Abstract
Hydrogen has been reported to have neuron protective effects due to its antioxidant properties, but the effects of hydrogen on cognitive impairment due to senescence-related brain alterations and the underlying mechanisms have not been characterized. In this study, we investigated the efficacies of drinking hydrogen water for prevention of spatial memory decline and age-related brain alterations using senescence-accelerated prone mouse 8 (SAMP8), which exhibits early aging syndromes including declining learning ability and memory. However, treatment with hydrogen water for 30 days prevented age-related declines in cognitive ability seen in SAMP8 as assessed by a water maze test and was associated with increased brain serotonin levels and elevated serum antioxidant activity. In addition, drinking hydrogen water for 18 weeks inhibited neurodegeneration in hippocampus, while marked loss of neurons was noted in control, aged brains of mice receiving regular water. On the basis of our results, hydrogen water merits further investigation for possible therapeutic/preventative use for age-related cognitive disorders.
Keywords: hydrogen water, magnesium stick, senescence, oxidative stress, cognitive disorder
Introduction
Many elderly individuals live with memory problems that are part of the normal aging process. Cognitive deficits, such as learning impairment and delayed amnesia, are striking, debilitating consequences of normal and pathological aging in humans. Multiple mechanisms are implicated in the development of age-associated memory impairment including age-related alterations in the central nervous system that may contribute to neuronal cell damage due to increased formation of reactive oxygen species (ROS) and a deteriorated antioxidant defense system [1, 2]. As the average life span continues to lengthen, it is critical to minimize the development of cognitive defects in elderly people.
Recent evidence suggests that molecular hydrogen potently protects the central nervous system by eliminating ROS [3]. Likewise, drinking hydrogen water (HW) prevented chronic physical restraint-induced impairments of learning tasks [4, 5]. More recently, hydrogen in drinking water reduced dopaminergic neuronal loss in a mouse model of Parkinson’s disease induced by administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [6]. Based on these observations, we hypothesized that oral intake of hydrogen via HW may reduce oxidative stress in the aging brain and ameliorate cognitive loss in aged mice.
In the present study, we have used a senescence-accelerated mouse (SAM) model, which is a well-accepted animal model of age-related cognitive impairment, to examine the influence of HW on brain activity and memory loss. SAMs were developed by selectively breeding AKR/J mice and are classified as senescence-prone or senescence-resistant based on senescence (as assessed by a scoring system) pathological phenotype, and lifespan [7, 8]. Senescence-accelerated prone mouse 8 (SAMP8) is an appropriate model of aging-associated senescence and has impairments in nonspatial learning and memory beginning as early as 2 months of age. The SAMP8 mice have a higher oxidative stress status that is partly caused by mitochondrial dysfunction and results in the excessive production of ROS and neurodegeneration [9]. Although protective effects of hydrogen have been postulated in several degenerative neuronal disease models, this study is the first report demonstrating the efficacies of hydrogen in a senescence-related cognitive disorder. Our method of HW production with a portable magnesium stick is safe, cost-effective and ideal for elderly people, as it can be administered without changing their lifestyle. Drinking HW daily may be a promising preventative approach for age-related neurodegenerative disease and have an enormous impact on future healthcare for the elderly.
Material and Methods
Hydrogen water
The magnesium stick used to produce hydrogen in the study is a plastic-shelled product consisting of metallic magnesium (99.9% pure) and natural stones in the polypropylene containers combined with ceramics (Doctor SUISOSUI®, Friendear, Tokyo, Japan). The product is capable of generating hydrogen when placed in drinking water by the following chemical reaction: Mg + 2H2O → Mg (OH)2 + H2. Hydrogen concentration in the water bottle was sequentially monitored using a hydrogen needle sensor (DHS-001, ABLE, Tokyo, Japan). Hydrogen concentration was maintained at levels between 0.55 and 0.65 mmol and pH was ranged between 7.7 and 8.3.
