Abstract
Objectives
Despite high rates of hepatitis C virus (HCV) infection, relatively few current or former injection drug users receive evaluation and treatment for HCV. Here, we demonstrate the feasibility and effectiveness of integrating HCV care and methadone maintenance treatment (MMT). We hypothesized that colocation of these services would result in improved access to and utilization of HCV care.
Methods
In this retrospective observational study, all patient charts from a single MMT clinic were reviewed 2 years after HCV care and MMT were integrated. Information obtained included screening for and counseling about HCV infection status, on-site HCV treatment and outcomes, and demographic and substance abuse data.
Results
Two hundred ninety-one patient charts were reviewed. Two hundred eighty-one (99%) patients were screened for HCV antibody (HCV-Ab), and 188 (65%) were positive. Forty-nine (17%) patients were HIV/HCV coinfected. Ninety-eight percent of the HCV-Ab-positive patients received HCV counseling. Hundred fifty-nine (85%) of the HCV-Ab-positive patients were eligible to receive further evaluation and treatment for HCV on site, and 125 (78%) accepted. Hundred eighteen (94%) patients were tested for chronic HCV, and 83 were determined to have chronic HCV. Twenty-five patients received liver biopsy; low-stage disease was found in 7 patients. Twenty-one patients initiated HCV treatment. Sustained viral response was achieved in 8 patients. Seventeen patients had contraindications to HCV treatment. Further workup was prevented or delayed in 45 patients for various reasons, most commonly due to personal choice (29 patients).
Conclusions
This study demonstrates that current and former injection drug users can be engaged successfully in evaluation and treatment of HCV infection when these services are collocated with MMT.
Keywords: hepatitis C, injection drug users, methadone maintenance, HIV/hepatitis C virus coinfection
It is estimated that between 4 and 5 million people in the United States are infected with hepatitis C virus (HCV), and injection drug use is the principal risk factor driving this epidemic.1,2 Up to 90% of current and former injection drug users (IDUs) in the United States have been infected with HCV; however, relatively few have been treated.3 Current guidelines recommend that persons with chronic HCV infection be considered potential candidates for treatment, including patients with substance use or psychiatric disorders.4 A growing number of studies worldwide demonstrate that HCV treatment in current and former IDUs can be successful.5–13
Despite expressing interest in HCV treatment,14 opioid-dependent patients referred to hepatology clinics in the United States often do not have satisfactory outcomes.15–17 Prior studies have shown that linking substance abuse treatment with on-site primary medical care has improved outcomes for both tuberculosis and HIV.18,19 In a recent survey of methadone maintenance care providers, 31% expressed willingness to provide colocated hepatitis C treatment given proper training and resources.20 Methadone maintenance treatment programs (MMTPs) provide a unique opportunity to improve access to HCV care in a population with an extremely high prevalence of chronic HCV infection.21 The MMTP described in this study, located in Bronx, NY and affiliated with a major medical center, had been offering HCV antibody (HCV-Ab) screening since 2001. Despite a high prevalence of HCV infection and availability of referrals to the affiliated medical center and others in the area, we observed that few patients were accessing diagnosis and treatment of chronic HCV. We also noted increasing morbidity and mortality due to liver disease, accounting for approximately 25% of the deaths in our MMTPs from 2001 to 2002 (Cohen R. Medical Director, Division of Substance Abuse, Albert Einstein College of Medicine, personal communication). Therefore, we implemented a program of comprehensive HCV diagnosis and treatment by primary care providers, colocated with MMT. Here, we describe the outcomes after the program’s first 2 years at 1 MMTP clinic.
METHODS
Treatment Setting
The Division of Substance Abuse of the Department of Psychiatry and Behavioral Sciences at Albert Einstein College of Medicine operates 9 MMTP clinics in 4 Bronx communities, serving approximately 3400 adults with opioid dependence. All patients receive MMT for opioid dependence, and Medicaid-insured patients may also receive primary medical care (including HIV care) and psychiatric care. In one of these MMTP clinics, a program of comprehensive on-site hepatitis C care was initiated in July 2003. Before the program’s initiation, patients identified as HCV-Ab positive at the MMTP were referred for further evaluation to primary care or hepatology clinics at an affiliated hospital or other area medical centers.
