Abstract
Reduced coenzyme Q10 (CoQ10H2) is known as a potent antioxidant in biological systems. However, it is not yet known whether CoQ9H2 could act as an antioxidant in human cells. The aim of this study is to assess whether exogenously added CoQ9 can protect human liver cells against injuries induced by a water-soluble radical initiator, 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH) and a lipid-soluble radical initiator, 2,2'-azobis(2,4-dimethylvaleronitrile) (AMVN). CoQ9-enriched cells were obtained by treatment of HepG2 cells with 10 µM CoQ9 liposomes for 24 h. CoQ9-enriched cells were exposed to 10 mM AAPH and 500 µM AMVN over 4 h and 24 h, respectively. The loss of viability after treatment with AAPH or AMVN was much less in CoQ9-enriched cells than in naive HepG2 cells. The decrease in glutathione and the increase in thiobarbituric acid-reactive substance after treatment with AAPH or AMVN were also suppressed in CoQ9-enriched cells. The incubation of CoQ9-enriched cells with AAPH or AMVN led to a decrease in cellular CoQ9H2 and reciprocal increase in cellular CoQ9 resulting from its antioxidant function. Taken together, it was demonstrated for the first time that exogenously added CoQ9 could prevent oxidative stress-mediated damage to human cells by virtue of its antioxidant activity.
Keywords: coenzyme Q9, free radical, human liver cells, antioxidant
Introduction
Coenzyme Q (CoQ) is a quinone derivative with an isoprenoid tail. CoQ homologs (CoQn) containing 1–13 isoprene units occur in nature, and in mammals the most common forms contain 9 (CoQ9) and 10 (CoQ10) isoprene units [1, 2]. CoQ9 is the predominant form in mouse and rat, and CoQ10 is predominant in rabbit, guinea pig, dog, pig and human [3]. As for the physiological significance of CoQn, its role as an electron-carrying component of mitochondrial respiratory chain is well established [4, 5]. A number of in vitro and in vivo studies have revealed antioxidant function of CoQ10H2, reduced form of CoQ10 [6–16]. However, it is not yet known whether CoQ9H2, a minor homolog in human, could play a role as an antioxidant in human cells.
2,2'-Azobis(2-amidinopropane) dihydrochloride (AAPH) and 2,2'-azobis(2,4-dimethylvaleronitrile) (AMVN) are a water-soluble and a lipid-soluble radical initiators, respectively which undergo spontaneous thermal decomposition to form carbon-centered radicals [17]. These radicals can initiate a chain reaction of lipid peroxidation to generate lipid peroxides in the presence of oxygen and polyunsaturated fatty acids. AAPH and AMVN have therefore been used to produce free-radical stresses.
We have reported that exogenously added CoQ10 can protected rat hepatocytes against cell death by AAPH [18]. However, it remains to be elucidated whether exogenously added CoQ9 can prevent oxidative damage to human cells, which have a considerable amount of CoQ10 but trace of CoQ9. In the present study, we determined the sensitivities of CoQ9-enriched human hepatic cells to oxidative stress induced by AAPH and AMVN.
Materials and Methods
Chemicals
AAPH and dimyristoyl-phosphatidylcholine (DMPC) were purchased from Wako Pure Chemical Ind. (Osaka, Japan). AMVN was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Chromatographically pure CoQ9 and CoQ10 were generous gifts from Eisai Co. (Tokyo, Japan). Fetal calf serum (FCS) was purchased from PAA Laboratories GmbH (Linz, Austria). Dulbecco’s modified eagle medium (DMEM) was obtained from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). All other chemicals used were of analytical grade.
Cell culture and establishment of CoQ9-enriched human liver cells
HepG2 human hepatoma cell line was obtained from Japan Health Science Foundation (Osaka, Japan), and grown in DMEM supplemented with 10% heat-inactivated FCS, 2 mM glutamine and antibiotics (100 units/ml penicillin, 100 µg/ml streptomycin) at 37°C in a humidified incubator with 5% CO2. Cells from passages 3–7 were used for the experiments. The cells were seeded in 94-mm dishes at a cell density of 5 × 105 cells/dish and incubated for 24 h at 37°C in an atmosphere of 5% CO2/95% air. After non-adherent cells were removed by washing with culture medium, attached cells were enriched with CoQ9 (see below) before induction of free radical injuries.
