Abstract
Endosome trafficking and function require acidification by the vacuolar ATPase (V-ATPase). Electrogenic proton (H+) transport reduces the pH and creates a net positive charge in the endosomal lumen. Concomitant chloride (Cl−) influx has been proposed to occur via ClC Cl−/H+ exchangers. This maintains charge balance and drives Cl− accumulation, which may itself be critical to endosome function. Production of reactive oxygen species (ROS) in response to cytokines occurs within specialized endosomes that form in response to receptor occupation. ROS production requires an NADPH oxidase (Nox) and the ClC-3 Cl−/H+ exchanger. Like the V-ATPase, Nox activity is highly electrogenic, but separates charge with an opposite polarity (lumen negative). Here we review established paradigms of early endosomal ion transport focusing on the relation between the V-ATPase and ClC proteins. Electrophysiologic constraints on Nox-mediated vesicular ROS production are then considered. The potential for ClC-3 to participate in charge neutralization of both proton (V-ATPase) and electron (Nox) transport is discussed. It is proposed that uncompensated charge separation generated by Nox enzymatic activity could be used to drive secondary transport into negatively charged vesicles. Further experimentation will be necessary to establish firmly the biochemistry and functional implications of endosomal ROS production. Antioxid. Redox Signal. 11, 1335–1347.
Introduction
Tremendous diversity exists among pools of intracellular vesicles. These compartments may appear to be dispersed randomly through the cytoplasm but are often localized to specific subcellular regions. As described elsewhere in this issue of Antioxidants & Redox Signaling, intracellular vesicles can have very different and highly specialized functions. Vesicle subtypes are identified and characterized by their lipid and protein composition. For example, the presence of vesicle-specific Rab guanosine triphosphatases (Rab GTPases), which contribute to the regulation of membrane traffic, distinguish early (Rab5) from late (Rab7) or recycling (Rab11) endosomes (36). Intravesicular pH and Cl− concentrations vary widely between these compartments (64, 92). These differences are likely to have important functional implications and to reflect differences in the presence or activity of specific ion-transport mechanisms.
The critical role of endosome acidification in membrane trafficking has long been appreciated (64). The vacuolar ATPase (V-ATPase), an electrogenic proton pump, is primarily responsible for this process (Fig. 1A). Secondary transport into vesicles can be driven by either the pH gradient or the charge separation generated by the V-ATPase. As vesicles mature from early to late endosomes and then to lysosomes, the free proton concentration increases by more than 2 orders of magnitude (pH decreases by 2 units) (25).
FIG. 1.
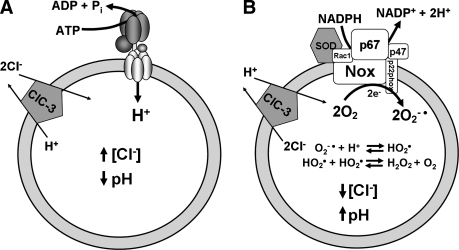
(A) The V-ATPase moves protons into endosomes by using energy derived from ATP hydrolysis. This results in net positive charge accumulation in the lumen of the vesicle. (B) The NADPH oxidase (Nox) transfers electrons from reduced NADPH to cytoplasmic FAD and then through two heme groups. In the lumen of the endosome, the electrons are passed to molecular oxygen to form two molecules of superoxide anion. This results in net negative charge accumulation. After regeneration of NADPH by the pentose phosphate shunt, two protons are created in the cytoplasm. The Nox protein NADPH complex is depicted without the associated membrane and cytoplasmic proteins that are required for function.
Just like the plasma membrane, the lipid bilayer of endosomes creates constraints on ion transport that are related to charge separation and may actually be magnified by the small volume of the endosome. In the absence of compensatory current flow, active transport of protons into the endosome by the V-ATPase creates a net positive intravesicular charge with respect to the cytoplasm. The higher the input resistance of the endosomal membrane, the greater the degree of charge separation that will develop. This resistance depends on the specific types and activities of ion channels and transporters in the membrane.
Ion channels are trafficked by a variety of mechanisms and therefore must cluster in specific regions of the membrane before internalization [see (48) and (49) for reviews]. Depending on the activity of these internalized channels, endosome membrane characteristics and input resistance may differ significantly from that of the plasma membrane. Because of the very small size of these vesicles, the magnitude of this resistance cannot be directly assessed.
Large potential differences across the vesicular membrane can be used to drive secondary transport, but may also limit the activity of the transporter that generated the gradient. For instance, V-ATPase activity is inhibited at positive intravesicular potentials, and this retards acidification. Cl− movement into vesicles facilitates acidification by providing an electrical shunt that can compensate for charge separation created by the V-ATPase. This process has been extensively modeled, and voltage gating of this Cl− conductance is critical (33). In support of this concept, acidification of clathrin-coated vesicles has been shown to be inhibited by either anion channel blockers or replacement of Cl− with impermeant anions (92, 93, 104).
Specifically, members of the ClC-3, -4, and -5 and the ClC-6 and -7 branches of the ClC family of anion channels/transporters have been proposed to participate in this process [40, 41, 55, 95, 96; see (46) for review]. The ClC family includes nine members (ClC-1 through 7, ClC-Ka, and ClC-Kb) (47). The ClC-3, -4, and -5 branch of the family is the most closely related to the more primitive ClCs (ClC-ec1 in Escherichia coli, GEF-1 in yeast). ClC-3 is expressed in virtually every cell type, and the amino acid sequence is 99.7% conserved from mouse to human. These observations suggest that the intracellular ClC proteins in general and ClC-3, in particular, serve a very basic and critical function.
A subgroup of Rab5-positive early endosomes contains NADPH oxidases (54, 65). These vesicles are formed as cytokine receptors are endocytosed and have been termed “signaling endosomes” (65, 105) or “redoxosomes” (Oakley et al., this issue of ARS). ROS produced in this compartment play a critical role in cytokine signaling in epithelial cells (54), neutrophils (67, 68) and vascular smooth muscle cells (65).
The catalytic subunits of NADPH oxidases, the Nox homologues, are electrogenic electron pumps, passing electrons from NADPH on the cytosolic side of the membrane to a flavin adenine dinucleotide (FAD), through two heme groups located within transmembrane domains, and finally to molecular oxygen located on the intravesicular side of the membrane (Fig. 1B). Like the V-ATPase, NADPH oxidases are capable of separating large amounts of charge if current flow is uncompensated. In the plasma membrane of neutrophils, charge compensation is provided by a proton channel (20). If this pathway is inhibited, Nox2 activation can depolarize the plasma membrane by as much as +180 mV (23). It is fascinating that, just as in vesicle acidification by the V-ATPase, intracellular Nox activity and cytokine signaling also are highly dependent on the presence of ClC-3 (65, 67, 68).
Very little is known as to how electron flow through Nox enzymes affects the electrophysiology of the signaling endosome. However, based on the electrogenic nature of Nox enzymes, the membrane potential of endosomes containing an active NADPH oxidase must differ markedly from that of endosomes lacking an oxidase. It is unclear how a dominantly negative intravesicular charge will alter the biology of the compartment. It has been presumed that the primary function of endosomal ROS generation is to initiate a redox-based “signal.”
However, it is important also to consider the possibility that the transmembrane potential difference generated by Nox activity could be used to drive secondary transport and thereby alter the composition of the lumen. In addition, the abundance and type of ROS produced will determine the redox potential of the compartment. Therefore, Nox activity may generate a ROS “signal,” modify the composition of endosomal electrolytes, and change the function of endosomal proteins. The requirement of protons for ROS metabolism suggests that endosome pH is critical, and the interplay between proton and electron movement into endosomes is likely to be very complex.
Much of what is known about ion transport across membranes of intracellular vesicles has been learned by using ion- or voltage-sensitive fluorescent dyes, either in intact cells or in purified vesicle preparations (93). These types of experiments have significant inherent limitations. Whole-cell experiments preclude control of cytoplasmic signaling events and ion concentrations, and vesicular transport is subject to the dynamic changes in cytoplasmic environment that are associated with cellular metabolism. Studies using purified vesicles are easier to control; however, critical factors affecting ion-transporter activity may be absent, or present in the wrong concentration. Localization of Nox proteins to the early endosome has only recently been appreciated. Therefore, previous studies using purified endosomal vesicles have not addressed the contribution of Nox proteins to the biology of the compartment, because no reason existed to include NADPH as substrate in these experiments.
This review focuses on the biophysical challenges and constraints associated with generation of ROS in endosomes. We explore mechanisms of endosomal acidification and consider the potential impact of ROS production by NADPH oxidases on these processes. Specific attention is paid to anion transport, charge compensation, and the dependence of both acidification and ROS production on ClC-3. Issues related to Nox-dependent generation of ROS in endosomes may also be relevant to other subcellular compartments in which the NADPH oxidase is activated.