HCV Clinical Protocol
The full-time medical staff in this clinic included 1 physician trained in internal medicine and 1 physician assistant. An on-site psychiatrist was available on a part-time basis. Additional support was provided by nursing and substance abuse counseling staff, who had received between one half and 2 full days of in-service training on HCV. Peer support groups to discuss HCV and other health issues were available to all patients. Patients were screened for the presence of antibodies to hepatitis A, B, and C viruses on admission to the MMTP. Medical staff met with patients for basic HCV counseling during MMTP admission, at annual physical exams, or as requested by patients. Basic counseling consisted of explanation of the patient’s HCV serostatus, education about transmission of and prevention from HCV infection, counseling to eliminate or decrease alcohol use, and the need for further evaluation for the diagnosis and treatment of chronic HCV, where appropriate. Basic HCV counseling also included offer of vaccination for hepatitis A and B, when indicated. Vaccines were provided free to the MMTP clinic by the New York City Department of Health.
Medicaid-insured patients testing positive for HCV-Ab were offered further evaluation and treatment, beginning with HCV viral load testing to diagnose chronic HCV infection. Referral to an outside hepatologist was offered to those who declined on-site care, to uninsured patients, and to those with medical insurance not accepted by the MMTP clinic. Patients diagnosed with chronic HCV (positive HCV viral load) were then considered for on-site treatment with pegylated interferon and ribavirin. Referral to an affiliated hospital for ultrasound-guided liver biopsy was offered but not required for initiation of HCV treatment, in accordance with current management guidelines.22 Patients were informed that liver biopsy provides information on progression of disease that may help the patient and provider decide whether to undergo treatment. HIV-negative patients with longstanding chronic HCV and biopsy-determined low-stage disease were generally counseled that treatment was not indicated. Patients with positive viral loads who declined a biopsy but desired HCV treatment were offered treatment if there were no contraindications. Patients with active drug or alcohol use, HIV/HCV coinfection, hepatitis B virus/HCV coinfection, current psychiatric illness, or compensated cirrhosis were all eligible for HCV treatment. Contraindications to treatment were determined by clinical judgment of medical providers on a case-by-case basis. The primary goals for completion of the HCV evaluation process are initiation of treatment when indicated, confirmation by liver biopsy that treatment is not indicated, or determination that treatment cannot be initiated due to contraindications (eg, unstable HIV disease).
Treatment
Our standardized HCV treatment protocol used treatment with once-weekly pegylated interferon in combination with twice daily ribavirin for either 24 or 48 weeks. Pegylated interferon α-2a or α-2b were dosed according to established guidelines.22 The dose of ribavirin was weight-based (1000 mg if ≤75 kg or 1200 mg if >75 kg). HCV-mono-infected patients with genotypes 2 or 3 were treated for 24 weeks; all others were treated for 48 weeks.
All patients attended the MMTP at least once weekly for methadone dosing, and medical and phlebotomy visits were flagged when patients checked in to the clinic. Most patients received interferon injections on-site by the physician or physician assistant, although some self-administered injections when patients were comfortable using needles and providers agreed adherence was likely. In general, ribavirin was self administered; dispensing along with methadone was possible but only used in one case.
Patients generally discontinued HCV treatment at 12 weeks if an early viral response (EVR, defined below) was not achieved. At monthly clinical evaluations, the following were assessed: adverse events, adherence, use of adequate contraception, monthly urine pregnancy tests, interval medical history, interval substance abuse (by urine toxicology and self-report), adequacy of methadone dose, and symptoms of depression. Resources available to minimize treatment discontinuations included HCV support groups, peer educators, substance abuse counselors, and use of hematologic growth factors if indicated.21,22 Clinic providers consulted with off-site HCV experts at the affiliated medical center to discuss patient care issues, if necessary. Patients could be referred to off-site hepatology clinics if medical issues surrounding treatment were deemed too complicated for treatment in the MMT setting, such as end-stage renal disease, or for evaluation for retreatment when treatment at the MMT was not successful.
Study Design
We conducted a retrospective chart review of all patients enrolled in 1 MMTP clinic from July 2003 to July 2005. The cohort was defined by including all patients enrolled in the MMTP as of July 1, 2003, plus all new patients admitted through December 15, 2004. Patients admitted to the MMTP after that date were excluded, because adequate time might not be available to complete the HCV evaluation and treatment initiation protocol by the end of the period under review. Details of hepatitis C evaluation and treatment were reviewed from July 2003 through July 2005, comprising the first 2 years the HCV clinical protocol was in place. A single reviewer used a standardized instrument to extract data from the MMTP medical record, which included all medical and substance abuse treatment notes. All patients were characterized as Hispanic (any race), white (not Hispanic) or African American (not Hispanic), according to admission data based on self-report. Charts were reviewed for records of services provided on-site, including receipt of basic HCV counseling, determination of on-site HCV evaluation, eligibility for HCV treatment, laboratory data, urine toxicology tests, liver biopsy results, and HCV treatment initiation and outcomes. In addition, information about any HCV care outside the MMTP during or before the review period was noted.