Animals
Male SAMP8/TaSlc and SAM resistant 1 (SAMR1/TaSlc mice), normal aging controls, were obtained from the Council for SAM Research via Japan SLC, Inc. (Hamamatsu, Japan). Mice were housed in conventional animal facilities with 12:12 light/dark cycle and were kept in an air-conditioned room maintained at 23 ± 1°C with humidity of 55 ± 5%. Either HW or regular water (RW; HW that consequently degassed by gently stirring for 24 h) was given ad libitum starting at the age of 8 weeks. All animal experiments were carried out under approved guidelines provided by the animal use committee at Suzuka University of Medical Science in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health.
Morris water maze test
Morris water maze test was performed for seven days after 30 days of continuous administration of HW [10]. The Morris water maze was a 0.5 m high and 1.5 m diameter circular tank made by wood and painted in black. The tank was filled with 23°C water to a depth of 16 cm and circled by a white cloth curtain with four different black cardboard shapes hung equidistantly. Milk powder was used to render the water opaque. A transparent Plexiglas platform (12.5 cm × 12.5 cm × 15 cm) was fixed in the southeast corner of the pool, 1 cm below the water surface. The starting location and the platform were located in quadrant area A and their locations did not change during all training sessions.
First, a single habituation (pretraining) trial was performed one day before the training session. Each mouse was placed into the water for 15 s and guided to the platform. Three trials were performed. Memory acquisition (training) trials with the hidden platform in the water maze were performed 24 h after the last pretraining trial. The memory acquisition trials consisted of 7 consecutive days of testing with 2 trials per day. Each mouse was placed at the middle of Quadrant area B, C or D of the pool, facing the wall, and the escape latency (the time spent swimming to find the platform) was recorded for 120 s. The interval of between trials was 60–90 min to let mice recover physical capacity. The sequence of water-entering points was different in each trial, although the location of the platform was fixed. If the mouse failed to find the platform within 120 s, mouse was manually guided to the platform and allowed to stay on the platform for 10 s; escape latency was assigned as 120 s. On the day 9, 24 h after the last training session, the mice were tested in the water maze for retention of spatial memory after the platform was removed (probe test). The probe test consisted of two measurements of performance: quadrant time (the time spent at the platform’s former location) and platform crossings (the number of crossings over the platform’s former location), higher scores in these measurements indicate better performance. Again, each mouse was placed at the starting point and observed for 120 s.
Superoxide dismutase (SOD)-like activity in blood
The superoxide dismutase (SOD)-like activity in the serum, obtained after completion of water maze test, was determined using the nitro-blue-tetrazolium method. Basically, the SOD activity of the sample was determined by measuring the inhibition rate of diformazan production according to the protocol provided by the manufacture (Wako Pure Cemical Industries, Osaka, Japan).
Total reactive antioxidant potential in blood
Luminol (Sigma, St. Louis, MO) was used to assess total reactive antioxidant potential and is one of the most commonly employed methods to estimate the antioxidant capacity of samples. This method is based on the quenching of luminol-enhanced chemiluminescence derived from the thermolysis of 2,2'-azo-bis (2-aminopropane)-dihydrochloride (AAPH, Aldrich Chemical, Milwaukee, WI) as the free radical source.
Lipid peroxidation in brain
To assess lipid peroxidation, thiobarbituric acid-reactive substance (TBA-RS) was measured as previously described [11]. Homogenized brain samples were prepared with 10% (w/v) of 1.15% potassium chloride (KCl), and supplemented with 1% phosphoric acid and 0.67% TBA chemical reagent. After incubation for 45 min in a 100°C water bath, n-butanol was added to the samples and the samples were cooled to room temperature. Following centrifugal separation (3,000 rpm, 10 min), absorbance of the supernatant at 535 nm and 520 nm was measured and lipid peroxidation was calculated according to the formula: Lipid peroxidation value (nmol/ml) = f/F × 10 nmol/ml brain cell homogenate, F: absorbance (A535-520) of a reference, f: absorbance (A535-520) of a specimen standard.
