Abstract
Polaprezinc (PZ), a chelate compound consisting of zinc and l-carnosine (Car), is an anti-ulcer drug developed in Japan. In the present study, we investigated whether PZ suppresses mortality, pulmonary inflammation, and plasma nitric oxide (NO) and tumor necrosis factor (TNF)-α levels in endotoxin shock mice after peritoneal injection of lipopolysaccharide (LPS), and how PZ protects against LPS-induced endotoxin shock. PZ pretreatment inhibited the decrease in the survival rate of mice after LPS injection. PZ inhibited the increases in plasma NO as well as TNF-α after LPS. Compatibly, PZ suppressed LPS-induced inducible NO synthase mRNA transcription in the mouse lungs. PZ also improved LPS-induced lung injury. However, PZ did not enhance the induction of heat shock protein (HSP) 70 in the mouse lungs after LPS. Pretreatment of RAW264 cells with PZ suppressed the production of NO and TNF-α after LPS addition. This inhibition likely resulted from the inhibitory effect of PZ on LPS-mediated nuclear factor-κB (NF-κB) activation. Zinc sulfate, but not Car, suppressed NO production after LPS. These results indicate that PZ, in particular its zinc subcomponent, inhibits LPS-induced endotoxin shock via the inhibition of NF-κB activation and subsequent induction of proinflammatory products such as NO and TNF-α, but not HSP induction.
Keywords: polaprezinc, lipopolysaccharide, nuclear factor-κB, inducible nitric oxide synthase, heat shock protein
Introduction
Lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria is a substance responsible for endotoxin shock as well as a potent activator of macrophages [1]. LPS is well known to induce production of proinflammatory mediators such as nitric oxide (NO) and cytokines including tumor necrosis factor-α (TNF-α), leading to death from endotoxin shock in animal models [2, 3]. Moreover, in vitro studies have demonstrated that LPS enhances cytokine and/or NO production in macrophages [4], microglial cells [5] and endothelial cells [6]. LPS is recognized by Toll-like receptor (TLR) 4 on the surface of the host cells and its signal is transduced, resulting in the activation of NF-κB [7], which is known to regulate transcriptions of many inflammation-related proteins including cytokines [8].
Polaprezinc [N-(3-aminopropionyl)-l-histidinato zinc] (PZ), a chelating compound of zinc and l-carnosine (Car), is commonly used in the clinical field as a drug for gastric ulcers in Japan [9]. Previously, both in vitro and in vivo studies revealed that PZ prevented gastric mucosal injuries by a variety of stimuli [10–13]. This mucosal protection by PZ was attributed to its antioxidant activity [10, 11], membrane-stabilizing action [12] and stimulation of mucus production [13]. Recent in vitro studies have indicated the importance of anti-apoptotic action of PZ in its protection against gastrointestinal cell injuries [14, 15]. Furthermore, it has been reported that PZ protects against the damages of gastric and colonic mucosa by its potent heat shock protein (HSP)-inducing activity [16–18]. Our recent study demonstrated that PZ protected mouse primary cultured hepatocytes against acetaminophen insult due to HSP70 induction and inhibition of lipid peroxidation [19]. It is known that the overexpression of HSP70 inhibits the production of cytokines and/or NO in macrophages activated by LPS challenge [20]. Recently, we have found that oral administration of geranylgeranylacetone, a potent HSP inducer to rats before LPS injection induces HSP70 in several organs, inhibits production of proinflammatory cytokines and NO, and prevents organ damages, resulting in improvement of survival rates [21].
In the present study, we investigated whether PZ inhibited LPS-induced endotoxin shock in mice as well as LPS-mediated macrophage activation, whether the inhibition by PZ was related to HSP induction, and how PZ protected against LPS-mediated endotoxin shock.
Materials and Methods
Materials
PZ was kindly provided by Zeria Pharmaceutical Co., Ltd. (Tokyo, Japan). Zinc sulfate and Car were purchased from Sigma-Aldrich (St. Louis, MO). Salmonella enterica LPS serotype enteritidis was purchased from Sigma Chemical Co. (St. Louis, MO). Fetal calf serum (FCS) was purchased from PAA Laboratories GmbH (Linz, Austria). Rabbit anti-HSP70 polyclonal antibody was purchased from Medical & Biological Laboratories Co., Ltd. (Nagoya, Japan). Rabbit anti-NF-κB p65 (H-286) polyclonal antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Dulbecco’s modified eagle medium (DMEM) was obtained from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). All the other chemicals used were of analytical grade.
Animals
Male ddY mice (5 weeks old) were purchased from Japan SLC (Shizuoka, Japan) and housed 5–6 per cage in plastic cages. The animals were maintained on a 12-h light/dark cycle under controlled temperature (23 ± 3°C) and humidity (55 ± 5%) for a week before use in experiments. They were allowed free access to standard laboratory food and water.
All studies were approved by the Institutional Animal Care and Use Committee at Tottori University Faculty of Medicine, and were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Treatments of mice with PZ and LPS
LPS (40 mg/kg body weight) or vehicle (10% dimethyl sulfoxide [DMSO] in phosphate-buffered saline [PBS]) was administered intraperitoneally. The dose volume of LPS was 6 ml/kg. PZ was suspended in 0.5% carboxymethyl cellulose sodium salt solution and given orally at a dose of 100 mg/kg at 2 h before LPS administration. The dose volume of PZ solution was 5 ml/kg. Mice were monitored for survival over 96 h after LPS administration. Mice were anesthetized with diethyl ether and blood samples were taken from the right ventricle using a heparinized syringe with a needle 6, 12, 18, and 24 h after LPS administration. After the collection of blood, lungs were perfused with PBS, removed from mice, frozen immediately, and stored in liquid nitrogen until assayed.