For patients who received HCV treatment on-site, rates of EVR (at least 2 log decrease in HCV viral load at 12 weeks), end of treatment response (undetectable viral load at 24 or 48 weeks for genotypes 2/3 and 1/4, respectively), and sustained viral response (SVR; undetectable viral load 24 weeks after completion of therapy) were determined. Final treatment outcomes were reviewed for all patients initiating on-site HCV treatment from July 2003 through July 2005. Because response to treatment is not available until up to 18 months after treatment initiation, treatment outcomes were determined by December 2006. The study was approved by the Albert Einstein College of Medicine Committee on Clinical Investigations.
RESULTS
Demographic Characteristics
We reviewed the charts of 291 patients, representing the entire clinic census for the period under review (Table 1). Sixty percent were men, 60% were Hispanic, 27% African American, and 13% were white. The mean patient age was 47 years. Fifty-seven (20%) of the patients in the clinic were HIV-positive, and 49 (17%) were coinfected with both HIV and HCV.
TABLE 1.
Demographics and Viral Parameters for All Patients, Regardless of Site of HCV Care
| n | % | |
|---|---|---|
| Male | 175 | 60 (175/291) |
| Race/ethnicity | ||
| African American | 38 | 13 (38/291) |
| Hispanic | 174 | 60 (174/291) |
| White | 79 | 27 (79/291) |
| Screen for HCV-Ab and basic HCV counseling | 289 | 99 (289/291) |
| HCV-Ab positive | 188 | 65 (188/289) |
| HCV viral load recorded | 139 | 74 (139/188) |
| Detectable HCV RNA | 98 | 71 (98/139) |
| Genotype recorded in chart | 81 | 83 (81/98) |
| Genotype 1 | 63 | 78 (63/81) |
| Genotype 2 or 3 | 18 | 22 (18/81) |
| HIV positive | 57 | 20 (57/291) |
| HIV positive/HCV-Ab positive | 49 | 17 (49/291) |
Total clinic census, n = 291.
HCV, hepatitis C virus.
HCV Status
During the study period, 289 (99%) of the patients had HCV-Ab testing and received basic HCV counseling (defined in HCV Clinical Protocol, described earlier) by a physician or a physician assistant, including offer of hepatitis A and B vaccination when indicated (Table 1). Hundred eighty-eight (65%) had positive HCV-Ab tests. Of these 188 patients, HCV viral load results were available for 139 (74%); 118 patients were tested on-site and 21 by outside physicians. Ninety-eight (71%) of the patients receiving a HCV viral load test had detectable HCV virus. The rate of spontaneous clearance of HCV infection was, therefore, 29% in this sample. Forty-two of the 49 HCV-Ab-positive patients lacking a measurement of HCV viral load did not receive HCV care at the MMTP for reasons described later. HCV genotype was recorded for 81 patients; 63 (78%) had genotype 1, and 18 (22%) had genotype 2 or 3.
On-Site HCV Care
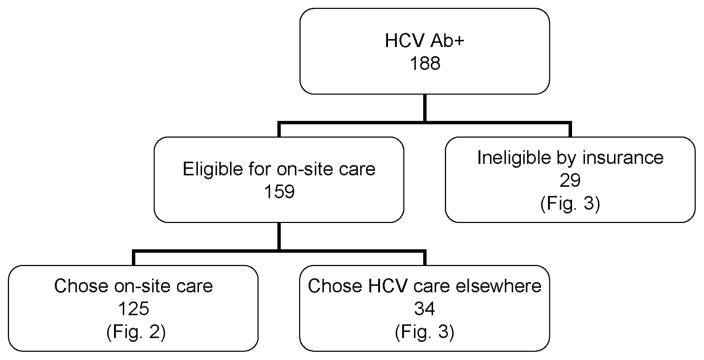
Of the188 HCV-Ab-positive patients, 159 (85%) were eligible for on-site HCV care based on their insurance status (insured by fee-for-service Medicaid or the Medicaid Managed Care Health Maintenance Organization in which the MMTP participated) (Fig. 1). Twenty-nine patients were not insurance eligible because they chose to remain in a noneligible Medicaid Health Maintenance Organization (16 patients), had other noneligible insurance (7 patients) or had no health insurance (6 patients). Thirty-four insurance-eligible patients (21%) elected to pursue HCV evaluation and treatment elsewhere.