Membrane Potential
The transmembrane potential of cytoplasmic vesicles has been extensively modeled, primarily with a focus on the interdependence of voltage and acidification (33, 85). Principal influences that have been considered include (a) the physical properties of the vesicle, such as size, shape, buffering capacity and lipid composition; (b) electrogenic ion transport (the V-ATPase and the Na-K ATPase); and (c) passive ion conductance.
The surface area–to-volume ratio of a vesicle directly affects ion transport. Smaller endosomes have a relatively larger surface area–to-volume ratio that will reduce membrane potential for a given charge density (85). Estimates of the diameter of early endosomes range between 100 and 500 nm (13, 70). For the purposes of this review, we base our calculations on vesicles with a diameter of 200 nm. During endocytosis, both soluble extracellular macromolecules and integral membrane proteins are incorporated into vesicles. These molecules tend to be negatively charged and have ionizable groups with the capacity to buffer changes in endosomal pH. The magnitude of this buffering capacity will alter the relation between proton-transport rate and pH change. Direct measurements of endosomal buffering capacity have yielded estimates of between 6 and 50 mM/pH unit (33, 92). Thus, to change pH, several orders of magnitude more protons must be moved into the vesicle than would be required to acidify an unbuffered solution. This is an important concept as we later consider the impact on pH of proton flux coupled to sodium or chloride transport.
A net negative charge is associated with cell-surface proteins. This has minimal impact on the composition of the relatively large volume of the extracellular space. However, after endocytosis, these fixed charges become concentrated into a much smaller space and give rise to a Donnan potential (27), which can drive diffusion of counterions into or out of the vesicle. Estimates of this force have ranged from −22 to −37 mV (101) in intracellular vesicles. This effect may significantly alter the ionic composition of the endosome lumen, even before any active transport has occurred. This effect has been proposed to account for the low Cl− concentration in endosomes that is measured immediately after formation (93).
The lipid composition of the vesicle membrane itself can also influence membrane potential. The cytoplasmic leaflet of the endosome tends to be more negatively charged because of a preponderance of acidic lipid headgroups (84). Estimates of the cytoplasmic surface potential range between −30 (10) and −50 mV (63). This surface potential may alter the surface concentration of ions dramatically. For example, concentrations of divalent and monovalent cations may be 5 to 50 times higher at the endosomal surface as compared with bulk concentrations (33).
The impact of the V-ATPase on vesicular membrane potential and pH has been demonstrated in many cell types (64) but has perhaps been best studied in synaptic vesicles, which can be considered specialized endosomes. Storage of neurotransmitters is accomplished by specific transport proteins that are powered by either the lumen-positive transmembrane potential or the proton chemical gradient generated by the V-ATPase (83). The relative magnitude of these two forces in a given vesicle is determined by the degree to which anion currents dissipate transmembrane potential. Higher Cl− permeability limits charge separation but facilitates acidification, thus creating a large proton chemical gradient. Conversely, limiting Cl− flux favors charge separation and enhances transmembrane potential. These principles have been proposed partially to underlie the phenotype of ClC-3–null mice, which undergo dramatic neurodegeneration of the hippocampus and retina (24, 94). Vesicular glutamate transport is driven primarily by voltage. ClC-3–null mice demonstrate impaired acidification of glutamatergic synaptic vesicles and an increase in the magnitude of glutamate-induced miniature excitatory postsynaptic potentials (94). These potentials are caused by the release of glutamate from a single vesicle, and the size of them therefore reflects the abundance of glutamate stored in each one. Larger excitatory potentials in ClC-3–null tissues are consistent with the idea that the loss of ClC-3 increases the transmembrane potential of the vesicles and thereby enhances glutamate uptake. The resulting increase in glutamatergic neurotransmission may favor excitotoxic effects and play a role in the neurodegenerative process.
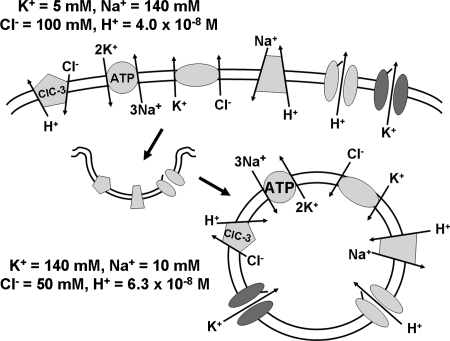
The sodium-potassium ATPase (Na+-K+ ATPase) also contributes to endosomal membrane potential (31). After endocytosis, this electrogenic transporter is oriented such that it moves three sodium ions into the vesicle in exchange for two potassium ions (Fig. 2). This creates a more-positive intravesicular charge, which will limit acidification (11). It has been speculated that this mechanism may be most important in the early endosome, in which the Na+-K+ ATPase is more highly expressed relative to the rest of the endosomal pathway. Na+-K+ ATPase activity may also be important for maintenance of endosome-to-cytoplasm Na+ and K+ gradients that are analogous to the gradients from the extracellular fluid to the cytoplasm. These gradients will contribute directly to vesicular membrane potential if ion-selective channels are present. They also can provide a driving force for secondary transport of other ions.
FIG. 2.
Membrane ion transporters and channels that may affect the electrophysiology of the signaling endosome. Endocytosis results in the extracellular face of these proteins being exposed to the lumen of the endosome. The intracellular ion concentrations provided for sodium (73) and Cl− (14) are estimates for vascular smooth muscle cells.
It is clear that multiple types of cation channels, including inward rectifier (Kir) (57) and voltage-dependent (Kv) potassium channels, and the epithelial sodium channel (ENaC) (56), are internalized through endocytosis. Membrane cycling may provide an important mechanism by which cell-surface channel activity is regulated. Membrane recycling of the Kv1.5 potassium channel occurs via Rab-dependent pathways including initial uptake into Rab5-positive early endosomes (15, 22). The voltage-dependent sodium channel NaV1.5 has a strictly intracellular distribution and localizes to Rab7-positive late endosomes in macrophages, where it regulates acidification by coupling sodium efflux to proton influx (12). It is likely that the ion channel composition of specific endosomal subtypes is highly variable and must be individually characterized.
The membrane potential of signaling endosomes cannot be directly measured. The volume of a sphere with a diameter of 200 nm is ∼4.2 × 10−18 L. The change in membrane potential produced by Nox activity will be a function of the abundance and activity of Nox protein relative to the surface area and input resistance of the membrane. Previous measurements of the depolarization induced by Nox2 activation were based on plasma membrane potential changes in intact neutrophils with a volume of ∼3 × 10−13 L (97). However, Nox2 protein is clearly recruited to phagosomes, which are much smaller (∼1.2 × 10−15 L), but still very large compared with signaling endosomes. Nox recruitment to a smaller compartment would be expected to enhance current density, but to a degree that will vary greatly with compartment size (71). In resting neutrophils, the majority of ClC-3 protein is found in secretory vesicles, and like Nox2, ClC-3 is also recruited to phagosomes (67). Consistent with this primarily intracellular localization, we observed no difference in the ability of ClC-3–null neutrophils to generate extracellular ROS, but the defect in intracellular ROS production is pronounced (67). In nonphagocytes, plasma membrane Nox1 is clustered in lipid-raft domains (98) from which it is recruited to signaling endosomes. Enrichment of Nox1 in endosomes could result in very high Nox1 current density, which would magnify the need for charge neutralization.
Acidification
The V-ATPase has long been recognized as the primary mechanism for endosomal acidification. It is a member of the family of large, multi-subunit complexes that function as ATP-driven proton pumps (4, 30). These complexes operate via a rotary mechanism in which ATP hydrolysis, carried out by a peripheral domain, drives rotation of a central integral membrane domain that is directly responsible for transport of protons across the membrane (19) (Fig. 1A). The coupling ratio between ATP consumption and H+ transport has been estimated to be in the range of 1:2 but can be modulated and is clearly pH dependent, with more energy required to move protons against higher pH gradients (8, 34). The reversal potential for H+ transport must therefore also vary. In the presence of bafilomycin A1, an inhibitor of V-ATPase, endosome acidification is severely limited (93). The rate and degree of acidification reflect a balance between active inward transport by the V-ATPase, the buffering capacity of the lumen, and passive outward proton movement via “leak” pathways. Potential leak pathways may include the electrically neutral Na+/H+ exchanger and proton channels. As discussed earlier, buffering capacity is a critical consideration. At neutral pH, a 4.2 × 10−18 L vesicle will contain very few free protons. The actual number of protons present must be considered on a time-averaged basis because at pH 7.0 (10−7 M protons), only 0.25 “free” protons are present per endosome!