Small unilamellar liposomes containing CoQ9 were prepared by dissolving 17 mg of DMPC in 1 ml of ethanol containing CoQ9 (1 mg/ml) at a CoQ9/DMPC molar ratio of 1:20. The solution was evaporated under N2 stream. The resulting film was redissolved in 1.26 ml of phosphate-buffered saline (PBS) to obtain 1 mM CoQ9, vortexed vigorously, and sonicated for 3 min. HepG2 cells were incubated at 37°C with varying concentrations of CoQ9 liposomes for different time periods to make CoQ9-enriched human liver cells.
Experimental protocol
AAPH and AMVN were dissolved in PBS and dimethyl sulfoxide (DMSO), respectively. CoQ9-enriched HepG2 cells and naive HepG2 cells were exposed to 10 mM AAPH and 500 µM AMVN over 4 h and 24 h, respectively to induce oxidative stress. The cells were harvested with rubber policeman, washed with PBS twice, and resuspended in PBS.
Measurement of CoQ9 and CoQ10
The cell suspension was transferred to a centrifuge tube and centrifuged at 1,500 × g for 5 min. The resulting pellet was washed with ice-cold PBS and stored at −80°C until assayed. The determination of cellular oxidized and reduced CoQ homologs (CoQ9, CoQ10, CoQ9H2 and CoQ10H2) was carried out as described previously [3, 19, 20]. Briefly, the frozen cells were homogenized with ice-cold water (1.3–1.5 ml/ sample) under an atmosphere of nitrogen gas, and then oxidized and reduced forms of CoQ9 and CoQ10 were extracted with a mixture of 2 volumes of ethanol and 5 volumes of n-hexane to 1 volume of homogenate, and further extracted two times. The n-hexane layers were collected and evaporated under nitrogen gas. The residue was redissolved in ethanol, and subjected to high-performance liquid chromatography (HPLC).
All standards used for HPLC were pure samples of CoQ9 and CoQ10. The reduced form of CoQ9 and CoQ10 were prepared by reduction with sodium borohydride.
Cell Viability
The viability of cells was determined using a commercially available WST-8 assay kit (Seikagaku Biobusiness Co., Tokyo, Japan) according to the manufacture’s instruction as described previously [21]. The cells were seeded in a 24-well plate at a cell density of 5 × 104 cells/well and incubated for 24 h after treatment with CoQ9 liposomes, and then exposed to 10 mM AAPH and 500 µM AMVN over 4 h and 24 h, respectively. Cell viability was assessed by measurement of the absorbance at 492 nm in a microplate reader (Biotrak II, Amersham) after incubation of cells in WST-8 solution for 1 h at 37°C.
Lipid peroxidation and glutathione assay
Cells were collected and washed with PBS twice. The cells were resuspended in PBS and lysed by freezing and thawing. Then, the cell lysates were homogenized in 0.05 M phosphate buffer (0.2 M NaH2PO4, 0.2 M Na2HPO4, 0.2 M Na-EDTA, pH 7.4) under N2 stream. Cellular lipid peroxidation was measured by a fuluorometric reaction with thiobarbituric acid as previously described [15, 21, 22]. Lipid peroxide content was expressed as the amount of malondialdehyde (MDA) equivalents using tetraethoxypropane as a standard.
Cellular reduced glutathione (GSH) contents were determined fluorometrically using Thio-Glo1® as previously described [23]. Briefly, cells treated with AAPH or AMVN were harvested with rubber policeman and washed twice with PBS. Thereafter cells were lysed by the same procedure as that in lipid peroxidation assay. Immediately after addition of 10 µM Thio-Glo1® to the cell lysates, fluorescence was measured in a CytoFluor II (Applied Biosystems, Foster city, CA) fluorescence microplate reader using excitation at 360 ± 40 nm and emission at 530 ± 25 nm.
Protein assay
Protein contents were determined by the method of Bradford [24] with bovine serum albumin as a standard.