FIGURE 1.
Site of hepatitis C virus (HCV) evaluation for HCV antibody-positive patients, n = 188.
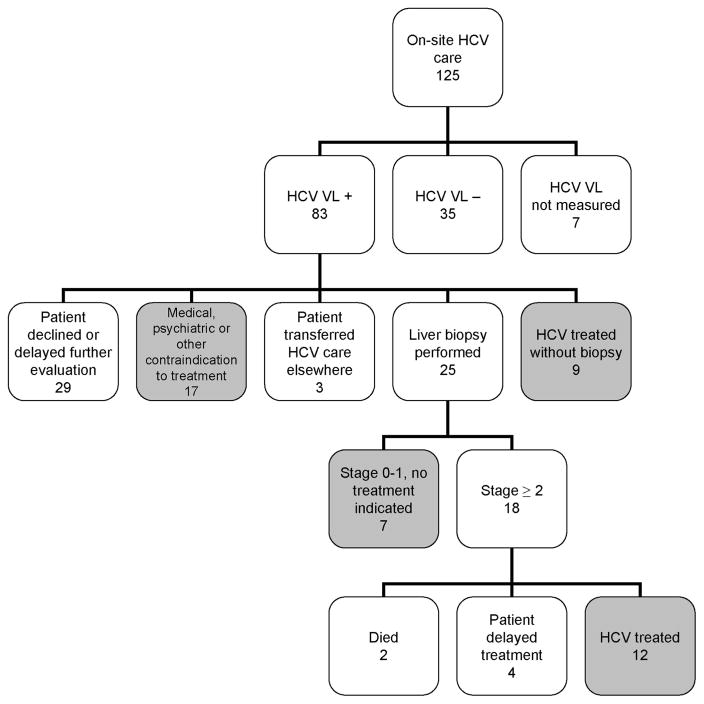
A total of 125 HCV-Ab-positive patients elected to receive on-site care at the MMTP (Figs. 1 and 2). Hundred eighteen patients (94%) had HCV viral load testing by the time of chart review and completed all or part of the HCV evaluation process. Of these 118 patients, 83 (70%) were diagnosed with chronic HCV infection, and 35 (30%) had spontaneous clearance of HCV. Of the 83 patients with chronic HCV, 24 (29%) were coinfected with HIV. Treatment with interferon-based chemotherapy or liver biopsy confirmation that treatment is not necessary was achieved within 2 years for 28 (34%) of the patients diagnosed with chronic HCV (Fig. 2, shaded boxes). Another 17 patients had contraindications to treatment as determined by medical providers; examples included unstable HIV disease, chronic kidney disease stage 3 or greater, unstable psychiatric disease, active alcohol use, and substance abuse affecting ability to adhere to treatment. Therefore, 45 patients (Fig. 2, shaded boxes) reached a primary goal for completion of HCV evaluation within 2 years, comprising 50% of the 90 patients potentially eligible for treatment (83 diagnosed with chronic HCV plus 7 patients whose viral loads were not determined; Fig. 2). One of the patients receiving on-site HCV care had undergone viral load testing, liver biopsy, and interferon-based treatment by an outside hepatologist before July 2003. This patient had virologic failure and was retreated at the MMTP, achieving an SVR.
FIGURE 2.
Outcomes of on-site hepatitis C virus (HCV) evaluation and treatment, n = 125. Shaded boxes comprise the 45 patients who reached a primary goal for completion of the HCV evaluation process.
Of the 83 patients with chronic HCV receiving on-site care, 25 had a liver biopsy by the date of chart review. Seven patients did not require treatment for HCV on the basis of very low stage disease (stage 0 or 1 by Ishak fibrosis scale).23 Three biopsies indicated cirrhosis (Ishak stage 6). Of the 18 patients with stage 2 to 6 fibrosis, 12 initiated treatment, 4 had elected to delay starting treatment by the time of chart review, 1 died of lung cancer, and 1 died during a psychiatric hospital admission (exact cause of death was not available).