The NHE3 Na+/H+ exchanger (74) is internalized via clathrin-coated pits (16) and modulates acidification of liver endosomes (100). Minimal data are available with respect to the dynamics of endosome sodium concentration. However, one might expect the initial sodium concentration to approximate that of extracellular fluid (∼140 mM) and significantly exceed that of the cytoplasm (∼10 mM). This gradient could provide a driving force for proton movement into endosomes via this exchanger (Fig. 2). This mechanism of acidification might be sustained if the Na+/K+ ATPase maintains a vesicle-to-cytoplasm sodium gradient. The importance of Na+/H+ exchange to endosome acidification has been well documented in yeast (9).
The genetic identity of endosomal proton channels has not been clearly established. In neutrophil phagosomes, they are thought to be the same as the plasma membrane proton channels that provide charge neutralization for Nox2 (20, 71). A candidate protein for this channel has been cloned and expressed (81, 88). Although these channels are best characterized in phagocytes, they are broadly expressed at the plasma membrane of many different cell types. As yet, it remains to be definitively proven that charge compensation for Nox2 activity can be attributed to this specific protein. The channel rectifies very strongly in the outward direction and therefore functionally should be able only to mediate proton efflux at the plasma membrane (Fig. 2). If endocytosed, this orientation should readily allow proton influx into endosomes, but may not allow protons readily to leak out of acidified vesicles. Passive proton influx could theoretically contribute to vesicle acidification if the NADPH oxidase was active and the endosome lumen, negatively charged. The cytoplasmic pH of neutrophils is transiently acidified, largely because of liberation of protons from NADPH by Nox activity (35). This enzymatic production of protons at the cytoplasmic face of signaling endosomes may provide an even stronger local gradient favoring passive endosome acidification via proton channels. It has been proposed and hotly debated whether the NADPH oxidase might have a secondary ability to conduct protons and provide its own charge balance (23, 61).
The effect of superoxide (O2−•) production inside the signaling endosome on the pH of the compartment is likely to be very complex. The Nox enzyme deposits superoxide within the endosome, whereas the protons that are liberated from NADPH in the process remain on the opposite, or cytosolic, side of the vesicular membrane. Two protons are consumed in the dismutation of superoxide, one to create hydroperoxyl radical [HO2•, pKa = 4.75 (7)], and the second to yield hydrogen peroxide [H2O2, pKa = 11.62; Fig. 3]. Spontaneous dismutation occurs rapidly (∼106 M/s) at neutral pH (78), but slows remarkably under alkaline conditions. The half-life of O2−• is prolonged approximately tenfold for every tenfold decrease in the free proton concentration (1-point increase in pH) between pH 6 and 14. The proton dependence of dismutation is nicely demonstrated by the observation that a 150 mM solution of potassium superoxide in DMSO showed less than 10% decomposition in a full day at room temperature (99). In the absence of another source of protons, superoxide will effectively “pull” protons from H2O, leaving OH− behind and thereby causing alkalinization. The overall supply of protons to the lumen of signaling endosomes during the oxidative burst is critical to the redox chemistry of the compartment, but no data have quantified this. If the pH of signaling endosomes is neutral or alkaline, biologically significant accumulation of O2−• may occur. An attempt to quantify the concentration of endosomal O2−• in TNF-α–induced endosomes has been made in this issue of ARS (52). Estimated O2−• production for a single vesicle with a 200-nm diameter was 4.3 × 10−22 moles or ∼260 molecules per second. The steady-state concentration of O2−• within the vesicle will depend on a number of factors, including vesicle size and pH. A 200-nm vesicle with the stated production rate was calculated to contain ∼14 μM O2−• at pH 7.4. This concentration increased to ∼30 μM at pH 8.0 and decreased to ∼4.4 μM at pH 5.0. The concept that biologically significant endosomal O2−• accumulation occurs is supported by the observation that superoxide exits endosomes through a DIDS-sensitive anion conductance (69). This implies that either superoxide accumulates in endosomes, or superoxide is somehow shielded from interaction with protons within the vesicle.
FIG. 3.
(A) Charge neutralization of the V-ATPase by ClC-3. Cl− enters the endosome, and H+ leaves with a stoichiometry of 2:1. Cl−/ H+ exchange can provide appropriate opposing current flow, but at a cost of removal of H+ from the compartment. The net result would be acidification and Cl− accumulation. (B) Charge neutralization of the NADPH oxidase by ClC-3. H+ enters the endosome, and Cl− leaves. Dismutation of superoxide consumes 2 H+ per molecule of the H2O2 produced. The net result of these processes would be alkalinization and Cl− depletion.
Nox2 activity has been proposed to provide a regulated means of retarding acidification in phagosomes of dendritic cells, which have a higher pH than those of macrophages (59). Proton consumption for H2O2 production maintains a less acidic environment. Reduced acidity has the important effect of limiting degradation of endocytosed protein and thereby facilitating antigen cross-presentation. It remains to be determined whether the V-ATPase and the NADPH oxidase can be active simultaneously in the signaling endosome. Theoretically, both enzymes might be relieved of charge constraints if their currents were of similar magnitude. This might lead to very efficient production of H2O2. However, if both pumps can be active at the same time, why would ClC-3 be required for either process?
Despite the relatively high rate constant for the spontaneous dismutation of superoxide, nature has developed an enzymatic mechanism, superoxide dismutase (SOD), which markedly accelerates the reaction rate to >109 M/s. Specific isoforms of SOD are localized to the mitochondria (Mn-SOD), the cytoplasm (CuZn-SOD), and the extracellular space (EC-SOD), and defects in these enzymes have profound physiological consequences (38, 58). EC-SOD binds to the surface of the plasma membrane via its heparin-binding domain and creates a mechanism by which SOD may be present inside early endosomes. EC-SOD is known to be internalized via clathrin-coated pits and localizes to EEA-1–positive early endosomes, whereas EC-SOD lacking a functional heparin-binding domain is not endocytosed (17). This localization seems to have functional significance, as carriers of a common genetic variant (EC-SODR213G) with disrupted heparin binding have a higher circulating plasma concentration of EC-SOD, but are at an increased risk for vascular disease (17). The functional significance of SOD inside this compartment remains to be determined. One might anticipate an acceleration of proton consumption and enhanced alkalinization.
SOD also localizes to the cytoplasmic side of the signaling endosome (Fig. 3). CuZn-SOD is recruited to early endosomes by cytokine activation, in which it directly binds to Rac1 and is required for activation of the Nox complex (42). Endosomal O2−• may be delivered to this cytoplasmic SOD by efflux through a DIDS-sensitive anion conductance (69). Oxidation of Rac1 by H2O2 reversibly uncouples SOD1 binding, thereby creating a potential feedback mechanism for redox-based sensing of O2−• levels. This interaction between SOD and Rac1 may play a critical role in familial ALS, in which failure of SOD to dissociate from and to inactivate the Nox complex may be linked to excess ROS production and long-term oxidative stress (42).
Chloride Transport
Although the importance of Cl− for vesicular acidification has been appreciated for decades, the identity of this anion pathway has only recently been proposed. It has been suggested that the intracellular ClC proteins (ClC-3, -4, -5, -6, and -7) provide shunt current for V-ATPase activity (46). The best-studied example of this relation is ClC-5. Mutations in this gene (CLCN5) cause Dent disease (OMIM 300008). ClC-5 is required for proper acidification and trafficking of early endosomes in renal proximal tubular epithelial cells (37, 40). Loss of ClC-5 function results in a complex series of downstream consequences that ultimately disrupt the endocytic pathway and lead to the formation of kidney stones (77). ClC-4 also localizes to intracellular vesicles in renal proximal tubules and cultured epithelial cells. Disruption of ClC-4 expression limits endosomal acidification and alters trafficking (66). Similarly, loss of ClC-3 in CHO cells (41) limits acidification of both transferrin-containing early/recycling endosomes and α2-macroglobulin–containing late endosomes, although not to the same extent as the anion channel blocker NPPB or bafilomycin A1. In pancreatic beta cells, impairment of ClC-3 interferes with terminal acidification and release of insulin granules (5, 95).
The proposed relation between the ClC proteins and the V-ATPase was intuitive when the ClCs were all assumed to be anion channels. The relation became less clear when it was determined that, like the bacterial protein ClC-ec1 (1), ClC-4 and -5 are not simple anion channels but Cl−/H+ antiporters (76, 89). Based on sequence homology, and in particular the presence a specific “gating” glutamate residue (2), it was inferred that ClC-3 through ClC-7 are all exchangers. In support of this, we provided direct evidence supporting exchanger function for ClC-3 (60). The best available evidence suggests a 2:1 Cl− for H+ stoichiometry of exchange for ClC-ec1 (72). Precise measurements of the coupling ratio of eukaryotic ClC antiporters are not available, and estimates have ranged between 1 and 5 (60, 76, 89). For the purposes of our discussion, we assume that the prokaryotic ratio of 2:1 applies.
