Abstract
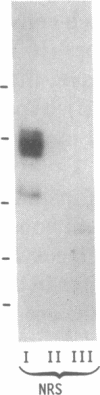
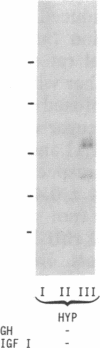
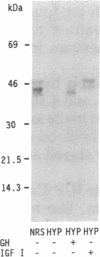
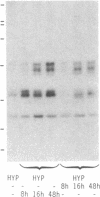
The physiology of the specific serum binding proteins which constitute the main storage pool for insulin-like growth factors (IGFs) in mammals is still incompletely understood. We have, therefore, investigated the regulation of these proteins in (i) hypophysectomized (hypox) rats infused with recombinant human growth hormone (rhGH) or recombinant human IGF 1 (rhIGF I) and (ii) streptozotocin-diabetic rats infused with insulin or rhIGF I. The main carrier protein, a GH-dependent complex of apparent molecular mass 200 kDa, contains N-glycosylated IGF-binding subunits (42, 45, and 49 kDa) that differ in their glycosyl but not in their protein moiety. These subunits are lacking in hypox and diabetic rats. They are induced by GH and insulin, respectively, and appear in the 200-kDa complex. Infusion of rhIGF I induces the subunits in both states; however, only in diabetic, not in hypox, rats do they form the 200-kDa complex. Glycosylated carrier protein subunits do not appear before 8 hr of rhIGF I infusion. During that period, hypox rats may become severely hypoglycemic. After 16 hr, glycosylated subunits are clearly induced, and blood sugar values are normal. We conclude: (i) The N-glycosylated subunits of the 200-kDa complex reflect the IGF I status. (ii) IGF I may mediate the induction of these subunits by GH. (iii) Significant association to the 200-kDa complex occurs only in the presence of GH. It is likely that GH, but not IGF I, induces a component, which itself does not bind IGF, but associates with the glycosylated IGF-binding subunits. (iv) The glycosylated subunits protect against IGF-induced hypoglycemia and may be involved in tissue-specific targeting of IGFs.
Full text
PDF
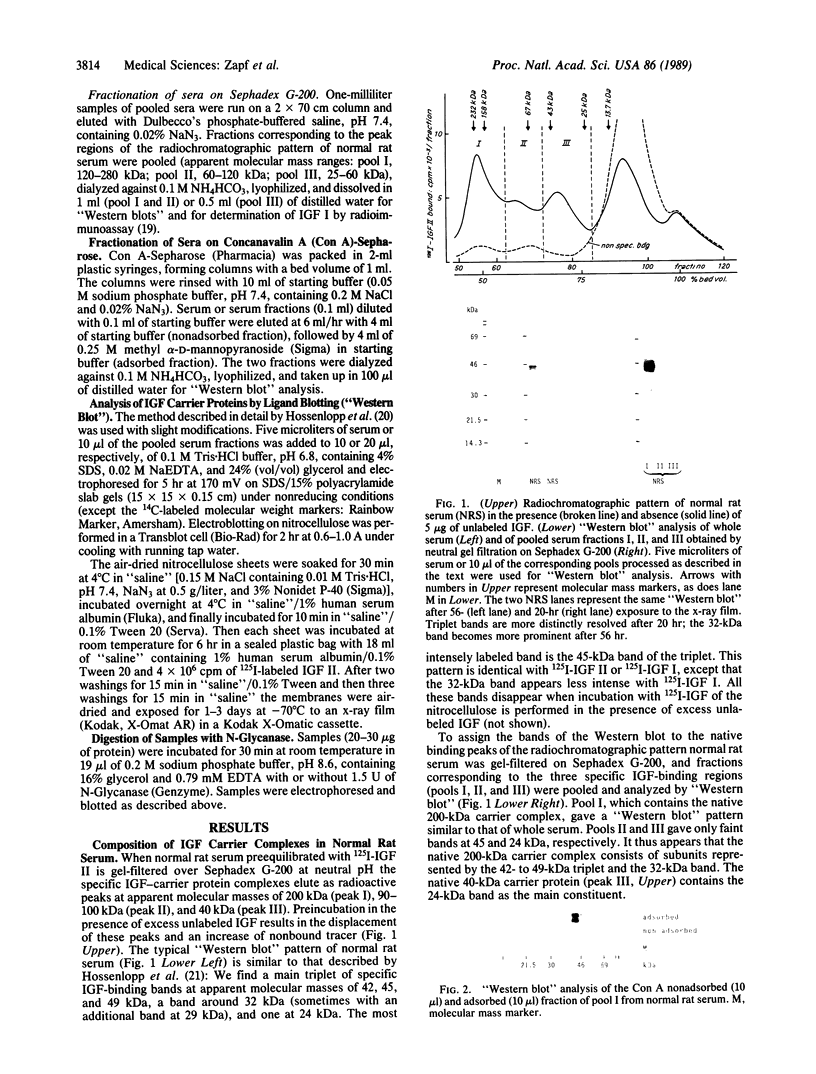
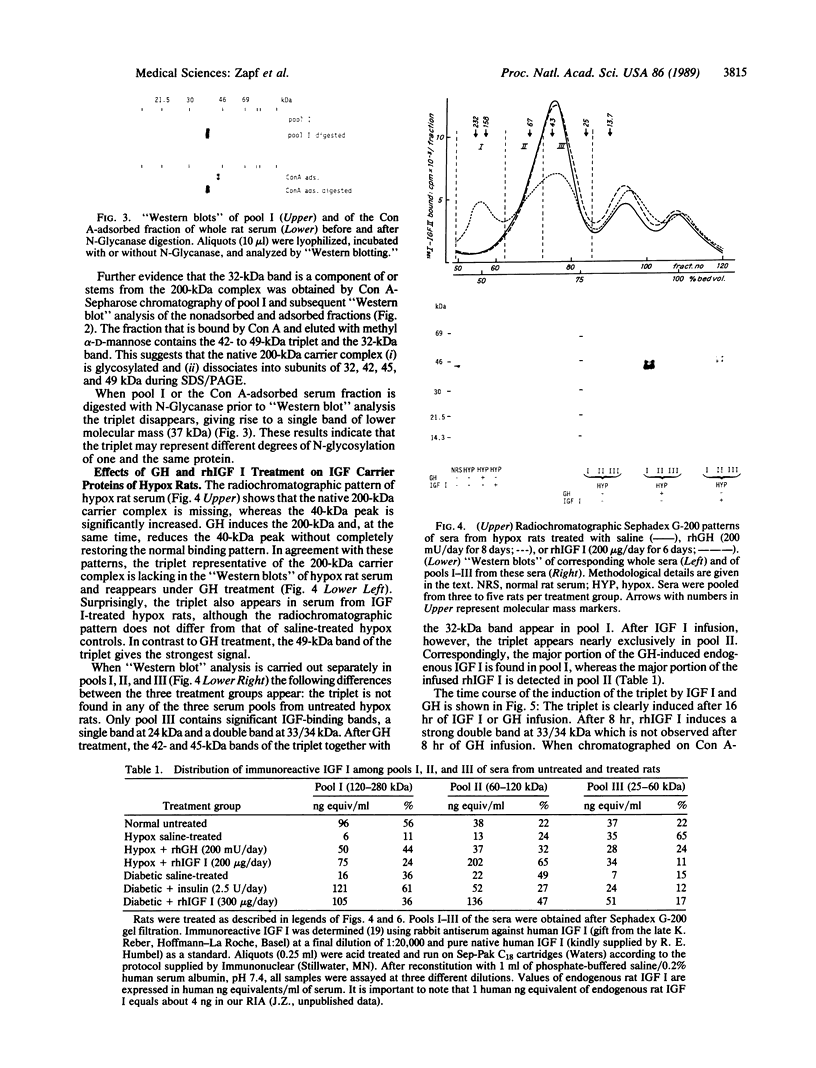
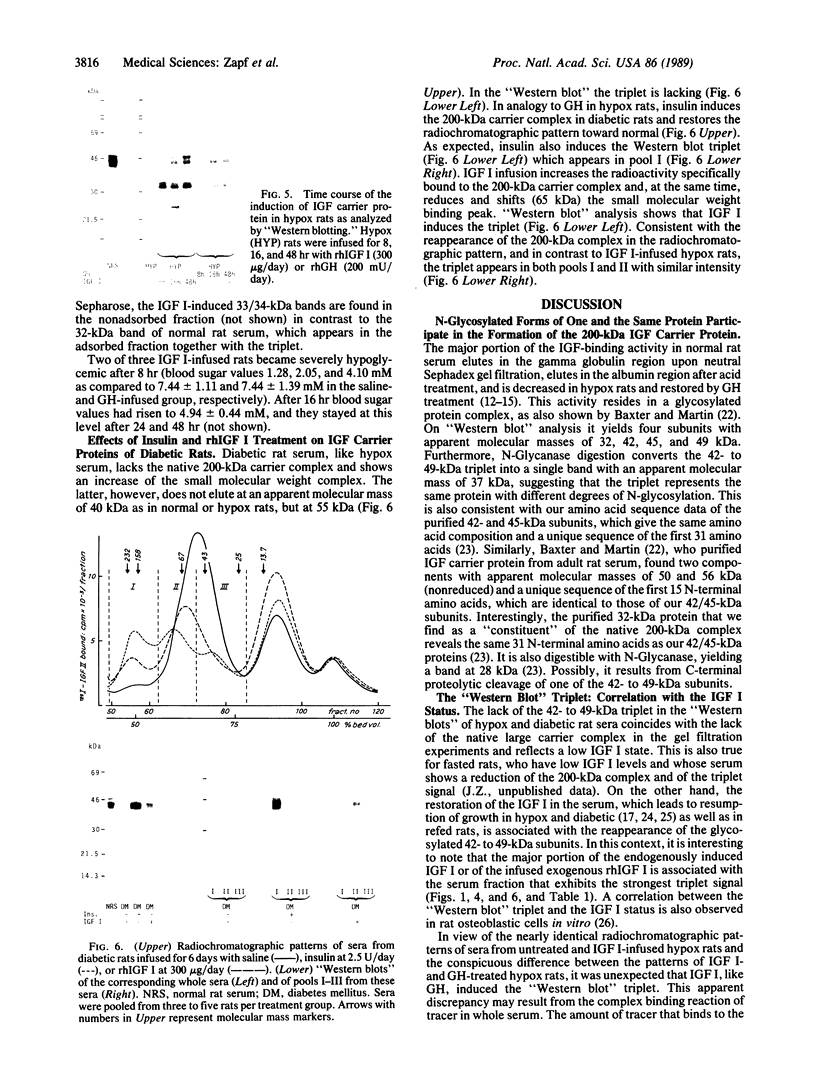

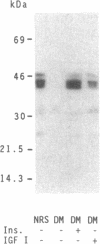
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Molitch M. E., Van Wyk J. J., Underwood L. E. Human growth hormone and somatomedin C suppress the spontaneous release of growth hormone in unanesthetized rats. Endocrinology. 1983 Oct;113(4):1319–1324. doi: 10.1210/endo-113-4-1319. [DOI] [PubMed] [Google Scholar]