Twenty-nine patients chose to decline or delay HCV treatment or biopsy after learning their HCV viral load test result, and the issue was readdressed by medical staff at least annually. It is expected that some of these patients will eventually agree to undergo biopsy or treatment for HCV. Reasons for declining or delaying included fear of treatment side effects, fear of pain from biopsy, desire to attend to personal and family issues before further evaluation, and denial that HCV disease is an important health problem. Three patients initially choosing on-site HCV care elected to transfer their HCV care elsewhere after the diagnosis of chronic HCV by viral load testing at the MMTP (Fig. 2). None of these 3 patients had received further workup by the date of chart review.
Twenty-one of the 83 patients with chronic HCV had initiated HCV treatment on-site by the date of chart review, and 7 (33%) of these were HIV and HCV coinfected (Table 2). Two of the coinfected patients were infected with HCV genotype 2, and the other 5 had genotype 1 HCV. Nine patients initiated treatment without having a liver biopsy (including one who had already had a biopsy in 1999, mentioned earlier). SVR was achieved in 8 patients (38% of those treated), of whom 5 were coinfected, and 6 had genotype 1 HCV. The racial and ethnic breakdown of patients treated and achieving SVR mirrored the entire cohort (Tables 1 and 2). Nine patients tolerated treatment but it was unsuccessful: 6 failed to achieve EVR at 12 weeks and treatment was stopped, and 3 achieved end of treatment response but had virologic rebound 6 months after completion of treatment. Four others stopped treatment for the following reasons: reversible renal failure due to ribavirin-induced anemia, persistent neutropenia in the setting of a skin abscess and pneumonia, suicidal ideation, and loss of health insurance coupled with exacerbation of polysubstance abuse.
TABLE 2.
Characteristics of Patients Treated On-Site and Treatment Outcomes, n = 21
| N (%) | |
|---|---|
| HCV monoinfection | 14 (67%) |
| Genotype 1 | 9 |
| Genotype 2 | 5 |
| HIV/HCV coinfection | 7 (33%) |
| Genotype 1 | 5 |
| Genotype 2 | 2 |
| Race/ethnicity | |
| African American | 2 (9%) |
| Hispanic | 13 (62%) |
| White | 6 (29%) |
| SVR total | 8 (38% of 21) |
| Coinfected HIV/HCV genotype 1 | 3 (60% of 5) |
| Coinfected HIV/HCV genotype 2 | 2 (100% of 2) |
| Monoinfected HCV genotype 1 | 3 (33% of 9) |
| Monoinfected HCV genotype 2 | 0 (0% of 5) |
| HCV genotype 1 total | 6 (43% of 14) |
| African American | 0 (0% of 2) |
| Hispanic | 6 (46% of 13) |
| White | 2 (33% of 6) |
| No EVR | 6 (29%) |
| ETR but virologic rebound | 3 (14%) |
| Stopped treatment early | 4 (19%) |
| Illicit substance use during treatment | 7 (33%) |
HCV, hepatitis C virus; SVR, sustained virologic response; EVR, early virologic response; ETR, end of treatment response.
Substance Use and Psychiatric Illness in Patients Treated On-Site for HCV
Of the 21 patients who initiated on-site HCV treatment, 10 (48%) reported use of illicit substances within 6 months before starting interferon-based therapy. Seven (33%) reported illicit drug use during therapy. Only one of these patients had to stop HCV treatment when substance abuse problems affected her ability to adhere to treatment. Seven (33%) reported a history of alcohol dependence, but no patients reported use of alcohol during treatment. Fourteen of the treated patients (67%) had a psychiatric disorder diagnosed by either the internist or psychiatrist before initiation of HCV treatment, and 13 (93%) of these saw a psychiatrist for mental health care during HCV treatment. Preexisting disorders included depression, generalized anxiety disorder, post-traumatic stress disorder, and attention deficit hyperactivity disorder.