For a Cl−/H+ antiporter to provide compensatory charge flow for the V-ATPase, Cl− must move into the endosome, and protons, out (Fig. 3A). Two puzzling aspects to this scenario exist: (a) this direction of current flow is not favored by the very strongly rectifying ClC currents, which generate very little detectable current in this direction, even at strong membrane voltages (Fig. 4); and (b) this system cannot achieve higher than 66% efficiency as a charge compensator for the V-ATPase. Assuming that three units of charge move through ClC-3 (72), one third of the total represents protons moving in the opposite direction from the V-ATPase. ClC-3–mediated proton efflux will therefore retard the V-ATPase–mediated acidification that it is proposed to facilitate. This question of efficiency has led to speculation that perhaps it is critically important to maintain a high endosome chloride concentration, and that this is achieved at the expense of acidification (28, 46). Stated another way, the proton electrochemical gradient generated through energy expended by the V-ATPase may be used for the explicit purpose of concentrating Cl− in this compartment.
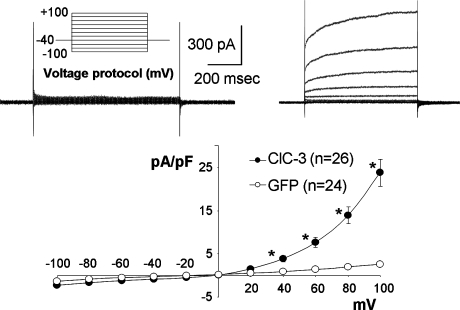
FIG. 4.
Rectification of ClC-3 favors outward current in HEK 293 cells overexpressing adenoviral ClC-3. Large currents are seen at positive intracellular potentials where Cl− is moving into the cell and H+ is moving out. After endocytosis, this becomes movement of Cl− out of the signaling endosome and H+ movement into the vesicle. These data are reprinted from (60). *The current in ClC-3–expressing cells is significantly larger than that in control, GFP-expressing cells (p < 0.05).
Why might vesicular Cl− concentration be important? Vesicular Cl− concentration is unexpectedly low (∼15–20 mM) as early as it can be measured (∼5 s) after endosome formation (93). This has been attributed to the Donnan potential of the endosome (92). Chloride accumulation subsequently parallels acidification, and endosomal Cl− increases again over a period of ∼30 min to the range of 40–60 mM. Similar to pH-dependent changes in protein function caused by protonation of critical amino acid residues, Cl− can interact with specific sites on chloride-sensitive proteins (CSPs) and alter their conformation or activity. Known CSPs include hemoglobin (79), cathepsin-C (18), an isoform of the Na+/H+ exchanger (87), the endosomal TRPV2 calcium channel (86), and many others (reviewed in ref. 28). CSPs may thereby transduce chloride concentration changes to the vesicular membrane.
Conversely, if the role of ClC-3 is to provide charge compensation for an NADPH oxidase, this would require ClC-3 to conduct ions in the opposite direction. In this scenario, ClC-3 current flow is in the direction that is favored by the rectification properties of the transporter (Fig. 3), and movement of protons is into the endosome. This would directly facilitate acidification and provide additional protons for H2O2 production. Cl−/H+ antiport also seems like a very efficient means for dissipation of the Donnan potential. In addition to relieving membrane depolarization and reducing vesicular Cl−, Cl−/H+ exchange driven by this force could “jump-start” endosomal acidification at no energetic expense. A significant issue for charge compensating the NADPH oxidase with a Cl−/H+ antiporter is whether the availability of Cl− in the endosome is sufficient to support the process. This is particularly a problem if Donnan effects reduce the concentration of Cl− inside the endosome immediately after formation. The amount of Cl− required for sustained ClC-3 activity will be directly related to the magnitude and duration of the electron current produced by the Nox protein. This issue of Cl− availability is discussed in detail later.
Electrophysiology of a ROS Signaling Endosome
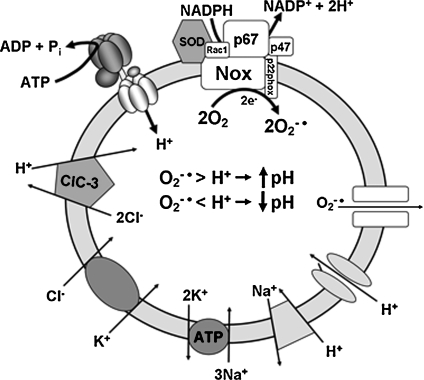
The electrophysiology of endosomes with positively charged lumens has already been extensively modeled with respect to acidification and Cl− handling (28, 33, 85). A signaling endosome with an active NADPH oxidase and a negatively charged lumen is a fundamentally different environment. These endosomes may represent completely different compartments, or alternatively, both modes of operation might apply to the same vesicle at different times. We must consider the possibility that superoxide production occurs immediately after endosome formation, and acidification is a slower and slightly delayed process. It is also possible that ClC-3 plays a role under both conditions by moving charge in opposite directions. We outline a working model for the electrophysiology of the signaling endosome (Fig. 5).
FIG. 5.
Potential contributors to the electrophysiology of the signaling endosome. It remains to be determined whether the V-ATPase and Nox enzymes operate in the same or different populations of vesicles. If they are present in the same compartment, the balance between superoxide production by Nox and proton accumulation by all potential mechanisms (V-ATPase, proton channels, Na+/H+ exchange, Cl−/H+ antiport) may determine both the pH and the redox state of the compartment. Control of the microenvironment of the signaling endosome may be critical to proper processing of endocytosed proteins and subsequent signal transduction. Rapid superoxide production associated with alkalinization of the vesicle lumen could allow significant accumulation of superoxide, which may then leave the endosome via an anion conductance. This mechanism may provide a controlled pathway for cytoplasmic ROS signaling by superoxide that is independent of H2O2 production.
How many ions are moving?
How many net ions must be transported across the endosomal membrane in the process of acidification and during dynamic changes in Cl− concentration? Are the observed changes in ion concentrations consistent with a fundamental role for coupled H+ and Cl− transport?
First we consider whether V-ATPase–mediated transport of protons into endosomes coupled to Cl−/H+ exchange is a plausible mechanism to drive the changes in vesicular pH and Cl− concentration that have been reported (40, 41). As we have already calculated, given the tiny volume of the endosome at neutral pH, fewer than one free proton is in the compartment at any instant in time. In contrast, when intravesicular chloride is ∼15 mM, as it is within seconds of endocytosis, ∼38,000 free Cl− ions are present. Over a ∼15-min period, the pH decreases to ∼6.0, and the number of Cl− ions approximately triples to a concentration of ∼45 mM. At this point, the compartment will contain ∼2.5 free protons and ∼114,000 Cl− ions. Therefore, to achieve the required amount of Cl− transport via ClC-dependent 2:1 Cl−/H+ exchange, the V-ATPase would have to make ∼38,000 protons available to the exchanger, so that H+ efflux from the compartment could be coupled to the influx of ∼76,000 Cl− ions. To reduce the pH, additional protons must be accumulated. The buffering capacity of endosomes has been estimated to be 35 to 50 mM/pH unit, based on measurement of alkalinization induced by NH4Cl (92). The number of protons required to alter the concentration in an unbuffered 4.2 × 10−18 L vesicle by 50 mM is ∼126,000. This means that a total of 38,000 + 126,000 = ∼164,000 protons are required both to increase the Cl− concentration from 15 to 45 mM and to reduce the pH by 1 unit. Roughly three fourths of these protons are buffered within the endosome, and one fourth are used to drive Cl− transport. These calculations illustrate the inefficiency of this coupled transport mechanism as a means either to acidify or to concentrate Cl− ions. If the primary goal is reducing pH, and the exchanger provides charge neutralization, extra protons must be pumped so that they are available to be exchanged for Cl−. If the goal is Cl− accumulation, the buffering capacity of the endosome must be overcome to create a significant pH gradient by which to drive this process. However, provided that the antiporter can support sufficient charge flow in this direction (not favored by rectification properties), the system is feasible.