Measurement of serum zinc concentration
Serum zinc concentrations were determined by atomic absorption spectrophotometry (Fukuyama Medical Laboratory Co., Ltd., Hiroshima, Japan). Serum zinc concentration was measured at 1, 2, 4 or 8 h after oral administration of PZ (10, 50 or 100 mg/kg body weight).
Cell culture and treatments
RAW 264 murine macrophage cell line was obtained from Riken BRC Cell Bank (Tsukuba, Japan), and grown in DMEM supplemented with 10% heat-inactivated FCS, 2 mM glutamine and antibiotics (100 units/ml penicillin, 100 µg/ml streptomycin) at 37°C in a humidified incubator with 5% CO2. All the experiments were performed using DMEM containing 10% FCS unless described specifically. The cells were seeded in a 24-well plate at a cell density of 5 × 105 cells/well and incubated for 2 h at 37°C in an atmosphere of 5% CO2/95% air. After non-adherent cells were removed by washing with culture medium, attached cells were exposed to LPS (100 ng/ml or 1 µg/ml) for periods of 24 h at 37°C under 5% CO2/95% air. LPS was dissolved in 10% DMSO in PBS. PZ was dissolved in 40 mM HCl. Zinc sulfate and Car were dissolved in distilled water. PZ, zinc sulfate or Car at concentrations of 50 or 100 µM was added into the medium 6 or 2 h before LPS administration.
Determination of NO
Plasma NO2/NO3 level or the nitrite in the culture medium was determined as an index of NO production spectrophotometrically by means of commercially available kits (Wako Pure Chemistry Industries, Osaka, Japan) [21, 22].
Determination of TNF-α
The TNF-α in plasma or the culture medium was determined with an enzyme-linked immunosorbent assay (ELISA) kit (BioSource International, Camarillo, CA) according to the manufacturer’s instructions [21, 22].
Western blot analysis
Contents of HSP70 or nuclear NF-κB p65 were determined by western blotting as described previously [23, 24]. Nuclear extracts were prepared by using NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Pierce Biothecnology, Rockford, IL) according to the manufacturer’s instructions. Frozen lung tissues or nuclear extracts from RAW 264 cells were homogenized and 40 µg of protein from each sample was separated by SDS polyacrylamide gel electrophoresis with 12.5% polyacrylamide gel. The gels were electroblotted onto polyvinylidene difluoride membranes. The membranes were incubated with rabbit anti-HSP70 polyclonal antibody (1:10000) or rabbit anti-NF-κB p65 antibody (1:2000), washed and then incubated with horseradish-peroxidase-conjugated goat anti-rabbit (Sigma Chemical Co., St. Louis, MO) antibody. The immunoblot was revealed with an ECLTM Western blotting analysis system (Amersham Pharmacia Biotech, Buckinghamshire, England). Western blots were quantified with Scion Image.
Real-time reverse transcriptase-polymerase chain reaction (Real-tme PCR)
Total RNA was extracted from lung using the RNeasy® Mini Kit (Qiagen Japan, Tokyo, Japan). Both reverse transcription and PCR were carried out in a single tube using a QuantiFast SYBR® Green RT-PCR Kit (Qiagen Japan). We used QuantiTect® Primer Assays (Quiagen Japan) for real-time PCR primers. The accession numbers of the primers are as follows: Nitric oxide synthase 2 (iNOS), Mm_Nos2_1_SG, QT00100275; Metallothionein 1, Mm_Mt1_1_SG, QT00265902; Metallothionein 2, Mm_Mt2_1_SG, QT00264278. A β-2 microglobulin primer (Mm_B2m_2_SG, QT01149547, Qiagen Japan) was used as an endogenous control. The relative amounts of all mRNAs were measured using a 7900HT Fast Real-Time PCR System (Applied Biosystems Japan, Tokyo, Japan).
Lung histopathology
For lung histopathological examination, the lungs were distended by infusion of 10% formalin into the trachea at a pressure of 15 cm H2O for 2 h, and fixed in 10% formalin for 24 h. Formalin-fixed lungs were embedded in paraffin, sectioned and stained with hematoxylin and eosin (H-E). To estimate the extent of damage, the specimen was observed under a light microscope.
Protein assay
Protein contents were determined by the method of Bradford [25] with bovine serum albumin as a standard.
Statistical evaluations
Data are expressed as mean ± standard error (SE). Changes in variables for different assays were analyzed by Student’s t test or one-way ANOVA. Survival studies were analyzed by Chi-square test. Differences were considered to be significant at p<0.05.