Intercerebral serotonin levels
Serotonin concentration in the brain was measured as previously described. In brief, homogenized brain was mixed with 0.2 N HClO4 (10 µl, 0.2 N HClO4/mg of tissue) using a supersonic wave crush. After centrifugal separation and neutralization, the supernatant was analyzed with EIA Serotonin Kit (IMMUNOTECH, Osaka, Japan).
Histopathological analysis
After long-term treatment with RW or HW for 18 weeks, the mice were deeply anesthetized with sodium pentobarbital, perfused transcardially with saline (1 mL/min) and then perfused with 10% buffered formalin for 15 min. The brain samples were post-fixed in 10% formalin for 24 h, stored in 10% sucrose in 0.1 M phosphate-buffered saline (PBS) for 4 h, 20% sucrose in 0.1 M PBS for 4 h and in 30% sucrose in 0.1 M PBS for overnight. Brain tissue was frozen in OCT (Optimal Cold Temperature, Sakura, Japan), cut into 9 µm sections and fixed with 50% ethanol for 30 min. Klüver-Barrera staining was performed and positively stained neurons in five individual high-power fields (400×) were counted.
Data analysis
Results were expressed as mean ± standard error. Statistical analysis was performed using analysis of variance (ANOVA, repeated measurements) as well as the F-test with Bonferroni post hoc group comparisons where appropriate. A probability level of p<0.05 was considered to be statistically significant.
Results
Drinking hydrogen water prevented loss of cognitive activity in aged SAMP8 mice
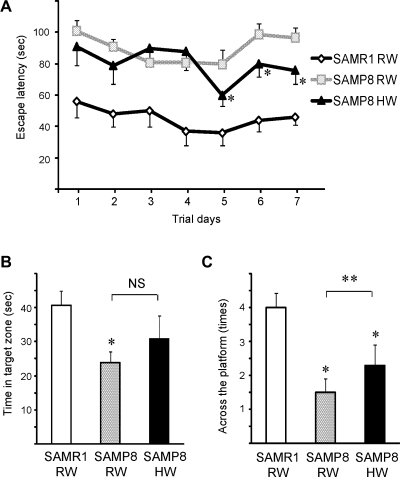
To evaluate spatial reference memory, the Morris water maze test to find a hidden platform in water was performed. SAMR1 mice and SMAP8 mice, both aged 8 weeks, were chronically treated with either RW or HW as drinking water for 30 days. No significant differences were observed in the swimming speeds of the SAMR1 mice and SMAP8 mice during the performance of pre-training and training trials (data not shown), indicating that motivational and sensory/motor influences on learning performance in the mice could be excluded.
During the 7-day course of acquisition training, arrival time to the platform of the SAMP8 mice was significantly delayed compared to that of SAMR1 regardless of which type of drinking water was administered. However, the escape latencies of SAMP8 mice treated with HW were significantly faster on days 5, 6 and 7 than the escape latencies of SAMP8 mice given RW, demonstrating that oral hydrogen intake ameliorated the cognitive deficits in SAMP8 (Fig. 1A). On day 8, following 7-day acquisition training, the quality of memory retention was also evaluated by time spent looking for the platform in a test where the platform had been removed. Both the time spent in the target zone (where the platform was located during training period) and the number of passes across the platform’s prior location indicated significant deterioration of the cognitive abilities of the SAMP8 mice. There was no statistically significant difference in time spent in the target zone between SAMP8 mice treated with HW and those treated with RW (Fig. 1B). However, the number of passes across the platform’s prior location was significantly higher in SAMP8 mice treated with HW compared with SAMP8 mice given RW, suggesting that HW prevented the loss of some cognitive abilities in the SAMP8 mice (Fig. 1C).
Fig. 1.