Off-Site HCV Care
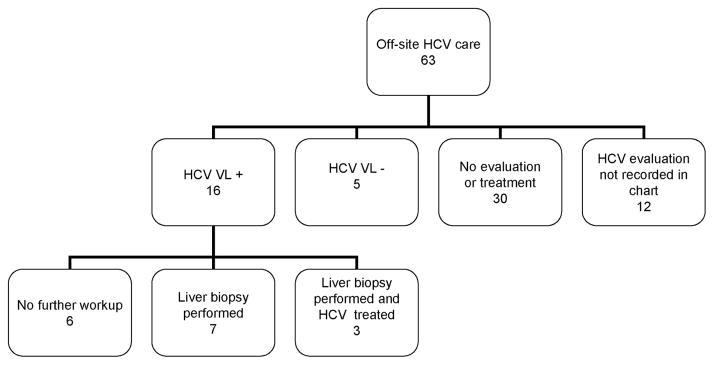
The 63 patients who were ineligible for on-site HCV care because of health insurance status or who chose to receive care off-site all received basic HCV counseling at the MMTP (described in HCV Clinical Protocol above). In many cases, laboratory data and patient self-reports of HCV care were recorded in the medical record, so some outcomes could be assessed (Fig. 3). These patients were referred by MMTP medical staff to outside primary care providers to access HCV care, and patients were informed that care at public hospitals is available to uninsured patients. Twenty-one patients (33%) were known to have initiated HCV care, including viral load measurement to diagnose chronic HCV. Of these, 7 had liver biopsies (6 stage 2 or higher), and 3 had been treated with interferon-based HCV therapy; SVR status was not available for those who received treatment. Thirty of these patients received no further evaluation or treatment for HCV outside the MMTP. Whether the remaining 12 patients sought further HCV care after basic counseling at the MMTP was not recorded.
FIGURE 3.
Outcomes of off-site HCV evaluation and treatment, n = 63; 34 patients were eligible for on-site HCV care but chose to receive care elsewhere, and 29 patients were not eligible for on-site care because of their health insurance coverage.
DISCUSSION
This study demonstrates that current and former IDUs can be engaged successfully in evaluation and treatment for HCV infection when these services are colocated with MMT. In our cohort of 188 HCV-Ab-positive patients, 118 patients had HCV care initiated on-site with viral load testing. Therefore, the MMTP program took the first step in HCV disease evaluation for 85% of the 139 HCV-Ab-positive clinic patients who had received HCV viral load testing by the time of chart review. Basic HCV counseling provided by the MMTP may also have contributed to the decision of 22 patients to seek HCV care elsewhere, although this effect was not measured. That 34 of 83 (41%) of the patients with chronic HCV who chose on-site care received liver biopsy and/or initiated interferon-based therapy in a relatively short time compares favorably with other published studies of comparable populations. In a Cleveland, Ohio study of an urban liver clinic in which only 66% of subjects had a history of injection drug use, 28% of 293 eligible patients received treatment for HCV.15 In a Bronx study of 228 opioid-dependent patients offered expedited referrals to liver specialists for HCV evaluation and treatment, only 54 (28%) kept appointments with a hepatologist, and 6 (3%) were treated.16 Because the patient population in the latter study was very similar to the cohort under review here, it seems unlikely that outside referral would have resulted in as high a rate of HCV evaluation as we have demonstrated.
Despite high rates of HIV coinfection, psychiatric comorbidity, and substance abuse in our sample, HCV treatment outcomes compare favorably with those found in clinical studies.24 –29 In this small sample, the rate of treatment success (SVR) in HIV/HCV coinfected patients is high. In studies with rigorous exclusion criteria not applied here, only 14% to 29% of coinfected patients with genotype 1, and 62% to 73% of coinfected patients with genotype 2 achieved SVR,26,27 compared with 60% and 100% in our study. Similarly, aggregate treatment success for genotype 1 patients treated here (43%) was comparable with SVR rates reported in clinical studies (42%– 46%).24,25 We observed an anomalously low treatment success rate in patients with genotype 2 HCV monoinfection (typically 80%),24 most of whom stopped treatment due to side effects or reasons other than treatment failure. It is notable that the majority of the study cohort and the group treated on-site were African American or Hispanic. Studies have demonstrated that nonwhite patients have lower rates of treatment success.28,29
We have demonstrated that integrating HCV care and MMT successfully reaches a population of patients who are far less likely to access traditional hepatology services. Co-location of HCV care and MMT removes many barriers to HCV care. Patients in MMT are automatically determined to be at high risk for HCV infection and receive screening; they may not reveal substance abuse history to other medical providers who may never become aware of their infection status. Many primary care providers may defer diagnosis of chronic HCV by viral load testing, and most defer treatment to specialists; on-site treatment removes the barrier of keeping an appointment with a hepatologist. On-site providers with access to colocated substance abuse, primary medical and psychiatric care may feel more comfortable initiating treatment in patients with substance abuse, medical and psychiatric comorbidities than outside specialists might. Adherence to HCV chemotherapy and monitoring of side effects is facilitated by frequent MMTP visits and on-site administration of interferon injections.