Statistical analysis
Data are expressed as means ± standard error (SE). Changes in variables for different assays were analyzed by Student’s t test or one-way ANOVA. Differences were considered to be significant at p<0.05.
Results
Enrichment of CoQ9 in HepG2 cells with CoQ9 liposomes
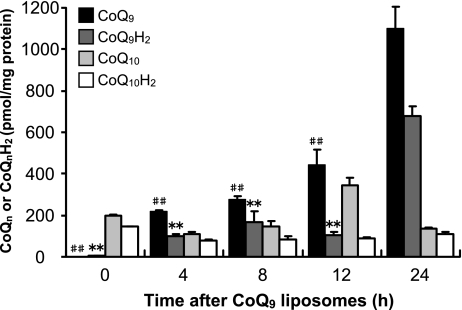
To establish CoQ9-enriched HepG2 cells (referred to as CoQ9-enriched cells below), intracellular concentration of CoQ9, CoQ10, CoQ9H2 and CoQ10H2 was measured at 0, 4, 8, 12 or 24 h after treatment of naive HepG2 cells (referred to as control cells below) with 10 µM CoQ9 liposomes (Fig. 1). Cellular CoQ9 and CoQ9H2 levels increased in a time-dependent manner and the levels reached a maximum 24 h after addition of CoQ9 liposomes. In contrast, there were few changes in cellular contents of CoQ10 and CoQ10H2 except for the CoQ10 content at 12 h.
Fig. 1.
Changes in contents of CoQn and CoQnH2 in HepG2 cells after exposure to CoQ9 liposomes. HepG2 cells were exposed to CoQ9 liposomes (10 µM) and harvested after 4, 8, 12, or 24 h. Intracellular CoQ9, CoQ9H2, CoQ10, and CoQ10H2 were measured by HPLC as described in Materials and Methods. Data points represent the means ± SE (n = 3). **p<0.01 vs CoQ9H2 at 24 h, ##p<0.01 vs CoQ9 at 24 h.
We next examined whether CoQ9 liposomes were really taken up by control cells, and whether they led to morphological changes of the cells. The cells were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with Oil-red-O and hematoxylin. Thereafter, the specimen was observed under a light microscope. Although CoQ9 liposomes were time-dependently taken up by the cells as shown by the increase in intracellular lipid droplets, there were no morphological changes in the cytosol as well as the nucleus until 24 h after treatment with CoQ9 liposomes (data not shown).
Given these results, we used the cells incubated with CoQ9 liposomes for 24 h as CoQ9-enriched cells in the following experiments.
Resistance of CoQ9-enriched cells to oxidative stress
To determine the concentration of AAPH and AMVN required to induce cell death in control cells, we treated the cells with AAPH (1 to 10 mM) and AMVN (200 to 500 µM) for 4 h and 24 h, respectively. Both AAPH and AMVN induced the decrease in cell viability in a dose-dependent manner and the decrease reached a maximum at 10 mM and 500 µM, respectively (data not shown).
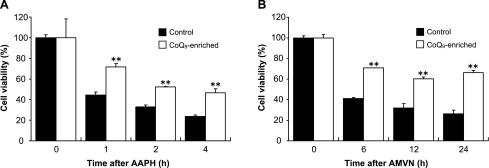
We next examined the time course of changes in cell viability in control cells and CoQ9-enriched cells following treatment with AAPH (10 mM) or AMVN (500 µM). The viability of control cells after AAPH treatment decreased in a time-dependent manner and was 24% of normal level after 4 h. On the other hand, the viability of CoQ9-enriched cells after AAPH was significantly higher than that of control cells during the incubation time period (Fig. 2A). Treatment of control cells and CoQ9-enriched cells with AMVN yielded similar results (Fig. 2B). These results suggested that CoQ9-enriched cells are strongly resistant to oxidative stress.
Fig. 2.
Changes in cell viability in control cells and CoQ9-enriched cells following treatment with AAPH (A) or AMVN (B). HepG2 cells were incubated for 24 h in the absence (for control cells) or presence (for CoQ9-enriched cells) of CoQ9 liposomes (10 µM), and then exposed to 10 mM AAPH and 500 µM AMVN over 4 h and 24 h, respectively. The cell viability was analyzed using WST-8 assay kit. Data points represent the means ± SE (n = 3). **p<0.01 vs control cells at the same incubation time.