Results
Changes in serum zinc concentration after PZ administration
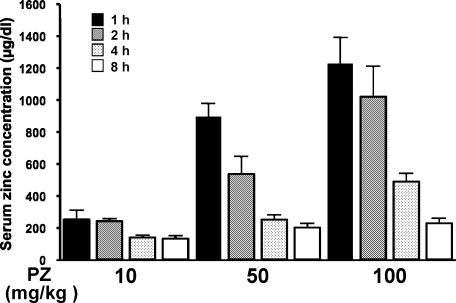
When healthy adult males received 300 mg of PZ in a fasting state, plasma zinc concentration increased linearly, reached a maximum (approximately 300 µg/dl) at 2 h after PZ intake, and thereafter reduced and became a normal level at 8 h after PZ (according to company data of Zeria Pharmaceutical). To determine optimal administration time and dose of PZ for mice, serum zinc concentration was measured at 1, 2, 4 or 8 h after oral treatment of mice with PZ (10, 50 or 100 mg/kg body weight) (Fig. 1). Serum zinc level increased in a dose-dependent manner and the level reached a maximum 1 h after administration of PZ to mice and subsequently decreased in a time-dependent manner.
Fig. 1.
Changes in serum zinc levels in mice after PZ intake. PZ (10, 50 or 100 mg/kg, p.o.) was given to mice, and blood samples were taken 1, 2, 4, or 8 h after PZ administration. Serum zinc concentration was measured as described in Materials and Methods. Data points represent the means ± SE (n = 6).
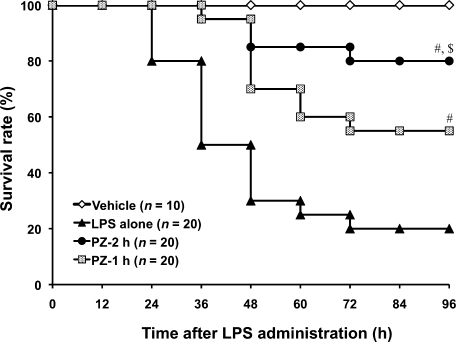
Effect of PZ pretreatment on survival rate in mice after LPS administration
The survival rate decreased to 20% at 96 h after LPS injection. Oral administrations of PZ (100 mg/kg) at 2 h and 1 h before LPS significantly increased survival rates to 80% and 55%, respectively (Fig. 2). Although serum zinc level in mice treated with PZ at 1 h before LPS was higher than that in 2-h treatment of mice with PZ (see Fig. 1), treatment with PZ at 2 h before LPS improved the survival rate better than 1-h treatment with PZ.
Fig. 2.
Time course of survival rate of mice treated with PZ followed by LPS. PZ (100 mg/kg) was given to mice 1 or 2 h before LPS (40 mg/kg, i.p.) administration. Mice were monitored for survival over 96 h after LPS administration. PZ-2 h and PZ-1 h received PZ at 2 h and 1 h before LPS administration, respectively. #p<0.01 vs LPS alone at 96 h after LPS. $p<0.01 vs PZ-1 h at 96 h after LPS.
Given these results, mice were administered 100 mg/kg of PZ at 2 h before LPS in the following experiments.
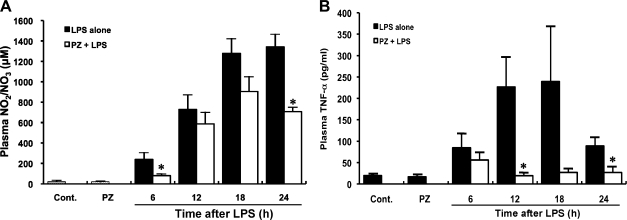
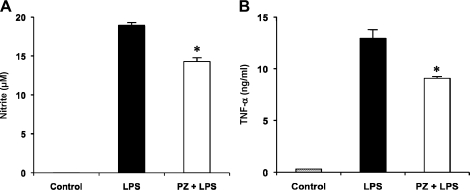
Effect of PZ pretreatment on plasma NO levels in mice after LPS administration
Plasma NO increased with time up to 24 h after LPS administration. Pretreatment of mice with PZ inhibited LPS-mediated NO production (Fig. 3A).
Fig. 3.
Inhibition by PZ of NO (A) and TNF-α (B) in plasma in mice injected with LPS. PZ (100 mg/kg) was given to mice 2 h before LPS (40 mg/kg, i.p.) administration. Blood samples were collected 6, 12, 18, 24 h after administration of LPS. Plasma NO and TNF-α concentrations were measured as described in Materials and Methods. Data points represent the means ± SE (n = 5). *p<0.01 vs LPS alone.
Effect of PZ pretreatment on plasma TNF-α levels in mice after LPS administration
Plasma TNF-α level also increased time-dependently, reached a maximum at 18 h and decreased at 24 h after LPS injection. Pretreatment of mice with PZ inhibited LPS-mediated TNF-α production (Fig. 3B).
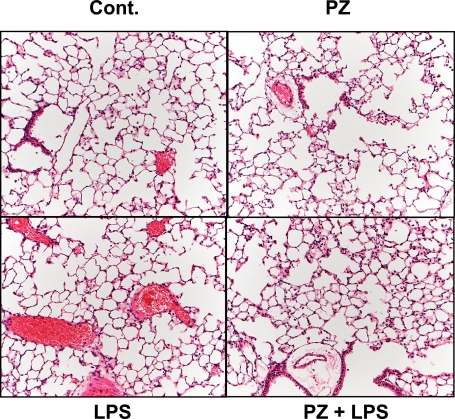
Effect of PZ pretreatment on lung histology in mice after LPS administration
LPS induced histological changes in the mouse lung such as inflammatory cell infiltration, peri-vascular edema and marked alveolar hemorrhage in comparison with control or PZ alone-treated lungs. PZ pretreatment improved such pulmonary damages remarkably (Fig. 4).
Fig. 4.