Next, if we assume that an intra-endosomal Donnan potential drives the initial rapid decrease in endosomal Cl− concentration via Cl−/H+ exchange, we can then ask if this effect would be sufficient to significantly change the vesicular pH. In this scenario, antiporter current is moving in its favored direction. If extracellular Cl− is normally ∼100 mM and decreases to ∼15 mM by this mechanism (40, 41), this 85 mM change in Cl− concentration could drive a 42.5 mM increase in unbuffered vesicular proton concentration. As before, based on the buffering capacity of early endosomes, this should decrease the vesicular pH by ∼1 pH unit, a pretty good “jump start” to the acidification process. Of note, this initial low endosomal Cl− concentration was still observed in endosomes from ClC-3–deficient hepatocytes (41), suggesting that if this mechanism is involved, it is mediated by a different Cl−/H+ exchanger, at least in this cell type.
Now we must estimate the capacity of an active NADPH oxidase to affect these processes. What is the magnitude of the Nox-mediated electron current that moves into the signaling endosome to produce O2−•? Direct measurements of the density of current in intracellular vesicles have not been made; however, modeling and measurement of superoxide production in whole neutrophils and phagosomes suggest that Nox2 produces “enormous quantities” of O2−• [reviewed in (71)]. Stimulated human neutrophils can generate ∼10 nmoles of O2−• per minute per million cells. This translates to a total flux of ∼108 electrons per second per cell or ∼16 pA of current across the plasma membrane of a neutrophil of average size (90). Direct measurements of PMA-induced electron current by using perforated-patch whole-cell recording in human neutrophils yielded slightly smaller currents (2.3 ± 1.5 pA), which may have been limited by the in vitro conditions and low temperature (20°C) at which the experiments were performed (21). Intraphagosomal electron current and O2−• production have been presumed to be much higher. Production estimates range from 2.5 to 10 mM per second (39, 103), and cumulatively, ∼4 M electron-equivalents may be introduced into the phagosome during an oxidative burst (82). Previous estimates of the rate of superoxide production in nonphagocytes suggested that they produce no more than 1–10% as much as phagocytes. This is due in part to the lower expression level of NADPH oxidase in nonphagocytes (50). However, if even a fraction of neutrophil phagosomal ROS production is occurring in the more-confined space of the signaling endosome, the impact on vesicular electrophysiology might be equally important. In this issue of ARS, Li et al. estimate the total flux of O2−• for a single signaling endosome to be ∼0.1 mM per second, clearly less than that of phagosomes. However, if this production rate is considered as ∼260 O2−• molecules per second in a vesicle with a 200-nm diameter, this translates to ∼234,000 molecules/endosome over a 15-min period. This is more than the number of protons that we calculated would be needed to alter pH and Cl− concentration over the same time period. This level of O2−• production and the associated protons required for the production of H2O2 seems likely to significantly alter vesicular pH. Regardless of the precise rate of O2−• production, if the supply of O2−• outstrips the influx of protons, this will alkalinize the compartment. In this setting, the buffering capacity of the endosome would be called on to function in the opposite direction, with protons being released from endosomal proteins and H2O2 to provide a substrate for the dismutation of O2−•.
Endosomal pH
Nox activity depolarizes the membrane and generates cytoplasmic protons, causing local acidification. This will favor passive proton flux into endosomes via three possible mechanisms: ClC-3, Na+/H+ antiport, and proton channels. These passive mechanisms, perhaps combined with V-ATPase activity, can contribute protons during this phase. However, the pH is likely to remain neutral or even to become slightly alkaline if superoxide production is rapid. It is the supply of protons, compared with the rate of O2−• generation, that will determine the vesicular concentrations of superoxide, H2O2, and the pH. If superoxide production outstrips the influx of protons from outside the compartment, alkalinization will occur. However, the pH may return rapidly toward neutral or become acidic as soon as the oxidative burst terminates, provided that significant proton transport into the vesicle continues. If large quantities of hydrogen peroxide are produced and diffuse out of the endosome, this may also constitute one kind of redox “signal.” Ample evidence indicates this has important biologic effects, including recruitment of TRAF6 to the endosome during cytokine signaling downstream of NF-κB and p38 MAP kinase (53, 54, 65, 68).
Another way of looking at this process is to consider that endosomal H2O2 generation, coupled to diffusion of H2O2 into the cytoplasm and subsequent inactivation by glutathione peroxidase or catalase, amounts to a mechanism for removal of protons from the endosomal lumen. It is possible that deprotonation of endogenous proteins in a transiently alkaline environment may have independent biologic effects.
Endosomal ROS levels
Are steady-state levels of endosomal O2−• or H2O2 biologically significant? Modeling of ROS production in phagosomes has yielded specific predictions regarding intraphagosomal ROS concentrations (103). Important factors include (a) the ability of H2O2, but not O2−•, readily to diffuse out of the compartment; and (b) the fact that neutrophils express myeloperoxidase (MPO), which uses H2O2 and Cl− to produce HOCl for microbial killing. These estimates yield steady-state phagosome O2−• concentrations of ∼25 μM and H2O2 concentrations in the low micromolar range. If MPO is removed from the calculations (as is appropriate for our discussion of endosomes in nonphagocytes), these levels increase to >100 μM O2−• and ∼30 μM H2O2. These numbers are surprisingly similar to the previously discussed ∼1- to 30-μM estimates of O2−• concentration in TNF-α–induced endosomes (52). Anion channel–mediated efflux of O2−• from the endosome might also contribute to reducing the steady-state O2−• level in the endosome (69) and could provide yet another mechanism of charge neutralization for oxidase activity. It has been proposed that ClC-3 itself mediates transmembrane superoxide flux (29, 43), raising the possibility that O2−• can substitute for Cl− and exit the endosome by this mechanism. Another issue worth considering is that hydroperoxyl radical [HO2•; pKa = 4.88 (6)], the protonated intermediate between O2−• and H2O2, is not ionized and could theoretically diffuse passively out of the endosome and revert to superoxide in a more-alkaline cytosol.
Endosomal membrane potential
As previously discussed, the negatively charged lumen of the signaling endosome may drive electrogenic Cl−/H+ antiport (Cl− out, H+ in) and perhaps also the passive flux of other ions, including protons. Under these circumstances, one would not expect V-ATPase activity to be constrained by charge. The plasma membrane of neutrophils is only modestly depolarized during the oxidative burst as long as normal mechanisms are available for charge neutralization (45). By analogy, we would predict that vesicular depolarization should not be excessive if ClC-3 is functional.
This raises a very interesting question. We have proposed that endosomal membrane potential will become very lumen negative if the Nox complex is activated but Cl−/H+ antiport is inhibited. Can Nox activity then be used as a “battery,” creating a voltage difference that then modifies protein function or drives secondary transport (Fig. 6)? This function of the NADPH oxidase would be analogous to how, in the absence of charge compensation, the V-ATPase creates a voltage difference of the opposite polarity that is then used to drive neurotransmitter uptake (62, 83). Traditional assessment of Nox activity by ROS detection might not be very useful under these conditions because O2−• production would be minimal. The magnitude of this potential difference would be controlled by interplay between activation of the oxidase, regulation of charge-compensation pathways, and rates of secondary-transport pathways that would consume the energy provided by NADPH oxidation. An inherent appeal exists in the concept that cells have the flexibility to transport charged molecules into or out of vesicular compartments by using either a proton- or electron-motive force as appropriate. Obviously, additional investigation will be required to determine whether this functional, but electrochemically constrained state, can be sustained by the NADPH oxidase, and whether this state has any specific secondary functions.
FIG. 6.
Proposed electrophysiology of the vesicle in the absence of charge neutralization. If the NADPH oxidase is activated without charge neutralization, oxidase activity, and therefore ROS production, will be low. However, a large potential difference (lumen negative) could theoretically be maintained. Under these conditions, activity of the V-ATPase should not be constrained, and a variety of secondary ion-transport mechanisms, including ion channels or co-transporters of larger charged molecules, could be facilitated. From the viewpoint of membrane proteins, this state would be the equivalent to that of a depolarized plasma membrane.
Endosomal chloride
In vascular smooth muscle cells and neutrophils, the absence of ClC-3 or the presence of an anion channel blocker limits vesicular NADPH oxidase activity (65, 67). We have hypothesized that the close relation between ClC-3 and Nox activity is based on a requirement for charge neutralization. The profound impairment of ROS production in the absence of ClC-3 in vascular smooth muscle cells suggests that these cells do not have an effective alternative mechanism to provide charge neutralization for Nox enzymatic activity. This must include the V-ATPase, suggesting either that these two electrogenic transporters are not simultaneously active or that the amount of charge moved by the oxidase outstrips the ability of the V-ATPase to keep up. Consistent with this, bafilomycin A1 does not interfere with cytokine-induced ROS signaling in vascular smooth muscle cells (65).