It is also important to note that even the patients with chronic HCV who did not achieve one of the primary goals for completion of HCV evaluation by the end of the study period (treatment, biopsy demonstration that treatment is not indicated, or determination that treatment is contraindicated) may have benefited from the HCV evaluation process. Patients may become more motivated to stabilize HIV treatment or mitigate substance abuse if they want to receive potentially curative treatment for HCV infection. Although not quantified here, we expect that in the future some of the patients deferring biopsy or treatment will eventually pursue treatment as they learn more about HCV and are in contact with others who have undergone treatment.
A limitation of this study is that there is no control group to compare outcomes achieved in the on-site HCV care program with usual care. However, it is notable that only 1 of the patients receiving care on-site had received treatment of HCV before the introduction of the program in July 2003. None of the 125 patients receiving on-site HCV care, with one exception, had received appropriate evaluation of HCV by any healthcare provider despite high risk for HCV infection. It is also useful to compare the outcomes of the 63 HCV-Ab-positive patients receiving HCV care outside the MMTP. This heterogeneous group included patients with greater engagement with health care outside the MMTP who accessed hepatologists independently, as well as uninsured patients for whom extra effort was required to access the care available to them in the public hospital system. By the end of the review period, only one third of these patients were known to have received HCV evaluation (Fig. 3), compared with 94% of those evaluated on-site with viral load testing. The remaining two thirds of the patients receiving off-site care either had no further HCV care or information about HCV care was not recorded in the MMTP chart.
CONCLUSIONS
We have demonstrated that specialized HCV treatment services can be provided successfully by trained generalists in a community-based substance abuse treatment setting. The frequent patient contact necessitated by MMT guidelines is well suited for ongoing monitoring of other intensive medical regimens, such as HCV treatment. Our study supports the notion that colocation of services is likely to be more effective at reaching socioeconomically challenged populations with a variety of comorbidities. Given the success of the initial programs, we now routinely offer HCV care to patients receiving primary medical care at all MMTPs in our system. Randomized prospective trials, such as one underway in Syracuse, New York,30 will permit direct comparisons of rates of HCV evaluation, treatment acceptance, and success in the MMT setting versus usual modes of medical care delivery.
Acknowledgments
Supported by NIH Grant R25 DA 14551 and Center for AIDS Research Grant P30 A151519 (awarded to the Albert Einstein College of Medicine of Yeshiva University), New York State Office of Alcoholism and Substance Abuse Services Grant C-002464 (Division of Substance Abuse), New York State Department of Health AIDS Institute (HIV primary care program), and Centers for Disease Control and Prevention Grant CDC U50 CCU22419201 (HCV support groups).
The authors thank Roy Cohen, MD, Kennedy Okyere, RPA, Ira Marion, MS, and Sarah Church, PhD, for their expertise and/or support.
References
- 1.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Edlin BR, Carden MR. Injection drug users: The overlooked core of the hepatitis C epidemic. Clin Infect Dis. 2006;42:673–676. doi: 10.1086/499960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephenson J. Former addicts face barriers to treatment for HCV. JAMA. 2001;285:1003–1005. doi: 10.1001/jama.285.8.1003. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health. National Institutes of Health Consensus Development Conference Statement, Management of Hepatitis C. Bethesda, MD: National Institues of Health Consensus; ; 2002. pp. 1–44. [Google Scholar]
- 5.Backmund M, Meyer K, Von Zielonka M, et al. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 6.Sylvestre DL. Treating hepatitis C in methadone maintenance patients: An interim analysis. Drug Alcohol Depend. 2002;67:117–123. doi: 10.1016/s0376-8716(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 7.Dalgard O, Bjoro K, Hellum K, et al. Treatment of chronic hepatitis C in injecting drug users: 5 years’ follow-up. Eur Addict Res. 2002;8:45–49. doi: 10.1159/000049487. [DOI] [PubMed] [Google Scholar]