Effect of PZ on lung injury in mice 24 h after LPS administration. Mice were treated with PZ (100 mg/kg, p.o.) 2 h before LPS (40 mg/kg, i.p.) administration. Lung histopathological analysis was performed as described in Materials and Methods. Cont. (upper left): lung from control mouse; PZ (upper right): lung from mouse treated with PZ (100 mg/kg) alone; LPS (lower left): lung from mouse treated with LPS alone; PZ + LPS (lower right): lung from mouse treated with PZ at 2 h before LPS (H-E, magnification ×20).
Effect of PZ pretreatment on expression of HSP70 in mouse lung after LPS
Although PZ is known to be a potent HSP inducer in stomach and colon [16–18], PZ did not enhance the induction of pulmonary HSP70 after LPS administration (Fig. 5).
Fig. 5.
Effect of PZ pretreatment on HSP70 induction in the mouse lung 24 h after LPS administration. PZ (100 mg/kg) was given to mice 2 h before LPS (40 mg/kg, i.p.) administration. HSP70 level was analyzed by western blotting. Representative western blots of HSP70 from 3 separate experiments are shown.
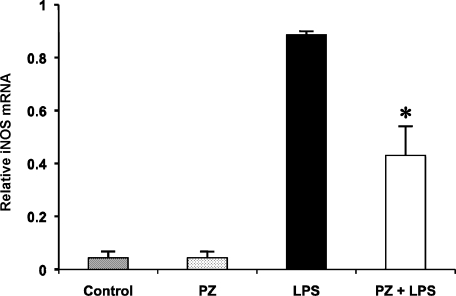
Effect of PZ pretreatment on iNOS gene expression in mouse lung after LPS
We determined the effect of PZ on expression of iNOS mRNA 6 h after LPS injection (Fig. 6). LPS treatment increased iNOS mRNA level in the lung approximately 20-fold of normal level. PZ administration 2 h before LPS significantly reduced the increase in iNOS mRNA gene expression by around 50%. In contrast, there was no difference of metallothionein-I (MT-I) and metallothionein-II (MT-II) mRNA levels between treatment with LPS alone and pretreatment with PZ at 2 h before LPS although MT-I and MT-II gene expressions increased remarkably in both groups (data not shown).
Fig. 6.
Effect of PZ on the expression of iNOS mRNA in the mouse lung 6 h after LPS. PZ (100 mg/kg) was given to mice 2 h before LPS (40 mg/kg, i.p.) administration. The level of iNOS mRNA in the mouse lung was measured as described in Materials and Methods. Data points represent the means ± SE (n = 5). *p<0.05 vs LPS alone.
Effect of PZ on LPS-mediated production of NO and TNF-α in RAW 264 cells
Treatment of RAW 264 cells with PZ (100 µM) at 6 h before LPS addition significantly suppressed NO production at 24 h as well as TNF-α production at 4 h after LPS (Fig. 7A and B).
Fig. 7.
Inhibitory effect of PZ on LPS-mediated production of NO (A) and TNF-α (B) in RAW 264 cells. Cells were incubated in the presence of LPS (100 ng/ml) for 24 h (A) or 4 h (B) at 37°C under a 5% CO2 and 95% air atmosphere. PZ (100 µM) was added 6 h before the addition of LPS. The levels of NO and TNF-α were measured as described in Materials and Methods. Data points represent the means ± SE (n = 3). *p<0.05 vs LPS alone-treated cells.
Effect of PZ on LPS-mediated NF-κB activation in RAW 264 cells
LPS is known to induce the expressions of iNOS and inflammatory cytokines such as TNF-α, IL-1β, IL-6 and IFN-γ [8], via activation of NF-κB. We next examined by western blot analysis whether PZ suppressed LPS-induced nuclear translocation of NF-κB in RAW 264 cells. LPS (100 ng/ml) caused the translocation of NF-κB p65 into the nucleus in a time-dependent manner, and PZ pretreatment 6 h before LPS significantly inhibited the nuclear translocation at 24 h after LPS addition (Fig. 8).
Fig. 8.
Inhibitory effect of PZ on LPS-mediated NF-κB activation in RAW 264 cells. Cells were incubated in the presence of LPS (100 ng/ml) for 24 h at 37°C under a 5% CO2 and 95% air atmosphere. PZ (100 µM) was added 6 h before the addition of LPS. Nuclear translocation of NF-κB p65 as an index of NF-κB activation was analyzed by western blotting, quantified densitometrically as described in Materials and Methods, and expressed as relative density to control cells. Data points represent the means ± SE (n = 3). *p<0.05 vs LPS alone-treated cells at 24 h after LPS.
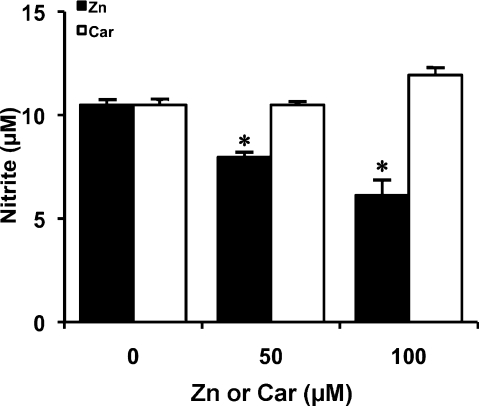
Effect of zinc sulfate or Car on LPS-mediated NO production in RAW 264 cells
Since PZ consists of zinc and Car, we investigated the effect of zinc or Car alone on LPS-induced NO production in RAW 264 cells. Treatment of the cells with zinc sulfate also inhibited NO production at 24 h after LPS addition in a dose-dependent manner. In contrast, Car failed to inhibit NO production (Fig. 9).
