. Acta. 1986;882:145–153. doi: 10.1016/0304-4165(86)90149-2. [DOI] [PubMed] [Google Scholar]
- 21.Niwano Y., Sato M., Kohno M., Niwano Y., Sato E., Kohno M., Matsuyama Y., Kim D., Oda T. Antioxidant properties of aqueous extracts from red tide plankton cultures. Biosci. Biotechnol. Biochem. 2007;71:1145–1153. doi: 10.1271/bbb.60593. [DOI] [PubMed] [Google Scholar]
- 22.Saito K., Kohno M., Yoshizaki F., Niwano Y. Extensive screening for edible herbal extracts with potent scavenging activity against superoxide anions. Plant. Foods Hum. Nutr. 2008;63:65–70. doi: 10.1007/s11130-008-0071-2. [DOI] [PubMed] [Google Scholar]
- 23.Baier J., Maisch T., Regensburger J., Pöllmann C., Bäumler W. Optical detection of singlet oxygen produced by fatty acids and phospholipids under ultraviolet A irradiation. J. Biomed. Opt. 2008;13:044029-1-7. doi: 10.1117/1.2960553. [DOI] [PubMed] [Google Scholar]
- 24.Baier J., Maisch T., Maier M., Landthaler M., Bäumler W. Direct detection of singlet oxygen generated by UVA irradiation in human cells and skin. J. Invest. Dermatol. 2007;127:1498–1506. doi: 10.1038/sj.jid.5700741. [DOI] [PubMed] [Google Scholar]
- 25.Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J. Biol. Chem. 1970;245:4053–4057. [PubMed] [Google Scholar]
- 26.Piatt J.F., Cheema A.S., O’Brien P.J. Peroxidase catalyzed singlet oxygen formation from hydrogen peroxide. FEBS Lett. 1977;74:251–254. doi: 10.1016/0014-5793(77)80857-0. [DOI] [PubMed] [Google Scholar]
- 27.Sachindra N.M., Sato E., Maeda H., Hosokawa M., Niwano Y., Kohno M., Miyashita K. J. Agric. Food Chem. 2007;55:8516–8522. doi: 10.1021/jf071848a. [DOI] [PubMed] [Google Scholar]
- 28.Kohno M., Minuta Y., Kusai M., Masumizu T., Makino K. Measurements of superoxide anion radical and superoxide anion scavenging activity by electron spin resonance spectroscopy coupled with DMPO spin trapping. Bull. Chem. Soc. Jpn. 1994;67:1085–1090. [Google Scholar]
- 29.Watts R.W., Watts J.A., Seegmiller J.E. Xanthine oxidase activity in human tissues and its inhibition by allopurinol (4-hydroxypyrazolo[3,4-d]pyrimidine) J. Lab. Clin. Med. 1965;66:688–697. [PubMed] [Google Scholar]
- 30.Di Mascio P., Devasagayam T.P., Kaiser S., Sies H. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem. Soc. Trans. 1990;18:1054–1056. doi: 10.1042/bst0181054. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi S., Zhang W., Wakamatsu K., Ito S., Hearing V.J., Kraemer K.H., Brash D.E. Melanin acts as a potent UVB photosensitizer to cause an atypical mode of cell death in murine skin. Pros. Natl. Acad. Sci. U.S.A. 2004;101:15076–15081. doi: 10.1073/pnas.0403994101. [DOI] [PMC free article] [PubMed] [Google Scholar]