Lipid peroxidation in CoQ9-enriched cells exposed to oxidative stress
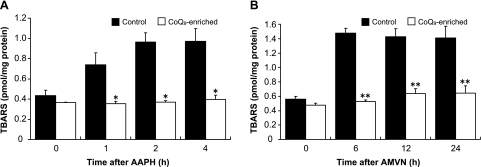
We examined the effect of CoQ9 enrichment on lipid peroxidation in control cells after AAPH (10 mM) exposure (Fig. 3A). Thiobarbituric acid-reactive substance (TBARS) level in control cells increased in a time-dependent manner up to 2 h after AAPH, and thereafter reached the plateau. The TBARS level was 2.5-fold of normal level at 4 h after AAPH. In contrast, CoQ9-enriched cells kept normal TBARS levels during the incubation time period. TBARS level in control cells increased to 2.8-fold of normal level at 6 h after AMVN (500 µM) exposure, and thereafter reached the plateau (Fig. 3B). However, CoQ9-enriched cells kept almost normal TBARS levels during the incubation time period.
Fig. 3.
Changes in lipid peroxidation in control cells and CoQ9-enriched cells following treatment with AAPH (A) or AMVN (B). Control and CoQ9-enriched cells were incubated as described in the legend to Fig. 2. Cellular TBARS content was measured as described in Materials and Methods. Data points represent the means ± SE (n = 3). *p<0.05, **p<0.01 vs control cells at the same incubation time.
Changes in GSH level in CoQ9-enriched cells exposed to oxidative stress
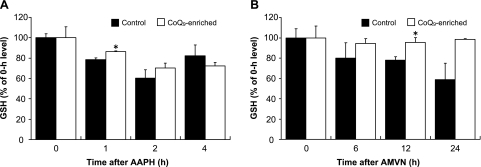
Since GSH is the major intracellular reductant, we measured its concentration in control cells and CoQ9-enriched cells over 4 h period of time after AAPH (10 mM) exposure (Fig. 4A). Intracellular GSH in control cells significantly decreased by 20% and 40% at 1 h and 2 h, respectively after treatment with AAPH. The GSH level in CoQ9-enriched cells was significantly higher than that in control cells at 1 h after AAPH. When control cells were incubated with 500 µM AMVN over 24 h, cellular GSH content significantly decreased by 20% at 12 h after AMVN (Fig. 4B). At that time, the GSH content in CoQ9-enriched cells was significantly higher than that in control cells.
Fig. 4.
Changes in GSH in control cells and CoQ9-enriched cells following treatment with AAPH (A) or AMVN (B). Control and CoQ9-enriched cells were incubated as described in the legend to Fig. 2. Cellular GSH content was measured as described in Materials and Methods. Data points represent the means ± SE (n = 3). *p<0.05 vs control cells at the same incubation time.
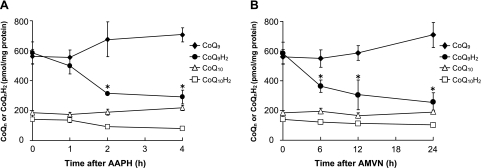
Changes in the concentration of CoQ9, CoQ10, CoQ9H2 and CoQ10H2 in CoQ9-enriched cells exposed to oxidative stress
To determine whether CoQ9H2 derived from intracellular conversion of CoQ9 taken up acts as an antioxidant, we measured the concentration of CoQ9, CoQ10, CoQ9H2 and CoQ10H2 in CoQ9-enriched cells over 4 h period of time after AAPH (10 mM) exposure (Fig. 5A). The concentration of CoQ9H2 in CoQ9-enriched cells decreased linearly from 1 to 4 h after addition of AAPH, whereas that of CoQ9 tended to increase reciprocally (Fig. 5A). In contrast, the concentration of CoQ10H2 in CoQ9-enriched cells did not decrease significantly during the incubation time period. When CoQ9-enriched cells were incubated with 500 µM AMVN over 24 h, the concentration of cellular CoQ9H2 decreased linearly from 6 to 24 h after AMVN exposure with a reciprocal increase in cellular CoQ9 (Fig. 5B). The concentration of CoQ10H2, however, did not decrease significantly over 24 h following AMVN addition.