- 8.Van Thiel DH, Anantharaju A, Creech S, et al. Response to treatment of hepatitis C in individuals with a recent history of intravenous drug abuse. Am J Gastroenterol. 2003;98:2281–2288. doi: 10.1111/j.1572-0241.2003.07702.x. [DOI] [PubMed] [Google Scholar]
- 9.Cournot M, Gilbert A, Castel F, et al. Management of hepatitis C inactive drug users: Experience of an addiction care hepatology unit. Gastroenterol Clin Biol. 2004;28:533–539. doi: 10.1016/s0399-8320(04)95008-7. [DOI] [PubMed] [Google Scholar]
- 10.Mauss S, Berger F, Goelz J, et al. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40:120–124. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- 11.Huber M, Weber R, Oppliger P, et al. Interferon alpha-2a plus ribavirin 1,000/1,200 mg versus interferon alpha-2a plus ribavirin 600 mg for chronic hepatitis C infection in patients on opiate maintenance treatment: An open-label randomized multicenter trial. Infection. 2005;33:25–29. doi: 10.1007/s15010-005-4043-2. [DOI] [PubMed] [Google Scholar]
- 12.Sylvestre DL. Treating hepatitis C virus infection in active substance users. Clin Infect Dis. 2005;40:S321–S324. doi: 10.1086/427447. [DOI] [PubMed] [Google Scholar]
- 13.Robaeys G, Van Vlierberghe H, Mathei C, et al. Similar compliance and effect of treatment in chronic hepatitis C resulting from intervenous drug use in comparison with other infection causes. Eur J Gatroenterol Hepatol. 2006;18:159–166. doi: 10.1097/00042737-200602000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Walley AY, White MC, Kushel MB, et al. Knowledge of and interest in hepatitis C treatment at a methadone clinic. J Subst Abuse Treat. 2005;28:181–187. doi: 10.1016/j.jsat.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Falck-Ytter Y, Kale H, Mullen KD, et al. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136:288–292. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 16.Fishbein DA, Lo Y, Reinus JF, et al. Factors associated with successful referral for clinical care of drug users with chronic hepatitis C who have or are at risk for HIV infection. J Acquir Immune Defic Syndr. 2004;37:1367–1375. doi: 10.1097/01.qai.0000131932.21612.49. [DOI] [PubMed] [Google Scholar]
- 17.Schackman BR, Teixeira PA, Beeder AB, et al. Offers of hepatitis C care do not lead to treatment. J Urban Health. 2007;84:455–458. doi: 10.1007/s11524-007-9180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selwyn PA, Feingold AR, Iezza A, et al. Primary care for patients with human immunodeficiency virus (HIV) infection in a methadone maintenance treatment program. Ann Intern Med. 1989;111:761–763. doi: 10.7326/0003-4819-111-9-761. [DOI] [PubMed] [Google Scholar]
- 19.Gourevitch MN, Wasserman W, Panero MS, et al. Successful adherence to observed prophylaxis and treatment of tuberculosis among drug users in a methadone program. J Addict Dis. 1996;15:93–104. doi: 10.1300/J069v15n01_07. [DOI] [PubMed] [Google Scholar]
- 20.Litwin AH, Kunins HV, Berg KM, et al. Hepatitis C management by addiction medicine physicians: Results from a national survey. J Subst Abuse Treat. 2007;33:99–105. doi: 10.1016/j.jsat.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litwin AH, Soloway I, Gourevitch MN, et al. Integrating services for injection drug users infected with hepatitis C virus with methadone maintenance treatment: Challenges and opportunities. Clin Infect Dis. 2005;40:S339–S345. doi: 10.1086/427450. [DOI] [PubMed] [Google Scholar]
- 22.Strader DB, Wright T, Thomas DL, et al. AASLD Practice Guideline: Diagnosis, management and treatment of chronic hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 23.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 24.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon lafa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomized trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 25.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 26.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 27.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bräu E, Bini EJ, Currie S, et al. Black patients with chronic hepatitis C have a lower sustained viral response rate than non-Blacks with genotype 1, but the same with genotypes 2/3, and this is not explained by more frequent dose reductions of interferon and ribavirin. J Viral Hepat. 2006;13:242–249. doi: 10.1111/j.1365-2893.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Torres M, Jeffers LJ, Sheikh MY, et al. Peginterferon alfa-2a and ribavirin in Latino and non-Latino Whites with hepatitis C. New Engl J Med. 2009;360:257–267. doi: 10.1056/NEJMoa0805062. [DOI] [PubMed] [Google Scholar]
- 30.Batki SL. Improving hepatitis C treatment in injection drug users. NIDA clinical trial; identifier NCT00148031. Available at: www.clinicaltrials.gov.