- Baxter R. C. Characterization of the acid-labile subunit of the growth hormone-dependent insulin-like growth factor binding protein complex. J Clin Endocrinol Metab. 1988 Aug;67(2):265–272. doi: 10.1210/jcem-67-2-265. [DOI] [PubMed] [Google Scholar]
- Baxter R. C., Martin J. L. Binding proteins for insulin-like growth factors in adult rat serum. Comparison with other human and rat binding proteins. Biochem Biophys Res Commun. 1987 Aug 31;147(1):408–415. doi: 10.1016/s0006-291x(87)80136-5. [DOI] [PubMed] [Google Scholar]
- Cohen K. L., Nissley S. P. The serum half-life of somatomedin activity: evidence for growth hormone dependence. Acta Endocrinol (Copenh) 1976 Oct;83(2):243–258. doi: 10.1530/acta.0.0830243. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Raben M. S., Salmon W. D., Jr, van den Brande J. L., van Wyk J. J. Somatomedin: proposed designation for sulphation factor. Nature. 1972 Jan 14;235(5333):107–107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Mariz I. K., Blethen S. L. Inhibition of access of bound somatomedin to membrane receptor and immunobinding sites: a comparison of radioreceptor and radioimmunoassay of somatomedin in native and acid-ethanol-extracted serum. J Clin Endocrinol Metab. 1980 Oct;51(4):781–788. doi: 10.1210/jcem-51-4-781. [DOI] [PubMed] [Google Scholar]
- Elgin R. G., Busby W. H., Jr, Clemmons D. R. An insulin-like growth factor (IGF) binding protein enhances the biologic response to IGF-I. Proc Natl Acad Sci U S A. 1987 May;84(10):3254–3258. doi: 10.1073/pnas.84.10.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlanetto R. W. The somatomedin C binding protein: evidence for a heterologous subunit structure. J Clin Endocrinol Metab. 1980 Jul;51(1):12–19. doi: 10.1210/jcem-51-1-12. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Froesch E. R. Short-term metabolic effects of recombinant human insulin-like growth factor I in healthy adults. N Engl J Med. 1987 Jul 16;317(3):137–140. doi: 10.1056/NEJM198707163170303. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Scheiwiller E., Froesch E. R. Recombinant human insulin-like growth factor I stimulates growth and has distinct effects on organ size in hypophysectomized rats. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4889–4893. doi: 10.1073/pnas.85.13.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz R. L. Plasma forms of somatomedin and the binding protein phenomenon. Clin Endocrinol Metab. 