Fig. 5.
Changes in contents of CoQn and CoQnH2 in control cells and CoQ9-enriched cells following treatment with AAPH (A) or AMVN (B). Control and CoQ9-enriched cells were incubated as described in the legend to Fig. 2. The concentrations of CoQn and CoQnH2 in the cells were determined by HPLC as described in Materials and Methods. Data points represent the means ± SE (n = 3). *p<0.05 vs 0-h incubation time.
Discussion
The present study demonstrated for the first time that exogenously added CoQ9 was converted to CoQ9H2 in human liver cells and subsequently acted as an antioxidant to suppress lipid peroxidation in the cells, resulting in protection of the cells against free radical-induced injuries.
Previous in vivo and in vitro studies showed that exogenously administered CoQ10 prevented a variety of injuries associated with oxidative stress [9–16]. In an experiment using endotoxicemic mice, administered CoQ10 was converted to CoQ10H2 in the liver, suppressed hepatic lipid peroxidation, spared endogenous CoQ9H2, and increased the survival rate of mice via its antioxidant function [12]. In this context, recent in vitro study has revealed that exogenously added CoQ10H2 suppressed the secretion of pro-inflammatory cytokine TNF-α as well as different chemokines in LPS-stimulated human monocytic THP-1 cells [25]. Furthermore, CoQ10 pretreatment was shown to protect rat liver and kidney against injuries caused by ischemia-reperfusion [10, 11], and orthotopic liver transplantation [13], and also to protect canine heart against reperfusion injury following cold preservation [14]. We have shown that pretreatment with CoQ10 resulted in an increase in hepatic CoQ10H2 and a marked reduction in hepatic lipid peroxide content and plasma alanine aminotransferase activity without affecting hepatic GSH after acetaminophen injection [15]. In addition to the in vivo study, we have demonstrated that exogenously added CoQ10 was actually taken up by rat hepatocytes and reduced to CoQ10H2 in the cells to protect against AAPH-induced cell death [18].
As described above, exogenously added CoQn has to be reduced to CoQnH2 in the cells to exhibit its antioxidant activity. Very important therefore is the presence of enzymes, which can reduce CoQn and/or CoQn semiquinone radicals. It is well known that mitochondrial CoQnH2 is efficiently regenerated by the respiratory chain [26]. On the other hand, to date, the following enzymes are proposed as reduction enzymes for non-mitochondrial CoQn: NAD(P)H oxidoreductase (DT diaphorase) [27]; NADPH-dependent CoQ reductase [28]. The DT diaphorase is unique since it can directly reduce CoQn by two-electron transfer without intermediate formation of semiquinone, but it is less efficient for longer isoprenoid side chain length, i.e. those with 9 or 10 isoprene units [29]. NADH-dependent CoQ reductase is also a two-electron reducing enzyme, located in the cytosol, and can reduce CoQn with a long isoprenoid side chain such as CoQ9 and CoQ10, to CoQnH2 [28]. Furthermore, lipoamide dehydrogenase in the matrix surface of the mitochondria, thioredoxine reductase 1 in the cytosol and nuclei, glutathione reductase in the cytosol also could reduce CoQ10 to CoQ10H2 [28]. However, it remains unknown which reductase plays a primary role in the reduction of CoQn.
We previously examined the difference in antioxidant activity between endogenous CoQ9H2 and CoQ10H2 using rat and guinea pig hepatocytes, which have CoQ9 and CoQ10, respectively as a primary CoQ homolog [3]. We found that endogenous CoQ9H2 constantly acted as a potent antioxidant in rat as well as guinea pig hepatocytes exposed to AAPH, whereas endogenous CoQ10H2 did so mainly in cells such as guinea pig hepatocytes, in which CoQ10 was the predominant CoQ homolog. Since endogenous CoQ9H2 is a potent lipid-soluble antioxidant, supplementation with CoQ9 is strongly suggested to be a promising antidote for oxidative stresses. Recent study based on the measurement of CoQ9 and CoQ9H2 has reported that exogenously added CoQ9 exhibits antioxidant activity in mice, which have CoQ9 as a major CoQ homolog [30].