Fig. 9.
Effect of PZ subcomponents on LPS-mediated NO production in RAW 264 cells. Cells were incubated in the presence of LPS (100 ng/ml) for 24 h at 37°C under a 5% CO2 and 95% air atmosphere. Zinc sulfate (Zn) or Car was added to the culture at the concentration of 50 or 100 µM 2 h before starting the 24-incubation with LPS. NO level was measured as described in Materials and Methods. Data points represent the means ± SE (n = 3). *p<0.05 vs LPS alone-treated cells.
Discussion
In the present study, PZ pretreatment improved the survival rate of mice treated with LPS dramatically. Multiple organ failure (MOF) is considered to be a direct cause of death by sepsis and/or endotoxemia [26]. Since acute lung injury (ALI), a significant cause of morbidity and mortality, often occurred in the context of MOF [27], we carried out lung histological examinations. These experiments illustrated that LPS caused severe lung damage compatible with ALI and PZ suppressed the damage remarkably (Fig. 4). We also examined whether LPS caused hepatic and renal injuries in mice. LPS increased plasma alanine aminotransferase (ALT) as an index of liver injury to just only 5-fold of normal level at 18 h after the injection (data not shown), although the ALT level was increased to several hundreds fold of normal level in the cases of lethal hepatic injuries such as acetaminophen-induced hepatotoxicity [28, 29]. In contrast, LPS did not increase plasma creatinine levels in our experimental model (data not shown).
NF-κB is activated in response to LPS and the expression of iNOS and other proinflammatory gene products is enhanced, leading to overproduction of NO and proinflammatory cytokines including TNF-α [8]. NO reacts with superoxide radical to produce peroxynitrite (ONOO−) at a rate near the diffusion-controlled limit (7 × 109 M−1s−1) [30]. Therefore, these two reactive species must be generated in the same cells as shown in macrophages and neutrophils or exist near to interact each other. Production of ONOO−, a highly reactive nitrogen species, causes protein nitration followed by cell death [30]. TNF-α is one of the early proinflammatory cytokines secreted during LPS-induced endotoxin shock and is primarily secreted by macrophages and neutrophils [31]. TNF-α has been reported to induce cytokine-induced neutrophil chemoattractant-1 in rat gastric mucosal cells in a NF-κB-dependent manner [32]. PZ pretreatment inhibited the production of NO and TNF-α induced by administration of LPS to mice (Fig. 3A and B). PZ-mediated inhibition of LPS-induced NO production likely resulted from the inhibition of iNOS mRNA transcription. As mentioned above, NF-κB is involved in the induction of iNOS. It has been reported that PZ inhibits NF-κB activation in gastric epithelial cells [33] and rat colonic mucosa [34]. Therefore, we determined the effect of PZ on NF-κB activation in RAW 264 macrophage cells after LPS challenge. PZ significantly inhibited LPS-stimulated activation of NF-κB, resulting in the inhibition of NO and TNF-α production in RAW 264 cells (Figs. 7 and 8). Zinc, one of PZ subcomponents, has a dual effect on the secretion of proinflammatory cytokines, i.e., potentiation and suppression [35]. This is explained by a concentration-dependent process in which low zinc doses potentiate the LPS effect, whereas high concentrations inhibit monokine secretion [35]. Zinc is well known to inhibit NF-κB activation by a variety of stimuli [35–37]. In contrast, it has been reported that zinc plays an important role in activation of NF-κB in HUT-78, a Th0 human malignant lymphoblastoid cell line [38]. Since zinc, but not Car, suppressed LPS-induced NO production in RAW 264 cells (Fig. 9), the inhibition of NF-κB activation by PZ is likely ascribed to zinc subcomponent. There are at least two potential mechanisms underlying the inhibition by zinc against NF-κB activation as follows: (1) Zinc induces protein kinase A activation and subsequently inhibits nuclear translocation of NF-κB after LPS challenge [35]; (2) Zinc inhibits NF-κB-induced expression of inflammatory cytokines through the induction of A20, a zinc-finger transactivating factor [39].
It has been shown that the production of proinflammatory cytokines is inhibited by the HSP70 induction [2, 40]. Furthermore, overexpressed HSP70 potentiates resistance to apoptosis and oxidative stress [41]. Recently, PZ is reported to induce HSP70 (HSP72) in rat gastric mucosa, mouse colonic mucosa, human colon cells and mouse hepatocytes, and protect against injuries induced by a variety of stimuli [16–19, 42]. Therefore, we examined whether HSP70 induction was involved in the protection by PZ of LPS-induced lung injury. However, PZ did not enhance LPS-mediated HSP70 protein expression in the mouse lung. Van Molle et al. revealed that zinc induced HSP70 expression in mice, particularly in the liver and small intestine, but HSP70 expression in the lung remained rather low [43]. They explained that this is a more specific expression pattern compared with whole-body heat shock, which also induced HSP70 in other organs such as colon and lung. Several investigators have reported that NF-κB transcription could be inhibited by induction of HSP72 through stabilization of IκB and prevention of its phosphorylation [44, 45]. Recent study demonstrated that expression of HSP72 was significantly up-regulated and NF-κB activation was markedly inhibited in the colonic mucosa in rats pretreated with PZ [34]. Therefore, it was considered that the induction of HSP70 was not involved in mechanisms of NF-κB suppression by PZ in our experiments. Since previous studies reported on tissues and/or cells of gastrointestinal tract and liver, this discrepancy might be due to the difference of organ. The further studies should be required for fully understanding the mechanisms.