The effects of HW on behavior were assessed using the Morris water maze. Mice were trained in the water maze for 7 days. The escape latencies of SAMP8 mice treated with HW were significantly shorter than those of age-matched SAMP8 given degassed HW at trial days 5, 6 and 7 (A). Time in target zone (B) and number of passes across the platform’s prior location (C) based on the probe tests performed at day 8. (n = 6–8 for each group, *p<0.05, in comparison to age-matched SAMR1/RW, #p<0.05, SAMP8/RW vs SAMP8/HW; RW, regular water; HW, hydrogen water; NS, no significant difference).
Mice receiving daily hydrogen water ad libitum had increased serum antioxidant properties
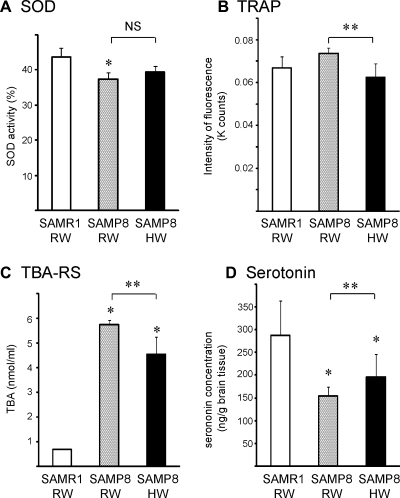
Serum SOD-like activity was significantly reduced in 13-week old SAMP8 mice treated with RW as compared with age-matched SAMR1 mice. Drinking HW for 30 days (from 8 to 12 weeks of age) had marginal effects on SOD-like activity in SAMP8 mice (Fig. 2A). Oxidation of luminol by AAPH-derived ROS was assessed to evaluate non-enzymatic antioxidant defenses using serum from the same time points. Less luminol-fluorescence in the AAPH-luminol system corresponds to more total reactive antioxidant potential, an indicator of non-enzymatic antioxidant defense. As shown in Fig. 2B, drinking HW for 30 days significantly activated antioxidant activity in the serum. Lipid peroxidation is particularly toxic to neurons because it alters cell membrane properties as well as the functions of membrane-bound receptors, ion channels, and signaling molecules [12]. Considering the possible antioxidant efficacies of HW, we evaluated brain lipid peroxidation in 13-week old mice after 30-day treatment with HW. The levels of TBA-RS, a marker of lipid peroxidation, were markedly elevated in the brains of SAMP8 mice compared with those in SAMR1 mice. Drinking HW significantly reduced lipid peroxidation in the brains of SAMP8 mice (Fig. 2C).
Fig. 2.
Serum analysis for SOD-like activity levels (A) and total reactive antioxidant potential assessed by the AAPH/luminol system (B) were performed using the serum taken after 30-day treatment with HW or RW. Brain TBA-RS (C) and serotonin (D) levels were also determined after 30-day treatment with HW or RW. (n = 4–6 for each group, *p<0.05, in comparison to age-matched SAMR1/RW, #p<0.05, SAMP8/RW vs SAMP8/HW; RW, regular water; HW, hydrogen water; NS, no significant difference; TRAP, total reactive antioxidant potential; TBA-RS, thiobarbituric acid-reactive substance).
Oral administration of hydrogen water prevented reduction of brain serotonin levels
Serotonin, a monoamine neurotransmitter, is synthesized in serotonergic neurons in central nervous system where has various roles including cognitive functions, such as learning and memory. Brain serotonin levels were significantly lower in SAMP8 mice compared with SAMR1 mice. This decrease of serotonin in the brain was significantly reduced in mice receiving HW orally for 30 days (Fig. 2D).