In this study, we administered CoQ9 to the human liver cells in which CoQ10 is predominant, and determined the antioxidant activity of extrinsic CoQ9H2 in the cells exposed to a water-soluble or a lipid-soluble radical initiator. We used two kinds of azo compounds well known as radical initiators, i.e. AAPH and AMVN [17]. AAPH generates carbon radicals primarily and constantly through thermal decomposition in the aqueous phase, and the radicals thus formed react with oxygen rapidly to give peroxyl radicals. This azo compound is therefore a useful tool for studying the cell damage by extracellular free radicals. On the other hand, AMVN is a lipid-soluble and water-insoluble azo compound, and generates radicals initially within the lipid region of the membranes. Therefore, we can investigate the effect of extrinsic CoQ9H2 on two different kinds of free radical-mediated damages. CoQ9-enriched cells were much more resistant to extracellular free radical-mediated as well as intramembranous free radical-mediated oxidative stress compared with control cells. In this context, suppression of lipid peroxidation was well correlated with the linear decrease in abundant CoQ9H2 with reciprocal increase in CoQ9 in CoQ9-enriched cells, indicating that a loss of CoQ9H2 in the cells exposed to two different kinds of radical initiators was caused by its acting as an antioxidant. Moreover, CoQ10H2 content in CoQ9-enriched cells remained unchanged during the exposure to the radical initiators, suggesting that extrinsic CoQ9H2 could spare endogenous CoQ10H2 by its serving as a primary antioxidant.
While CoQ10 administration was reported to enhance endogenous CoQ9 by a mechanism that remains to be elucidated [31], it has been shown that dietary CoQ10 did not influence the endogenous biosynthesis of CoQ9 [32]. There was no difference in the CoQ10 levels between control cells and CoQ9-enriched cells. These discrepancies may be ascribed to the differences in the duration of CoQ administration and/or in CoQ homologs administered. However, the further studies should be required.
Extrinsic CoQ9H2 was the same potent antioxidant as endogenous CoQ9H2 even when it was administered to the cells that have CoQ10 as a predominant homolog. It is evident from the studies using rodent models that exogenously added CoQ10 is converted to CoQ10H2 and subsequently plays an antioxidant role in various tissues, which have CoQ9 as a predominant homolog. Therefore, CoQ9 supplementation for mammals that have mainly CoQ10 is potentially one of promising antioxidative procedures like CoQ10 supplementation. The previous study has revealed that CoQ10 is taken up from the intestine into the circulation with a low rate in rats, and only about 2–4% can be recovered [32]. Accordingly, in the case of CoQ9 supplementation in vivo, absorption efficiency of CoQ9 from the intestine in CoQ10-predominant mammals has to be elucidated. Furthermore, CoQ9 taken up by the cells has to be delivered to the cellular compartments appropriately. In this connection, saposin B, a CoQ10-binding and transfer protein, has been shown to also bind to CoQ9 [33].