These observations are in contrast to what has been observed in MCF-7 mammary epithelial cells, in which anion channel blockers do not prevent ROS production, but rather appear to impede efflux of O2−• from endosomes (69). In neutrophils, ClC-3 is very important for intracellular ROS production, but the process is not completely ClC-3 dependent (67, 68). The mechanisms of charge neutralization of endosomal NADPH oxidases may differ between cell types, or even in the same cell responding to different stimuli.
As noted earlier, for ClC-3 to provide charge neutralization for Nox current, Cl− availability within the endosome must be adequate. If Nox activity is sustained, the concentration of Cl− in the endosome could become limiting. Cl− depletion may provide a mechanism that limits the duration and intensity of Nox activity, or alternatively, Cl− may be replenished from an alternative source. One mechanism by which the Cl− concentration of the endosome could be sustained during the oxidative burst might be an electrically neutral K+/Cl− cotransporter. Although we are not aware that any of the KCC family of transporters has been definitively characterized as an endosomal protein, immunostaining in HEK293 cells for the ubiquitously expressed KCC1 protein yields granular immunostaining of the cytoplasm, which could be endosomal (51).
Immediately after endocytosis, the cytosolic concentrations of both K+ and Cl− exceed their respective concentrations in the vesicle. Extracellular K+ is far lower than intracellular, and we already discussed the surprisingly low initial endosomal Cl− concentration. In addition, both vascular smooth muscle (14) and neutrophils (91) actively accumulate cytoplasmic Cl− and have surprisingly high cytosolic Cl− concentrations.
A useful exercise in evaluating the potential role of ClC-3 is to consider the biologic implications if it provided all of the charge neutralization for superoxide production. Three molecules of NADPH consumed by a Nox enzyme yield six endosomal electrons (i.e., six superoxide molecules) and, after processing of NADP+ through the pentose phosphate shunt, six cytoplasmic protons. Two cycles of ClC-3 will move a total of four Cl− ions out of and two protons into the endosome to balance completely the charge separation induced by the oxidase. Both charge and osmotic imbalances will result, and these will change dynamically as both protons and O2−• ions “disappear” through production of H2O2. These effects must be balanced by movement of other ions such as K+, which has also been shown to be able to contribute to charge neutralization of the oxidase (82). This highly dynamic nature of ROS metabolism limits specific predictions.
Implications
We have speculated as to how compartmentalization of ROS generated by Nox family NADPH oxidases facilitates redox-mediated cellular signaling. A complex set of variables arises as one attempts to understand a system of O2−• and H2O2 production that facilitates “signaling.” It also is important to consider the broad range of effects of vesicular Nox activation and to entertain the possibility that ROS production for “signaling” or microbial killing may not be the only goals of Nox activation. The NADPH oxidase expends significant metabolic energy to produce these ROS, and in the process, significant charge is separated by electron transfer across an insulating membrane. Energy stored as NADPH can thereby be converted to voltage. As we have discussed at length, this system will modify the ionic composition and redox potential of the early endosome. These local environmental changes may be important for the regulation of kinase and phosphatase activity (3) and for the proper processing of endocytosed proteins. This may include the TNF-receptor itself, which is internalized within the signaling endosome (32, 102). Both TNFR1 and TNFR2 have several cysteine-rich modules in their extracellular domain. Exposure to high concentrations of a thiol oxidant (1 mM diamide) promotes self-association of these domains, which activates ligand-independent TNF signaling.
Perhaps even more interesting, although a low concentration of diamide (1 μM) did not provoke self-association, it did enhance TNF-induced signaling, suggesting that ligand-dependent responses are also redox dependent (75). The critical importance of a redox environment for TNF-receptor signaling is further supported by observations that the redox state alters the affinity of TNF receptors for TNF (26, 44).
An unavoidable side effect of the need for a microenvironment with a redox state that differs significantly from that of the cytoplasm is the creation of potentially toxic ROS intermediates, which then must be biochemically “managed.” Potential problems include numerous unintended redox reactions with metals or nitric oxide. These reactions yield highly reactive intermediates, including hydroxyl radical and peroxynitrite (see ref. 80 for review). Proteins both inside and immediately outside of the signaling endosome must either be exposed to these ROS or be protected from redox reactions by specific, and probably highly localized antioxidant systems. If biologic systems need to undertake chemical reactions that require an altered pH and redox state, confinement of this process to a cytoplasmic vesicle is logical mechanism by which to achieve this.
It is intriguing to consider how inflammatory diseases or aging might lead to failure to of the mechanisms that confine and control these redox-active processes. This would result in “oxidative stress,” a common theme in the etiology of so many disease states. Defining the mechanisms and primary goals of endosomal ROS signaling is an exciting new frontier in free radical biology.
Acknowledgments
This material is based on work supported in part by the NIH (F.S.L., J.G.M., F.J.M.), by the Office of Research and Development, Department of Veterans Affairs (F.J.M.), and by the American Heart Association (F.S.L.).
Abbreviations
ClC, chloride channel; CSP, chloride-sensitive protein; DIDS, diisothiocyanostilbene sulfonic acid; DPI, diphenyleneiodonium; EC-SOD, extracellular superoxide dismutase; EEA-1, early endosomal antigen 1; FAD, flavin adenine dinucleotide; HO2•, hydroperoxyl radical; H2O, water; H2O2, hydrogen peroxide; HOCl, hypochlorous acid; IL-1β, interleukin 1-beta; KCC, K+/Cl− cotransporter; MPO, myeloperoxidase; NADPH, nicotinamide adenine dinucleotide phosphate, reduced; NADP+, nicotinamide adenine dinucleotide phosphate, oxidized; Na+-K+ ATPase, sodium-potassium ATPase; NF-κB, nuclear factor-kappa-B; NHE, sodium-hydrogen exchanger; Nox, NADPH oxidase; O2−•, superoxide anion; OH−, hydroxide ion; pA, picoampere; ROS, reactive oxygen species; SOD, superoxide dismutase; TNF, tumor necrosis factor; TRAF6, TNF-receptor–associated factor 6; V-ATPase, vacuolar ATPase.
References
- 1.Accardi A. Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427(6977):803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 2.Accardi A. Walden M. Nguitragool W. Jayaram H. Williams C. Miller C. Separate ion pathways in a Cl−/H+ exchanger. J Gen Physiol. 2005;126(6):563–570. doi: 10.1085/jgp.200509417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afanas'ev IB. Competition between superoxide and hydrogen peroxide signaling in heterolytic enzymatic processes. Med Hypotheses. 2006;66(6):1125–1128. doi: 10.1016/j.mehy.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Al-Awqati Q. Proton-translocating ATPases. Annu Rev Cell Biol. 1986;2:179–199. doi: 10.1146/annurev.cb.02.110186.001143. [DOI] [PubMed] [Google Scholar]
- 5.Barg S. Huang P. Eliasson L. Nelson DJ. Obermuller S. Rorsman P. Thevenod F. Renstrom E. Priming of insulin granules for exocytosis by granular Cl(−) uptake and acidification. J Cell Sci. 2001;114(Pt 11):2145–2154. doi: 10.1242/jcs.114.11.2145. [DOI] [PubMed] [Google Scholar]
- 6.Behar D. Czapski G. Rabini J. Dorfman LM. Schwartz HA. The acid dissociation constant and decay kinetics of perhydroxyl radical. J Phys Chem. 1970;74:3209–3214. [Google Scholar]
- 7.Bielski BH. Allen AO. Mechanism of the disproportionation of superoxide radicals. J Phys Chem. 1997;81:1048–1050. [Google Scholar]
- 8.Breton S. Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol. 2007;292:F1–F10. doi: 10.1152/ajprenal.00340.2006. [DOI] [PubMed] [Google Scholar]