The induction of MT is known as one of important biological activities of zinc, a PZ subcomponent. MT, an important protein induced by metals, is shown to play a protective role in a lot of experimental models [46]. MTs are a superfamily of small proteins that are present in virtually every living organism. In mice, only four functional murine MT genes are known (MT-I, MT-II, MT-III, and MT-IV), and highly inducible MT-I and MT-II isoforms are coordinately regulated [47]. Therefore, we examined the effect of PZ pretreatment on the increase in MT-I and MT-II mRNA levels in the mouse lung after LPS administration. However, it did not affect the MT gene expressions after LPS (data not shown). The study using MT-I/II knock out mice has demonstrated that endogenous MT protects against ALI induced by LPS possibly via a protective effect on the vascular integrity, but not through inhibition of the NF-κB pathway and subsequent proinflammatory cytokine expression [48]. Given that PZ inhibited NF-κB activation as well as increases in NO and TNF-α levels after LPS challenge, it is suggested that MT induction is not involved in the protective effect of PZ on LPS-induced endotoxin shock in our experimental model.
In conclusion, the present study has shown that PZ protects against LPS-induced endotoxin shock via inhibition of NF-κB activation and subsequent induction of proinflammatory products including NO and TNF-α.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Akira S. Mammalian Toll-like receptors. Curr. Opin. Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 2.Klosterhalfen B., Hauptmann S., Offner F.A., Amo-Takyi B., Tons C., Winkeltau G., Affify M., Kupper W., Kirkpatrick C.J., Mittermayer C. Induction of heat shock protein 70 by zinc-bis-(DL-hydrogenaspartate) reduces cytokine liberation, apoptosis, and mortality rate in a rat model of LD100 endotoxiemia. Shock. 1997;7:254–262. doi: 10.1097/00024382-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Carrillo-Vico A., Lardone P.J., Naji L., Fernández-Santos J.M., Martín-Lacave I., Guerrero J.M., Calvo J.R. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal Res. 2005;39:400–408. doi: 10.1111/j.1600-079X.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama T., Fujita M., Koide N., Mori I., Yoshida T., Mori H., Yokochi T. 2-Aminopurine inhibits lipopolysaccharide-induced nitric oxide production by preventing IFN-β production. Microbiol. Immunol. 2004;48:957–963. doi: 10.1111/j.1348-0421.2004.tb03625.x. [DOI] [PubMed] [Google Scholar]
- 5.Jantaratnotai N., Utaisincharoen P., Piyachaturawat P., Chongthammakun S., Sanvarinda Y. Inhibitory effect of Curcuma comosa on NO production and cytokine expression in LPS-activated microglia. Life Sci. 2006;78:571–577. doi: 10.1016/j.lfs.2005.04.065. [DOI] [PubMed] [Google Scholar]
- 6.Huang K.T., Kuo L., Liao J.C. Lipopolysaccharide activates endothelial nitric oxide synthase through protein tyrosine kinase. Biochem. Biophys. Res. Commun. 1998;245:33–37. doi: 10.1006/bbrc.1998.8384. [DOI] [PubMed] [Google Scholar]
- 7.Palsson-McDermott E.M., O’Neill L.A.J. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S.F., Malik A.B. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 9.Ito M., Tanaka T., Suzuki Y. Effect of N-(3-aminopropionyl)-l-histidinato zinc (Z-103) on healing and hydrocortisone-induced relapse of acetic acid ulcers in rats with limited food-intake-time. Jpn. J. Pharmacol. 1990;52:513–520. doi: 10.1254/jjp.52.513. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa T., Naito Y., Tanigawa T., Yoneta T., Kondo M. The antioxidant properties of a novel zinc-carnosine chelate compound, N-(3-aminopropionyl)-l-histidinato zinc. Biochim. Biophys. Acta. 1991;1115:15–22. doi: 10.1016/0304-4165(91)90005-2. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa T., Naito Y., Tanigawa T., Yoneta T., Yasuda M., Ueda S., Oyamada H., Kondo M. Effect of zinc-carnosine chelate compound (Z-103), a novel antioxidant, on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Free Radic. Res. Commun. 1991;14:289–296. doi: 10.3109/10715769109088958. [DOI] [PubMed] [Google Scholar]
- 12.Cho C.H., Luk C.T., Ogle C.W. The membrane-stabilizing action of zinc carnosine (Z-103) in stress-induced gastric ulceration in rats. Life Sci. 1991;49:189–194. doi: 10.1016/0024-3205(91)90321-2. [DOI] [PubMed] [Google Scholar]
- 13.Arakawa T., Satoh H., Nakamura A., Nebiki H., Fukuda T., Sakuma H., Nakamura H., Ishikawa M., Seiki M., Kobayashi K. Effects of zinc-l-carnosine on gastric mucosal and cell damage caused by ethanol in rats. Correlation with endogenous prostaglandin E2. Dig. Dis. Sci. 1990;35:559–566. doi: 10.1007/BF01540402. [DOI] [PubMed] [Google Scholar]