Drinking hydrogen water inhibited histopathological alterations of hippocampal CA1 and CA3
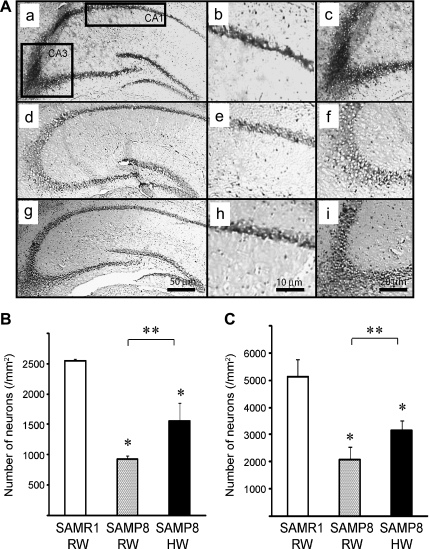
With aging, the brain undergoes synaptic loss in many areas, which has a large impact on cognitive decline. The hippocampus is necessary for several types of learning and memory formation in mammals [13]. Loss of hippocampal neurogenesis may attenuate cognitive functions [14]. Klüver-Barrera staining revealed neuron loss in 26-week old SAMP8 mice receiving RW. Treatment with HW for 10 weeks (between 8- and 26-weeks of age) significantly prevented neuronal loss in hippocampal areas CA1 and CA3 (Fig. 3A–C). Although amyloid is implicated in the pathogenesis of age-associated brain dysfunction [15], no amyloid deposits were observed on the hippocampus of 26-weeks old SAMP8 mice.
Fig. 3.
Panels (A) show representative photomicrographs of Klüver-Barrera’s stained brain sections of SAMR1 mice (a, b and c), SAMP8 mice treated with RW (d, e, and f) and SAMP8 mice treated with HW (g, h and i). Panels (b, e, and h) are representative images of CA-1 and panels (c, f, and i) are representative images typical images of CA-3, respectively. The number of Klüver-Barrera positive cells in CA-1 (B) and CA-3 (C) for each treatment group. (n = 4 for each group, *p<0.05, in comparison to age-matched SAMR1/RW, #p<0.05, SAMP8/RW vs SAMP8/HW; RW, regular water; HW, hydrogen water).
Discussion
Our data demonstrate that drinking HW, generated by magnesium stick, daily was effective in preventing age-related learning and memory impairments in a well-established animal model of accelerated senescence. Oral intake of drinking water containing a high concentration of hydrogen is a novel, safe and potent approach for preventing aging-related cognitive disorders.
Loss of cognitive abilities during aging, even in the absence of a specific neurodegenerative disease, is a complex process and disparate mechanisms have been proposed. However, oxidative stress, an imbalance between the levels of endogenous ROS and the antioxidant defense system, has been postulated to be a major cause of senescence-related cognitive decline [1, 16]. Oxidative stress on nervous tissue can produce damage by several interacting mechanisms, including increasing intracellular free calcium ions and release of excitatory amino acids. The central nervous system is particularly susceptible to oxidative stress due to its high oxygen consumption and low levels of antioxidant enzymes. Furthermore, the brain is rich in iron and copper, which catalyze the formation of highly reactive hydroxyl radicals from excess superoxide anions and hydroperoxide. This accelerates ROS reactions [17]. SAMP8 brain mitochondria demonstrate a higher redox state and more mitochondrial respiration with a lower respiration control ratio than the mitochondria of SAMR1 mouse brains, indicating that an inefficient hyperactive state can exist in the mitochondrial electron transport system before age-associated mitochondrial dysfunction develops [18].
Antioxidant therapeutic medical gas may be a reasonable approach for treatment of oxidative stress [19]. Hydrogen is one very promising gaseous agent that has come to the forefront of research during the last few years. In fact, there has been accumulating evidence that drinking HW reduces oxidative injury in various disease models. In this study, we demonstrate clear effects of HW on some markers of oxidative injury, such as brain TBA-RS and total reactive antioxidant potential as detected by the AAPH-luminol system in SAMP8 mice. Although apoptosis plays a role in some neurodenerative diseases, including Alzheimer’s disease [20] and Parkinson’s disease [21], Wu et al. reported that aging-related apoptosis is a minor occurrence in the senescent hippocampus of SAMP mice in the absence of external stimuli, and similar patterns of apoptosis were observed in SAMP8 and SAMR1 mice [22]. Indeed, we attempted to detect neuronal apoptosis by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay and saw very few TUNEL-positive cells in hippocampi of SAMP8 and SAMR1 mice (data not shown). Our results were consistent with the observation of Wu and colleagues and suggest that apoptosis is unlikely to be the cause of functional decline during aging in the SAMP 8 mice.