- 9.Brett CL. Tukaye DN. Mukherjee S. Rao R. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell. 2005;16:1396–1405. doi: 10.1091/mbc.E04-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahalan MD. Hall J. Alamethicin channels incorporated into frog node of Ranvier: calcium-induced inactivation and membrane surface charges. J Gen Physiol. 1982;79:411–436. doi: 10.1085/jgp.79.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cain CC. Sipe DM. Murphy RF. Regulation of endocytic pH by the Na+, K+-ATPase in living cells. Proc Natl Acad Sci U S A. 1989;86:544–548. doi: 10.1073/pnas.86.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrithers MD. Dib-Hajj S. Carrithers LM. Tokmoulina G. Pypaert M. Jonas EA. Waxman SG. Expression of the voltage-gated sodium channel NaV1.5 in the macrophage late endosome regulates endosomal acidification. J Immunol. 2007;178:7822–7832. doi: 10.4049/jimmunol.178.12.7822. [DOI] [PubMed] [Google Scholar]
- 13.Cataldo AM. Mathews PM. Boiteau AB. Hassinger LC. Peterhoff CM. Jiang Y. Mullaney K. Neve RL. Gruenberg J. Nixon RA. Down syndrome fibroblast model of Alzheimer-related endosome pathology: accelerated endocytosis promotes late endocytic defects. Am J Pathol. 2008;173:370–384. doi: 10.2353/ajpath.2008.071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chipperfield AR. Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol. 2005;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- 15.Choi WS. Khurana A. Mathur R. Viswanathan V. Steele DF. Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97:363–371. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- 16.Chow CW. Khurana S. Woodside M. Grinstein S. Orlowski J. The epithelial Na(+)/H(+) exchanger, NHE3, is internalized through a clathrin-mediated pathway. J Biol Chem. 1999;274:37551–37558. doi: 10.1074/jbc.274.53.37551. [DOI] [PubMed] [Google Scholar]
- 17.Chu Y. Piper R. Richardson S. Watanabe Y. Patel P. Heistad DD. Endocytosis of extracellular superoxide dismutase into endothelial cells: role of the heparin-binding domain. Arterioscler Thromb Vasc Biol. 2006;26:1985–1990. doi: 10.1161/01.ATV.0000234921.88489.5c. [DOI] [PubMed] [Google Scholar]
- 18.Cigic B. Pain RH. Location of the binding site for chloride ion activation of cathepsin C. Eur J Biochem. 1999;264:944–951. doi: 10.1046/j.1432-1327.1999.00697.x. [DOI] [PubMed] [Google Scholar]
- 19.Cipriano DJ. Wang Y. Bond S. Hinton A. Jefferies KC. Qi J. Forgac M. Structure and regulation of the vacuolar ATPases. Biochim Biophys Acta. 2008;1777:599–604. doi: 10.1016/j.bbabio.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 21.DeCoursey TE. Cherny VV. Zhou W. Thomas LL. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc Natl Acad Sci U S A. 2000;97:6885–6889. doi: 10.1073/pnas.100047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehghani Zadeh A. Xu H. Loewen ME. Noble GP. Steele DF. Fedida D. Internalized Kv1.5 traffics via Rab-dependent pathways. J Physiol. 2008;586:4793–4813. doi: 10.1113/jphysiol.2008.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demaurex N. Petheo GL. Electron and proton transport by NADPH oxidases. Phil Trans R Soc Lond B Biol Sci. 2005;360:2315–2325. doi: 10.1098/rstb.2005.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickerson LW. Bonthius DJ. Schutte BC. Yang B. Barna TJ. Bailey MC. Nehrke K. Williamson RA. Lamb FS. Altered GABAergic function accompanies hippocampal degeneration in mice lacking ClC-3 voltage-gated chloride channels. Brain Res. 2002;958:227–250. doi: 10.1016/s0006-8993(02)03519-9. [DOI] [PubMed] [Google Scholar]
- 25.Dikic I. Endosomes. Georgetown, Tex: Springer Science+Business Media; 2006. p. 157. [Google Scholar]
- 26.Dominici S. Pieri L. Paolicchi A. De Tata V. Zunino F. Pompella A. Endogenous oxidative stress induces distinct redox forms of tumor necrosis factor receptor-1 in melanoma cells. Ann N Y Acad Sci. 2004;1030:62–68. doi: 10.1196/annals.1329.008. [DOI] [PubMed] [Google Scholar]
- 27.Donnan FG. The theory of membrane equilibria. Chem Rev. 1925;1:73–90. [Google Scholar]
- 28.Faundez V. Hartzell HC. Intracellular chloride channels: determinants of function in the endosomal pathway. Sci STKE. 2004;233:re8. doi: 10.1126/stke.2332004re8. [DOI] [PubMed] [Google Scholar]
- 29.Fisher AB. Redox signaling across cell membranes. Antioxid Redox Signal. 2009;11:1349–1356. doi: 10.1089/ars.2008.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs R. Schmid S. Mellman I. A possible role for Na+, K+-ATPase in regulating ATP-dependent endosome acidification. Proc Natl Acad Sci U S A. 1989;86:539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgiou G. How to flip the (redox) switch. Cell. 2002;111:607–610. doi: 10.1016/s0092-8674(02)01165-0. [DOI] [PubMed] [Google Scholar]
- 33.Grabe M. Oster G. Regulation of organelle acidity. J Gen Physiol. 2001;117:329–344. doi: 10.1085/jgp.117.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grabe M. Wang H. Oster G. The mechanochemistry of V-ATPase proton pumps. Biophys J. 2007;78:2798–2813. doi: 10.1016/S0006-3495(00)76823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grinstein S. Furuya W. Cytoplasmic pH regulation in phorbol ester-activated human neutrophils. Am J Physiol. 1986;251:C55–C65. doi: 10.1152/ajpcell.1986.251.1.C55. [DOI] [PubMed] [Google Scholar]
- 36.Grosshans BL. Ortiz D. Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunther W. Luchow A. Cluzeaud F. Vandewalle A. Jentsch TJ. ClC-5, the chloride channel mutated in Dent's disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci U S A. 1998;95:8075–8080. doi: 10.1073/pnas.95.14.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutteridge JM. Halliwell B. Free radicals and antioxidants in the year 2000: a historical look to the future. Ann N Y Acad Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 39.Hampton MB. Kettle AJ. Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun. 1996;64:3512–3517. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara-Chikuma M. Wang Y. Guggino SE. Guggino WB. Verkman AS. Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem Biophys Res Commun. 2005;329:941–946. doi: 10.1016/j.bbrc.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 41.Hara-Chikuma M. Yang B. Sonawane ND. Sasaki S. Uchida S. Verkman AS. ClC-3 chloride channels facilitate endosomal acidification and chloride accumulation. J Biol Chem. 2005;280:1241–1247. doi: 10.1074/jbc.M407030200. [DOI] [PubMed] [Google Scholar]
- 42.Harraz MM. Marden JJ. Zhou W. Zhang Y. Williams A. Sharov VS. Nelson K. Luo M. Paulson H. Schoneich C. Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawkins BJ. Madesh M. Kirkpatrick CJ. Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayakawa M. Miyashita H. Sakamoto I. Kitagawa M. Tanaka H. Yasuda H. Karin M. Kikugawa K. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jankowski A. Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential: quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J Biol Chem. 1999;274:26098–26104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- 46.Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol. 2007;578(Pt 3):633–640. doi: 10.1113/jphysiol.2006.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol. 2008;43:3–36. doi: 10.1080/10409230701829110. [DOI] [PubMed] [Google Scholar]
- 48.Lai HC. Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- 49.Lakadamyali M. Rust MJ. Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lassegue B. Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 51.Lauf PK. Zhang J. Gagnon KB. Delpire E. Fyffe RE. Adragna NC. K-Cl cotransport: immunohistochemical and ion flux studies in human embryonic kidney (HEK293) cells transfected with full-length and C-terminal-domain-truncated KCC1 cDNAs. Cell Physiol Biochem. 2001;11:143–160. doi: 10.1159/000047802. [DOI] [PubMed] [Google Scholar]
- 52.Li Q. Spencer NY. Oakley FD. Englehardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-κB by TNF-α. Antioxid Redox Signal. 2009;11:1249–1263. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q. Zhang Y. Marden JJ. Banfi B. Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J. 2008;411:531–541. doi: 10.1042/BJ20071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Q. Harraz MM. Zhou W. Zhang LN. Ding W. Zhang Y. Eggleston T. Yeaman C. Banfi B. Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2001;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X. Wang T. Zhao Z. Weinman SA. The ClC-3 chloride channel promotes acidification of lysosomes in CHO- K1 and Huh-7 cells. Am J Physiol Cell Physiol. 2002;282:C1483–C1491. doi: 10.1152/ajpcell.00504.2001. [DOI] [PubMed] [Google Scholar]