In conclusion, the present study has shown that exogenously added CoQ9 protects human cells against oxidative stress via its potent antioxidant activity.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Crane F.L. In: Distribution in ubiquinones, in Biochemistry of Quinones. Morton R.A., editor. Academic Press; London: 1965. pp. 183–206. [Google Scholar]
- 2.Karr D.E., Bibb W.F., Moss C.W. Isoprenoid quinones of the genus Legionella. J. Clin. Microbiol. 1982;15:1044–1048. doi: 10.1128/jcm.15.6.1044-1048.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsura T., Yamada K., Kawasaki T. Difference in antioxidant activity between reduced coenzyme Q9 and coenzyme Q10 in the cell: studies with isolated rat and guinea pig hepatocytes treated with a water-soluble radical initiator. Biochim. Biophys. Acta. 1992;1123:309–315. doi: 10.1016/0005-2760(92)90012-k. [DOI] [PubMed] [Google Scholar]
- 4.Ernster L., Lee I.Y., Norling B., Persson B. Studies with ubiquinone-depleted submitochondrial particles. Essentiality of ubiquinone for the interaction of succinate dehydrogenase, NADH dehydrogenase, and cytochrome b. Eur. J. Biochem. 1969;9:299–310. doi: 10.1111/j.1432-1033.1969.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 5.Kröger A., Klingenberg M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur. J. Biochem. 1973;34:358–368. doi: 10.1111/j.1432-1033.1973.tb02767.x. [DOI] [PubMed] [Google Scholar]
- 6.Mellors A., Tappel A.L. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J. Biol. Chem. 1966;241:4353–4356. [PubMed] [Google Scholar]
- 7.Takayanagi R., Takeshige K., Minakami S. NADH- and NADHP-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochem. J. 1980;192:853–860. doi: 10.1042/bj1920853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto Y., Komuro E., Niki E. Antioxidant activity of ubiquinol in solution and phosphatidylcholine liposome. J. Ntr. Sci. Vitaminol. 1990;36:505–511. doi: 10.3177/jnsv.36.505. [DOI] [PubMed] [Google Scholar]
- 9.Stocker R., Bowry V.W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does α-tocopherol. Proc. Natl. Acad. Sci. U.S.A. 1991;88:1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takenaka M., Tatsukawa Y., Dohi K., Ezaki H., Matsukawa K., Kawasaki T. Protective effects of α-tocopherol and coenzyme Q10 on warm ischemic damages of the rat kidney. Transplantation. 1981;32:137–141. doi: 10.1097/00007890-198108000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Marubayashi S., Dohi K., Ezaki H., Hayashi K., Kawasaki T. Preservation of ischemic rat liver mitochondrial functions and liver viability with CoQ10. Surgery. 1982;91:631–637. [PubMed] [Google Scholar]
- 12.Sugino K., Dohi K., Yamada K., Kawasaki T. Changes in the levels of endogenous antioxidants in the liver of mice with experimental endotoxicemia and the protective effects of the antioxidants. Surgery. 1989;105:200–206. [PubMed] [Google Scholar]
- 13.Sumimoto K., Inagaki K., Ito H., Marubayashi S., Yamada K., Kawasaki T., Dohi K. Ischemic damage prevention by coenzyme Q10 treatment of the donor before orthotopic liver transplantation: biochemical and histological findings. Surgery. 1987;102:821–827. [PubMed] [Google Scholar]
- 14.Matsushima T., Sueda T., Matsuura Y., Kawasaki T. Protection by coenzyme Q10 of canine myocardial reperfusion injury after preservation. J. Thorac. Cardiovasc. Surg. 1992;103:945–951. [PubMed] [Google Scholar]
- 15.Amimoto T., Matsura T., Koyama S., Nakanishi T., Yamada K., Kajiyama J. Acetaminopheninduced hepatic injury in mice: the role of lipid peroxidation and effects of pretreatment with coenzyme Q10 and α-tocopherol. Free Radic. Biol. Med. 1995;19:169–176. doi: 10.1016/0891-5849(94)00233-a. [DOI] [PubMed] [Google Scholar]
- 16.Kettawan A., Takahashi T., Kongkachuichai R., Charoenkiatkul S., Kishi T., Okamoto T. Protective effect of coenzyme Q10 on decreased oxidative stress resistance induced by simvastatin. J. Clin. Biochem. Nutr. 2007;40:194–202. doi: 10.3164/jcbn.40.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niki E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990;186:100–108. doi: 10.1016/0076-6879(90)86095-d. [DOI] [PubMed] [Google Scholar]