1984 Mar;13(1):31–42. doi: 10.1016/s0300-595x(84)80007-9. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P., Seurin D., Segovia-Quinson B., Hardouin S., Binoux M. Analysis of serum insulin-like growth factor binding proteins using western blotting: use of the method for titration of the binding proteins and competitive binding studies. Anal Biochem. 1986 Apr;154(1):138–143. doi: 10.1016/0003-2697(86)90507-5. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P., Seurin D., Segovia B., Portolan G., Binoux M. Heterogeneity of insulin-like growth factor binding proteins between structure and affinity. 2. Forms released by human and rat liver in culture. Eur J Biochem. 1987 Dec 30;170(1-2):133–142. doi: 10.1111/j.1432-1033.1987.tb13677.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann U., Zapf J., Froesch E. R. Growth-hormone dependence of non-suppressible insulin-like activity (NSILA) and of NSILA-carrier protein in rats. Acta Endocrinol (Copenh) 1978 Apr;87(4):716–727. doi: 10.1530/acta.0.0870716. [DOI] [PubMed] [Google Scholar]
- Meuli C., Zapf J., Froesch E. R. NSILA-carrier protein abolishes the action of nonsuppressible insulin-like activity (NSILA-S) on perfused rat heart. Diabetologia. 1978 Apr;14(4):255–259. doi: 10.1007/BF01219425. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nessley S. P., Cohen K. L., Rechler M. M. Specific binding of a somatomedin-like polypeptide in rat serum depends on growth hormone. Nature. 1976 Sep 9;263(5573):137–140. doi: 10.1038/263137a0. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Passamani J., White R. M. Further characterization of growth hormone-dependent somatomedin-binding proteins in rat serum and demonstration of somatomedin-binding proteins produced by rat liver cells in culture. Endocrinology. 1979 Feb;104(2):536–546. doi: 10.1210/endo-104-2-536. [DOI] [PubMed] [Google Scholar]
- Scheiwiller E., Guler H. P., Merryweather J., Scandella C., Maerki W., Zapf J., Froesch E. R. Growth restoration of insulin-deficient diabetic rats by recombinant human insulin-like growth factor I. Nature. 1986 Sep 11;323(6084):169–171. doi: 10.1038/323169a0. [DOI] [PubMed] [Google Scholar]