- 14.Fujii Y., Matsura T., Kai M., Kawasaki H., Yamada K. Protection by polaprezinc, an anti-ulcer drug, against indomethacin-induced apoptosis in rat gastric mucosal cells. Jpn. J. Pharmacol. 2000;84:63–70. doi: 10.1254/jjp.84.63. [DOI] [PubMed] [Google Scholar]
- 15.Matsuu-Matsuyama M., Shichijo K., Okaichi K., Nakayama T., Nakashima M., Uemura T., Nino D., Sekine I. Protection by polaprezinc against radiation-induced apoptosis in rat jejunal crypt cells. J. Radiat. Res. 2008;49:341–347. doi: 10.1269/jrr.07114. [DOI] [PubMed] [Google Scholar]
- 16.Ohkawara T., Takeda H., Kato K., Miyashita K., Kato M., Iwanaga T., Asaka M. Polaprezinc (N-(3-aminopropionyl)-L-histidinato zinc) ameliorates dextran sulfate sodium-induced colitis in mice. Scand. J. Gastroenterol. 2005;40:1321–1327. doi: 10.1080/00365520510023530. [DOI] [PubMed] [Google Scholar]
- 17.Mikami K., Otaka M., Watanabe D., Goto T., Endoh A., Miura K., Ohshima S., Yoneyama K., Sato M., Shibuya T., Segawa D., Kataoka E., Yoshino R., Takeuchi S., Sato W., Odashima M., Watanabe S. Zinc L-carnosine protects against mucosal injury in portal hypertensive gastrophathy through induction of heat shock protein 72. J. Gastroenterol. Hepatol. 2006;21:1669–1674. doi: 10.1111/j.1440-1746.2006.04328.x. [DOI] [PubMed] [Google Scholar]
- 18.Otaka M., Konishi N., Odashima M., Jin M., Wada I., Matsuhashi T., Horikawa Y., Ohba R., Watanabe S. Is Mongolian gerbil really adequate host animal for study of Helicobacter pylori infection-induced gastritis and cancer? Biochem. Biophys. Res. Commun. 2006;347:297–300. doi: 10.1016/j.bbrc.2006.06.094. [DOI] [PubMed] [Google Scholar]
- 19.Nishida T., Ohata S., Kusumoto C., Mochida S., Nakada J., Inagaki Y., Ohta Y., Matsura T. Zinc supplementation with polaprezinc protects mouse hepatocytes against acetaminophen-induced toxicity via induction of heat shock protein 70. J. Clin. Biochem. Nutr. 2010;46:43–51. doi: 10.3164/jcbn.09-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X.Z., Fernandez-Prada C.M., Bhattacharjee A.K., Hoover D.L. Over-expression of HSP-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16:210–219. doi: 10.1006/cyto.2001.0959. [DOI] [PubMed] [Google Scholar]
- 21.Nakada J., Matsura T., Okazaki N., Nishida T., Togawa A., Minami Y., Inagaki Y., Ito H., Yamada K., Ishibe Y. Oral administration of geranylgeranylacetone improves survival rate in a rat endotoxin shock model: administration timing and HSP70 induction. Shock. 2005;24:482–487. doi: 10.1097/01.shk.0000180980.63247.a9. [DOI] [PubMed] [Google Scholar]
- 22.Mochida S., Matsura T., Yamashita A., Horie S., Ohata S., Kusumoto C., Nishida T., Minami Y., Inagaki Y., Ishibe Y., Nakada J., Ohta Y., Yamada K. Geranylgeranylacetone ameliorates inflammatory response to lipopolysaccharide (LPS) in murine macrophages: Inhibition of LPS binding to the cell surface. J. Clin. Biochem. Nutr. 2007;41:115–123. doi: 10.3164/jcbn.2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii Y., Matsura T., Kai M., Matsui H., Kawasaki H., Yamada K. Mitochondrial cytochrome c release and caspase-3-like protease activation during indomethacin-induced apoptosis in rat gastric mucosal cells. Proc. Soc. Exp. Biol. Med. 2000;224:102–108. doi: 10.1046/j.1525-1373.2000.22407.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsura T., Nishida T., Togawa A., Horie S., Kusumoto C., Ohata S., Nakada J., Ishibe Y., Yamada K., Ohta Y. Mechanisms of protection by melatonin against acetaminophen-induced liver injury in mice. J. Pineal Res. 2006;41:211–219. doi: 10.1111/j.1600-079X.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 26.Oberholzer A., Oberholzer C., Moldawer L.L. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 27.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 28.Sumioka I., Matsura T., Yamada K. Therapeutic effect of S-allylmercaptocysteine on acetaminophen-induced liver injury in mice. Eur. J. Pharmacol. 2001;433:177–185. doi: 10.1016/s0014-2999(01)01503-5. [DOI] [PubMed] [Google Scholar]
- 29.Nishida T., Matsura T., Nakada J., Togawa A., Kai M., Sumioka I., Minami Y., Inagaki Y., Ishibe Y., Ito H., Ohta Y., Yamada K. Geranylgeranylacetone protects against acetaminophen-induced hepatotoxicity by inducing heat shock protein 70. Toxicology. 2006;219:187–196. doi: 10.1016/j.tox.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 30.James L.P., Mayeux P.R., Hinson J.A. Acetaminophen-induced hepatotoxicity. Drug Metab. Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 31.Grivennikov S.I., Tumanov A.V., Liepinsh D.J., Kruglov A.A., Marakusha B.I., Shakhov A.N., Murakami T., Drutskaya L.N., Förster I., Clausen B.E., Tessarollo L., Ryffel B., Kuprash D.V., Nedospasov S.A. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22:93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Handa O., Naito Y., Takagi T., Shimozawa M., Kokura S., Yoshida N., Matsui H., Cepinskas G., Kvietys P.R., Yoshikawa T. Tumor necrosis factor-α-induced cytokine-induced neutrophil chemoattractant-1 (CINC-1) production by rat gastric epithelial cells: role of reactive oxygen species and nuclear factor-κB. J. Pharmacol. Exp. Ther. 2004;309:670–676. doi: 10.1124/jpet.103.062216. [DOI] [PubMed] [Google Scholar]