- 18.Matsura T., Yamada K., Kawasaki T. Protective effects of coenzyme Q10 and α-tocopherol against free radical-mediated liver injury. Redox Rerort. 1995;1:343–347. doi: 10.1080/13510002.1995.11747009. [DOI] [PubMed] [Google Scholar]
- 19.Matsura T., Yamada K., Kawasaki T. Changes in the content and intracellular distribution of coenzyme Q homologs in rabbit liver during growth. Biochim. Biophys. Acta. 1991;1083:277–282. doi: 10.1016/0005-2760(91)90083-t. [DOI] [PubMed] [Google Scholar]
- 20.Matsura T., Yamada K., Kawasaki T. Antioxidant role of cellular reduced coenzyme Q homologs and α-tocopherol in free radical-induced injury of hepatocytes isolated from rats fed diets with different vitamin E contents. Biochim. Biophys. Acta. 1992;1127:277–283. doi: 10.1016/0005-2760(92)90232-k. [DOI] [PubMed] [Google Scholar]
- 21.Nishida T., Ohata S., Kusumoto C., Mochida S., Nakada J., Inagaki Y., Ohta Y., Matsura T. Zinc supplementation with polaprezinc protects mouse hepatocytes against acetaminophen-induced toxicity via induction of heat shock protein 70. J. Clin. Biochem. Nutr. 2010;46:43–51. doi: 10.3164/jcbn.09-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishida T., Matsura T., Nakada J., Togawa A., Kai M., Sumioka I., Minami Y., Inagaki Y., Ishibe Y., Ito H., Ohta Y., Yamada K. Geranylgeranylacetone protects against acetaminophen-induced hepatotoxicity by inducing heat shock protein 70. Toxicology. 2006;219:187–196. doi: 10.1016/j.tox.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Matsura T., Serinkan B.F., Jiang J., Kagan V.E. Phosphatidylserine peroxidation/externalization during staurosporine-induced apoptosis in HL-60 cells. FEBS Lett. 2002;524:25–30. doi: 10.1016/s0014-5793(02)02990-3. [DOI] [PubMed] [Google Scholar]
- 24.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 25.Schmelzer C., Lorenz G., Rimbach G., Döring F. In vitro effects of the reduced form of coenzyme Q10 on secretion levels of TNF-α and chemokines in response to LPS in the human monocytic cell line THP-1. J. Clin. Biochem. Nutr. 2009;44:62–66. doi: 10.3164/jcbn.08-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Åberg F., Appelkvist E.L., Dallner G., Ernster L. Distribution and redox state of ubiquinones in rat and human tissues. Arch. Biochem. Biophys. 1992;295:230–234. doi: 10.1016/0003-9861(92)90511-t. [DOI] [PubMed] [Google Scholar]
- 27.Beyer R.E., Segura-Aguilar J., Di Bernardo S., Cavazzoni M., Fato R., Fiorentini D., Galli M.C., Setti M., Landi L., Lenaz G. The role of DT-diaphorase in the maintenance of the reduced antioxidant form of coenzyme Q in membrane systems. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2528–2532. doi: 10.1073/pnas.93.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi T., Okuno M., Okamoto T., Kishi T. NADH-dependent coenzyme Q reductase is the main enzyme responsible for the reduction of non-mitochondrial CoQ in cells. BioFactors. 2008;32:59–70. doi: 10.1002/biof.5520320108. [DOI] [PubMed] [Google Scholar]
- 29.Lind C., Cadenas E., Hochstein P., Ernster L. DT diaphorase: purification, properties, and function. Methods Enzymol. 1990;186:287–301. doi: 10.1016/0076-6879(90)86122-c. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida Y., Hayakawa M., Habuchi Y., Niki E. Evaluation of the dietary effects of coenzyme Q in vivo by the oxidative stress marker, hydroxyoctadecadienoic acid and its stereoisomer ratio. Biochim. Biophys. Acta. 2006;1760:1558–1568. doi: 10.1016/j.bbagen.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Sohal R.S., Forster M.J. Coenzyme Q, oxidative stress and aging. Mitochondrion. 2007;75:S103–S111. doi: 10.1016/j.mito.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Åberg F., Appelkvist E.L., Dallner G., Ernster L. Uptake of dietary coenzyme Q is limited in rats. J. Nutr. 1995;125:446–453. doi: 10.1093/jn/125.3.446. [DOI] [PubMed] [Google Scholar]
- 33.Jin G., Kubo H., Kashiba M., Horinouchi R., Hasegawa M., Suzuki M., Sagawa T., Oizumi M., Fujisawa A., Tsukamoto H., Yoshimura S., Yamamoto Y. Saposin B is a human coenzyme Q10-binding/transfer protein. J. Clin. Biochem. Nutr. 2008;42:167–174. doi: 10.3164/jcbn.2008024. [DOI] [PMC free article] [PubMed] [Google Scholar]