- Schmid C., Zapf J., Froesch E. R. Production of carrier proteins for insulin-like growth factors (IGFs) by rat osteoblastic cells. Regulation by IGF I and cortisol. FEBS Lett. 1989 Feb 27;244(2):328–332. doi: 10.1016/0014-5793(89)80556-3. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Hauri C., Steiner T., Froesch E. R. Comparison of in vivo effects of insulin-like growth factors I and II and of growth hormone in hypophysectomized rats. Acta Endocrinol (Copenh) 1985 Feb;108(2):167–174. doi: 10.1530/acta.0.1080167. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Humbel R. E., Froesch E. R. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982 Mar 18;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- Tannenbaum G. S. Growth hormone secretory dynamics in streptozotocin diabetes: evidence of a role for endogenous circulating somatostatin. Endocrinology. 1981 Jan;108(1):76–82. doi: 10.1210/endo-108-1-76. [DOI] [PubMed] [Google Scholar]
- Zapf J., Born W., Chang J. Y., James P., Froesch E. R., Fischer J. A. Isolation and NH2-terminal amino acid sequences of rat serum carrier proteins for insulin-like growth factors. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1187–1194. doi: 10.1016/s0006-291x(88)80758-7. [DOI] [PubMed] [Google Scholar]
- Zapf J., Froesch E. R., Humbel R. E. The insulin-like growth factors (IGF) of human serum: chemical and biological characterization and aspects of their possible physiological role. Curr Top Cell Regul. 1981;19:257–309. doi: 10.1016/b978-0-12-152819-5.50024-5. [DOI] [PubMed] [Google Scholar]
- Zapf J., Hauri C., Waldvogel M., Froesch E. R. Acute metabolic effects and half-lives of intravenously administered insulinlike growth factors I and II in normal and hypophysectomized rats. J Clin Invest. 1986 Jun;77(6):1768–1775. doi: 10.1172/JCI112500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Froesch E. R. In vivo effects of the insulin-like growth factors (IGFs) in the hypophysectomized rat: comparison with human growth hormone and the possible role of the specific IGF carrier proteins. Ciba Found Symp. 1985;116:169–187. doi: 10.1002/9780470720974.ch11. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Jagars G., Sand I., Grunwald J., Froesch E. R. Inhibition of the action of nonsuppressible insulin-like activity on isolated rat fat cells by binding to its carrier protein. J Clin Invest. 1979 May;63(5):1077–1084. doi: 10.1172/JCI109377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Walter H., Froesch E. R. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest. 1981 Nov;68(5):1321–1330. doi: 10.1172/JCI110379. [DOI] [PMC free article] [PubMed] [Google Scholar]