Animal models are inevitably used to study aging due to the limitations and individual differences in human subjects. Although several different, and sometimes overlapping, diseases occur with aging in humans, SAMP8 mice have been extensively studied using a variety of short-term and long-term memory tests and are recognized as an appropriate model to study alterations in general behavior and learning and memory impairments due to aging. The Morris water maze test is thought to be a sensitive assay for brain abnormalities, especially in the hippocampus [23].
Recent research on animals, as well as on humans, has been demonstrated that administration of antioxidants can attenuate cognitive decline. Lifestyle factors can influence the integrity of brain function during aging. Daily consumption of vegetables and fruits and weekly consumption of fish is associated with a decreased risk of dementia, as these foods contain a number of potentially neuroprotective substances, such as polyphenolic antioxidants [24, 25]. Likewise, vitamin E (α-tocopherol) was effective in preventing cognitive decline due to improvements in the cholinergic system and neurotransmission [26]. Dietary intervention with foods or drinking water may be ideal for elderly people because of its feasibility. As shown in the present study, the administration of hydrogen-rich water via a portable magnesium stick could easily be incorporated into routine life without complicating or changing lifestyle. On the basis of our results, we believe that hydrogen water merits further investigation for possible therapeutic/preventative use for age-related cognitive disorders.
Abbriviations
- AAPH
2,2'-azo-bis (2-aminopropane)-dihydrochloride
- ANOVA
analysis of variance
- HW
hydrogen water
- MDA
malondialdehyde
- OCT
Optimal Cold temperature
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- RW
regular water
- SAM
senescence-accelerated mouse
- SOD
superoxide dismutase
- TBA-RS
thiobarbituric acid-reactive substance
References
- 1.Harman D. Free-radical theory of aging. Increasing the functional life span. Ann. N.Y. Acad. Sci. 1994;717:1–15. doi: 10.1111/j.1749-6632.1994.tb12069.x. [DOI] [PubMed] [Google Scholar]
- 2.Villeponteau B., Cockrell R., Feng J. Nutraceutical interventions may delay aging and the age-related diseases. Exp. Gerontol. 2000;35:1405–1417. doi: 10.1016/s0531-5565(00)00182-0. [DOI] [PubMed] [Google Scholar]
- 3.Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Katsura K., Katayama Y., Asoh S., Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 4.Nagata K., Nakashima-Kamimura N., Mikami T., Ohsawa I., Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology. 2009;34:501–508. doi: 10.1038/npp.2008.95. [DOI] [PubMed] [Google Scholar]
- 5.Fu Y., Ito M., Fujita Y., Ito M., Ichihara M., Masuda A., Suzuki Y., Maesawa S., Kajita Y., Hirayama M., Ohsawa I., Ohta S., Ohno K. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett. 2009;453:81–85. doi: 10.1016/j.neulet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Fujita K., Seike T., Yutsudo N., Ohno M., Yamada H., Yamaguchi H., Sakumi K., Yamakawa Y., Kido M.A., Takaki A., Katafuchi T., Tanaka Y., Nakabeppu Y., Noda M. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of parkinson’s disease. PLoS. One. 2009;4:e7247. doi: 10.1371/journal.pone.0007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda T., Hosokawa M., Takeshita S., Irino M., Higuchi K., Matsushita T., Tomita Y., Yasuhira K., Hamamoto H., Shimizu K., Ishii M., Yamamuro T. A new murine model of accelerated senescence. Mech. Ageing. Dev. 1981;17:183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 8.Takeda T., Hosokawa M., Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of accelerated senescence. J. Am. Geriatr. Soc. 1991;39:911–919. doi: 10.1111/j.1532-5415.1991.tb04460.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiba Y., Shimada A., Kumagai N., Yoshikawa K., Ishii S., Furukawa A., Takei S., Sakura M., Kawamura N., Hosokawa M. The senescence-accelerated mouse (SAM): a higher oxidative stress and age-dependent degenerative diseases model. Neurochem. Res. 2009;34:679–687. doi: 10.1007/s11064-008-9812-8. [DOI] [PubMed] [Google Scholar]