- 56.Lu C. Pribanic S. Debonneville A. Jiang C. Rotin D. The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic. 2007;8:1246–1264. doi: 10.1111/j.1600-0854.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- 57.Ma D. Zerangue N. Raab-Graham K. Fried SR. Jan YN. Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33:715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 58.Maier CM. Chan PH. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist. 2002;8:323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- 59.Mantegazza AR. Savina A. Vermeulen M. Perez L. Geffner J. Hermine O. Rosenzweig SD. Faure F. Amigorena S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuda JJ. Filali MS. Volk KA. Collins MM. Moreland JG. Lamb FS. Overexpression of CLC-3 in HEK293T cells yields novel currents that are pH dependent. Am J Physiol Cell Physiol. 2008;294:C251–C262. doi: 10.1152/ajpcell.00338.2007. [DOI] [PubMed] [Google Scholar]
- 61.Maturana A. Krause KH. Demaurex N. NOX family NADPH oxidases: do they have built-in proton channels? J Gen Physiol. 2002;120:781–786. doi: 10.1085/jgp.20028713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maycox PR. Deckwerth T. Hell JW. Jahn R. Glutamate uptake by brain synaptic vesicles: energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem. 1988;263:15423–15428. [PubMed] [Google Scholar]
- 63.McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- 64.Mellman I. Fuchs R. Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 65.Miller FJ Jr. Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 66.Mohammad-Panah R. Ackerley C. Rommens J. Choudhury M. Wang Y. Bear CE. The chloride channel ClC-4 co-localizes with cystic fibrosis transmembrane conductance regulator and may mediate chloride flux across the apical membrane of intestinal epithelia. J Biol Chem. 2002;277:566–574. doi: 10.1074/jbc.M106968200. [DOI] [PubMed] [Google Scholar]
- 67.Moreland JG. Davis AP. Bailey G. Nauseef WM. Lamb FS. Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J Biol Chem. 2006;281:12277–12288. doi: 10.1074/jbc.M511030200. [DOI] [PubMed] [Google Scholar]
- 68.Moreland JG. Davis AP. Matsuda JJ. Hook JS. Bailey G. Nauseef WM. Lamb FS. Endotoxin priming of neutrophils requires NADPH oxidase-generated oxidants and is regulated by the anion transporter ClC-3. J Biol Chem. 2007;282:33958–33967. doi: 10.1074/jbc.M705289200. [DOI] [PubMed] [Google Scholar]
- 69.Mumbengegwi DR. Li Q. Li C. Bear CE. Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murk JL. Posthuma G. Koster AJ. Geuze HJ. Verkleij AJ. Kleijmeer MJ. Humbel BM. Influence of aldehyde fixation on the morphology of endosomes and lysosomes: quantitative analysis and electron tomography. J Microsc. 2003;212(Pt 1):81–90. doi: 10.1046/j.1365-2818.2003.01238.x. [DOI] [PubMed] [Google Scholar]
- 71.Murphy R. Decoursey TE. Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Nguitragool W. Miller C. Uncoupling of a CLC Cl−/H+ exchange transporter by polyatomic anions. J Mol Biol. 2006;362:682–690. doi: 10.1016/j.jmb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Okada K. Ishikawa S. Saito T. Enhancement of intracellular sodium by vasopressin in spontaneously hypertensive rats. Hypertension. 1993;22:300–305. doi: 10.1161/01.hyp.22.3.300. [DOI] [PubMed] [Google Scholar]
- 74.Orlowski J. Grinstein S. Na+/H+ exchangers of mammalian cells. J Biol Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- 75.Ozsoy HZ. Sivasubramanian N. Wieder ED. Pedersen S. Mann DL. Oxidative stress promotes ligand-independent and enhanced ligand-dependent tumor necrosis factor receptor signaling. J Biol Chem. 2008;283:23419–23428. doi: 10.1074/jbc.M802967200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Picollo A. Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420–423. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 77.Piwon N. Gunther W. Schwake M. Bosl MR. Jentsch TJ. CIC-5 Cl- channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- 78.Plonka A. Mayer J. Metodiewa D. Gebicki JL. Zgirski A. Grabska M. Superoxide radical dismutation by copper proteins. J Radioanalyt Nucl Chem. 1986;101:221–225. [Google Scholar]
- 79.Prange HD. Shoemaker JL Jr. Westen EA. Horstkotte DG. Pinshow B. Physiological consequences of oxygen-dependent chloride binding to hemoglobin. J Appl Physiol. 2001;91:33–38. doi: 10.1152/jappl.2001.91.1.33. [DOI] [PubMed] [Google Scholar]
- 80.Pryor WA. Houk KN. Foote CS. Fukuto JM. Ignarro LJ. Squadrito GL. Davies KJ. Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291:R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 81.Ramsey IS. Moran MM. Chong JA. Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reeves EP. Lu H. Jacobs HL. Messina CG. Bolsover S. Gabella G. Potma EO. Warley A. Roes J. Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 83.Reimer RJ. Fremeau RT Jr. Bellocchio EE. Edwards RH. The essence of excitation. Curr Opin Cell Biol. 2001;13:417–421. doi: 10.1016/s0955-0674(00)00230-1. [DOI] [PubMed] [Google Scholar]
- 84.Rothman JE. Lenard J. Membrane asymmetry. Science. 1977;195:743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- 85.Rybak SL. Lanni F. Murphy RF. Theoretical considerations on the role of membrane potential in the regulation of endosomal pH. Biophys J. 1997;73:674–687. doi: 10.1016/S0006-3495(97)78102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saito M. Hanson PI. Schlesinger P. Luminal chloride-dependent activation of endosome calcium channels: patch clamp study of enlarged endosomes. J Biol Chem. 2007;282:27327–27333. doi: 10.1074/jbc.M702557200. [DOI] [PubMed] [Google Scholar]
- 87.Sangan P. Rajendran VM. Geibel JP. Binder HJ. Cloning and expression of a chloride-dependent Na+-H+ exchanger. J Biol Chem. 2002;277:9668–9675. doi: 10.1074/jbc.M110852200. [DOI] [PubMed] [Google Scholar]
- 88.Sasaki M. Takagi M. Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 89.Scheel O. Zdebik AA. Lourdel S. Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–427. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 90.Shult PA. Graziano FM. Wallow IH. Busse WW. Comparison of superoxide generation and luminol-dependent chemiluminescence with eosinophils and neutrophils from normal individuals. J Lab Clin Med. 1985;106:638–645. [PubMed] [Google Scholar]
- 91.Simchowitz L. De Weer P. Chloride movements in human neutrophils: diffusion, exchange, and active transport. J Gen Physiol. 1986;88:167–194. doi: 10.1085/jgp.88.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sonawane ND. Verkman AS. Determinants of [Cl-] in recycling and late endosomes and Golgi complex measured using fluorescent ligands. J Cell Biol. 2003;160:1129–1238. doi: 10.1083/jcb.200211098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sonawane ND. Thiagarajah JR. Verkman AS. Chloride concentration in endosomes measured using a ratioable fluorescent Cl− indicator: evidence for chloride accumulation during acidification. J Biol Chem. 2002;277:5506–5513. doi: 10.1074/jbc.M110818200. [DOI] [PubMed] [Google Scholar]
- 94.Stobrawa SM. Breiderhoff T. Takamori S. Engel D. Schweizer M. Zdebik AA. Bosl MR. Ruether K. Jahn H. Draguhn A. John R. Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 95.Thevenod F. Ion channels in secretory granules of the pancreas and their role in exocytosis and release of secretory proteins. Am J Physiol Cell Physiol. 2002;283:C651–C672. doi: 10.1152/ajpcell.00600.2001. [DOI] [PubMed] [Google Scholar]
- 96.Thevenod F. Barg S. Braun M. Renstrom E. Rorsman P. The stimulatory effect of tolbutaminde on Ca++-dependent exocytosis is mediated by a 65 kDa MDR-like P-glycoprotein and ClC-3 Cl channels in granule membranes of insulin secreting B-cells. FASEB J. 2000;14:A109. [Google Scholar]
- 97.Ting-Beall HP. Needham D. Hochmuth RM. Volume and osmotic properties of human neutrophils. Blood. 1993;81:2774–2780. [PubMed] [Google Scholar]
- 98.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006(349):re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 99.Valentine JS. Curtis AB. A convenient preparation of solutions of superoxide anion and the reaction of superoxide anion with a copper (II) complex. J Am Chem Soc. 1975;97:224–226. doi: 10.1021/ja00834a058. [DOI] [PubMed] [Google Scholar]
- 100.Van Dyke RW. Na+/H+ exchange modulates acidification of early rat liver endocytic vesicles. Am J Physiol. 1995;269(4 Pt 1):C943–C954. doi: 10.1152/ajpcell.1995.269.4.C943. [DOI] [PubMed] [Google Scholar]
- 101.Van Dyke RW. Belcher JD. Acidification of three types of liver endocytic vesicles: similarities and differences. Am J Physiol. 1994;266(1 Pt 1):C81–C94. doi: 10.1152/ajpcell.1994.266.1.C81. [DOI] [PubMed] [Google Scholar]
- 102.Winterbourn CC. Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 103.Winterbourn CC. Hampton MB. Livesey JH. Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 104.Xie XS. Stone DK. Racker E. Determinants of clathrin-coated vesicle acidification. J Biol Chem. 1983;258:14834–14838. [PubMed] [Google Scholar]
- 105.Zweifel LS. Kuruvilla R. Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]