- 33.Shimada T., Watanabe N., Ohtsuka Y., Endoh M., Kojima K., Hiraishi H., Terano A. Polaprezinc down-regulated proinflammatory cytokine-induced nuclear factor-κB activation and interleukin-8 expression in gastric epithelial cells. J. Pharmacol. Exp. Ther. 1999;291:345–352. [PubMed] [Google Scholar]
- 34.Odashima M., Otaka M., Jin M., Wada I., Horikawa Y., Matsuhashi T., Ohba R., Hatakeyama N., Oyake J., Watanabe S. Zinc l-carnosine protects colonic mucosal injury through induction of heat shock protein 72 and suppression of NF-κB activation. Life Sci. 2006;79:2245–2250. doi: 10.1016/j.lfs.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 35.von Bülow V., Dubben S., Engelhardt G., Hebel S., Plümäkers B., Heine H., Rink L., Haase H. Zinc-dependent suppression of TNF-α production is mediated by protein kinase A-induced inhibition of Raf-1, IκB kinase β, and NF-κB. J. Immunol. 2007;179:4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Z., Wang L., Song Z., Saari J.T., McClain C.J., Kang Y.J. Abrogation of nuclear factor-κB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis factor-α production and liver injury. Am. J. Pathol. 2004;164:1547–1556. doi: 10.1016/s0002-9440(10)63713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szuster-Ciesielska A., Plewka K., Daniluk J., Kandefer-Szerszen M. Zinc supplementation attenuates ethanol- and acetaldehyde-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS) production and by influencing intracellular signaling. Biochem. Pharmacol. 2009;78:301–314. doi: 10.1016/j.bcp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Prasad A.S. Zinc: mechanisms of host defense. J. Nutr. 2007;137:1345–1349. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- 39.Prasad A.S., Bao B., Beck F.W.J., Sarkar F.H. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi Y., Sawa Y., Fukuyama N., Nakazawa H., Matsuda H. Preoperative glutamine administration induces heat-shock protein 70 expression and attenuates cardiopulmonary bypass-induced inflammatory response by regulating nitric oxide synthase activity. Circulation. 2002;106:2601–2607. doi: 10.1161/01.cir.0000035651.72240.07. [DOI] [PubMed] [Google Scholar]
- 41.Hirata I., Naito Y., Hayashi N., Mizushima K., Adachi S., Omatsu T., Okayama T., Kishimoto E., Ichikawa H., Takagi T., Kokura S., Otaka M., Yoshikawa T. Heat-shock protein 70-overexpressing gastric epithelial cells are resistant to indomethacin-induced apoptosis. Digestion. 2009;79:243–250. doi: 10.1159/000215352. [DOI] [PubMed] [Google Scholar]
- 42.Ohkawara T., Nishihira J., Nagashima R., Takeda H., Asaka M. Polaprezinc protects human colon cells from oxidative injury induced by hydrogen peroxide: relevant to cytoprotective heat shock proteins. World J. Gastroenterol. 2006;12:6178–6181. doi: 10.3748/wjg.v12.i38.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Molle W., Van Roy M., Van Bogaert T., Dejager L., Van Lint P., Vanlaere I., Sekikawa K., Kollias G., Libert C. Protection of zinc against tumor necrosis factor-induced lethal inflammation depends on heat shock protein 70 and allows safe antitumor therapy. Cancer Res. 2007;67:7301–7307. doi: 10.1158/0008-5472.CAN-06-4010. [DOI] [PubMed] [Google Scholar]
- 44.Schell M.T., Spitzer A.L., Johnson J.A., Lee D., Harris H.W. Heat shock inhibits NF-κB activation in a dose- and time-dependent manner. J. Surg. Res. 2005;129:90–93. doi: 10.1016/j.jss.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 45.King T.A., Ghazaleh R.A., Juhn S.K., Adams G.L., Ondrey F.G. Induction of heat shock protein 70 inhibits NF-kappa-B in squamous cell carcinoma. Otolaryngol. Head Neck Surg. 2005;133:70–79. doi: 10.1016/j.otohns.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 46.Formigari A., Irato P., Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: Biochemical and cytochemical aspects. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;146:443–459. doi: 10.1016/j.cbpc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Waeytens A., De Vos M., Laukens D. Evidence for a potential role of metallothioneins in inflammatory bowel diseases. Mediators Inflamm. 2009:729172. doi: 10.1155/2009/729172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takano H., Inoue K., Yanagisawa R., Sato M., Shimada A., Morita T., Sawada M., Nakamura K., Sanbonji C., Yoshikawa T. Protective role of metallothionein in acute lung injury induced by bacterial endotoxin. Thorax. 2004;59:1057–1062. doi: 10.1136/thx.2004.024232. [DOI] [PMC free article] [PubMed] [Google Scholar]