- 10.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 11.Park E.C., Yoon J.B., Seong J.S., Choi K.S., Kong E.S., Kim Y.J., Park Y.M., Park E.M. Effect of ionizing radiation on rat tissue: proteomic and biochemical analysis. Prep. Biochem. Biotechnol. 2006;36:19–35. doi: 10.1080/10826060500388470. [DOI] [PubMed] [Google Scholar]
- 12.Sultana R., Perluigi M., Butterfield D.A. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid. Redox Signal. 2006;8:2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- 13.Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 14.Bruel-Jungerman E., Rampon C., Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev. Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- 15.Banks W.A., Farr S.A., Morley J.E., Wolf K.M., Geylis V., Steinitz M. Anti-amyloid beta protein antibody passage across the blood-brain barrier in the SAMP8 mouse model of Alzheimer’s disease: an age-related selective uptake with reversal of learning impairment. Exp. Neurol. 2007;206:248–256. doi: 10.1016/j.expneurol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller M., Grobbee D.E., Aleman A., Bots M., van der Schouw Y.T. Cardiovascular disease and cognitive performance in middle-aged and elderly men. Atherosclerosis. 2007;190:143–149. doi: 10.1016/j.atherosclerosis.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa T., Takahashi J.A., Fujibayashi Y., Fujisawa H., Zhu B., Nishimura Y., Ohnishi K., Higuchi K., Hashimoto N., Hosokawa M. An early stage mechanism of the age-associated mitochondrial dysfunction in the brain of SAMP8 mice; an age-associated neurodegeneration animal model. Neurosci. Lett. 1998;254:69–72. doi: 10.1016/s0304-3940(98)00646-6. [DOI] [PubMed] [Google Scholar]
- 19.Nakao A., Sugimoto R., Billiar T.R., McCurry K.R. Therapeutic Antioxidant Medical Gas. J. Clin. Biochem. Nutr. 2009;44:1–13. doi: 10.3164/jcbn.08-193R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotman C.W., Poon W.W., Rissman R.A., Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J. Neuropathol. Exp. Neurol. 2005;64:104–112. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- 21.Koller W.C., Cersosimo M.G. Neuroprotection in Parkinson’s disease: an elusive goal. Curr. Neurol. Neurosci. Rep. 2004;4:277–283. doi: 10.1007/s11910-004-0052-2. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y., Zhang A.Q., Wai M.S., Lai H.W., Wu S.X., Yew D.T. Changes of apoptosis-related proteins in hippocampus of SAM mouse in development and aging. Neurobiol. Aging. 2006;27:782 e1–782 e10. doi: 10.1016/j.neurobiolaging.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Skelton R.W., McNamara R.K. Bilateral knife cuts to the perforant path disrupt spatial learning in the Morris water maze. Hippocampus. 1992;2:73–80. doi: 10.1002/hipo.450020110. [DOI] [PubMed] [Google Scholar]
- 24.Barberger-Gateau P., Raffaitin C., Letenneur L., Berr C., Tzourio C., Dartigues J.F., Alperovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69:1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 25.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur. J. Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Asha Devi S. Aging brain: prevention of oxidative stress by vitamin E and exercise. Scientific World Journal. 2009;9:366–372. doi: 10.1100/tsw.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]