Abstract
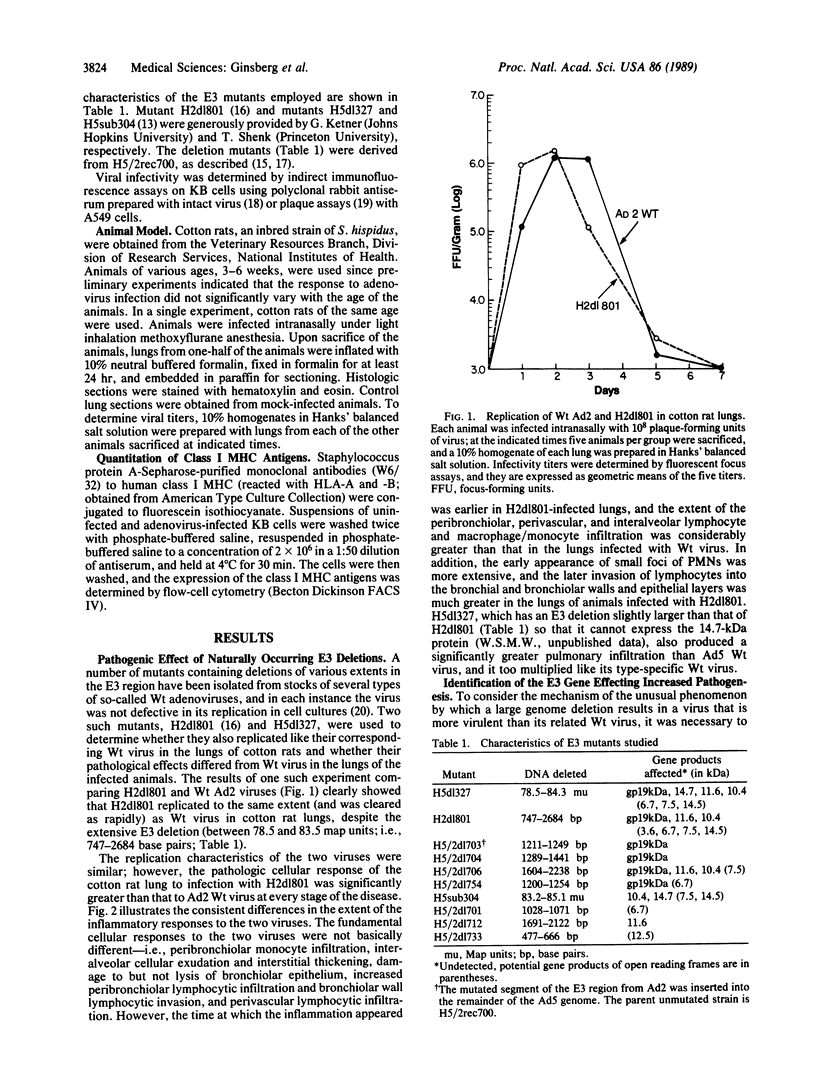
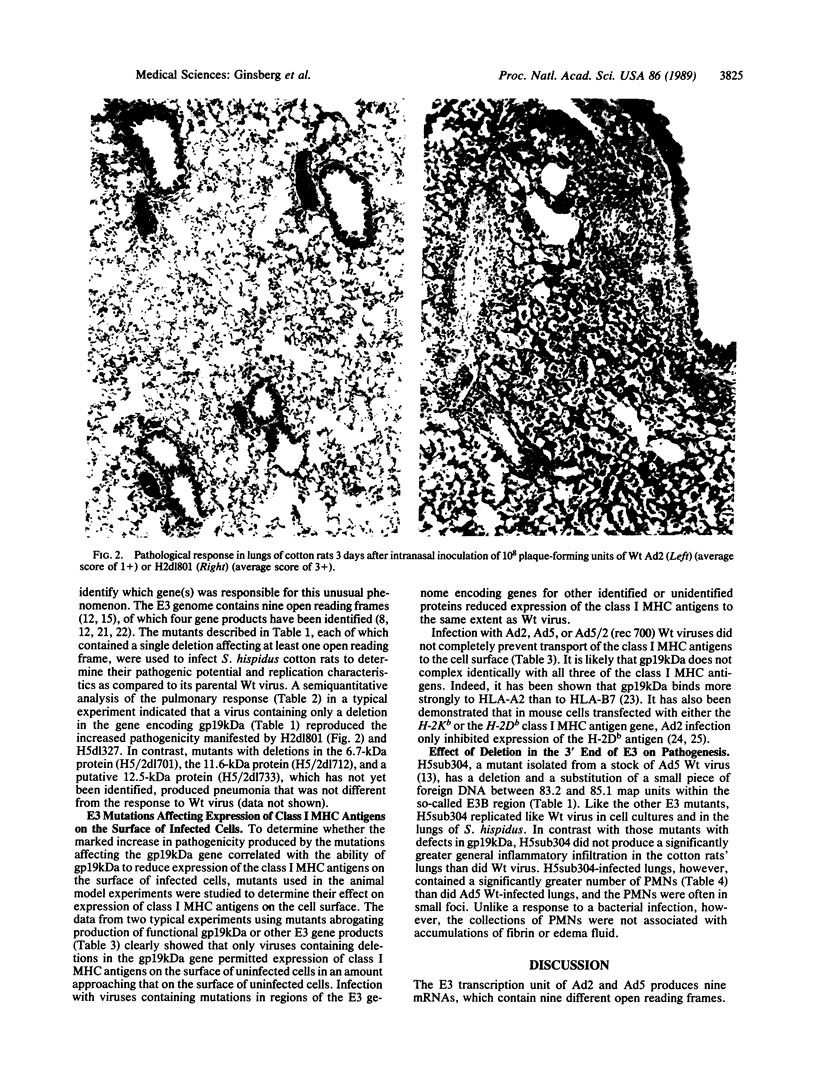
The cotton rat Sigmodon hispidus has provided an animal model of adenovirus pneumonia that permits investigation of the viral gene products required to produce the disease and the molecular mechanisms effecting the damage. This study was carried out to test the hypothesis that early region 3 (E3) of the adenovirus genome plays a critical role in pathogenesis of the virus's disease process even though none of its gene products are essential for its replication. Mutants whose E3 region is largely deleted (i.e., H2dl801 and H5dl327) replicated like wild-type virus in the cotton rats' lungs, but the lymphocyte and macrophage/monocyte inflammatory response was markedly increased. Viruses containing mutations that ablated production of the 19-kDa glycoprotein had the same effect as H2dl801 and H5dl327. However, mutants with deletions in the other E3 open reading frames, some of which encode known proteins, did not differ from wild-type virus in their pathogenic properties. The 19-kDa glycoprotein markedly reduces expression of the class I major histocompatibility complex antigens on the surface of infected cells. A complete correlation was found between those mutants that had increased pathogenic effects and those that lost the ability to reduce transport of the class I major histocompatibility complex antigens to surface of infected cells (i.e., all mutants unable to express the 19-kDa glycoprotein). H5sub304, which has a deletion between 83.2 and 85.1 map units in the E3B region and expresses the 19-kDa glycoprotein, did not increase the extent of pneumonia but qualitatively changed the inflammatory response in that increased numbers of polymorphonuclear leukocytes accumulated, often in small foci.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984 Apr;50(1):202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S., Darnell J. E., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985 Oct;5(10):2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat B. M., Brady H. A., Pursley M. H., Wold W. S. Deletion mutants that alter differential RNA processing in the E3 complex transcription unit of adenovirus. J Mol Biol. 1986 Aug 20;190(4):543–557. doi: 10.1016/0022-2836(86)90240-8. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. The E3/19K protein of adenovirus type 2 binds to the domains of histocompatibility antigens required for CTL recognition. EMBO J. 1987 Jul;6(7):2019–2026. doi: 10.1002/j.1460-2075.1987.tb02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg S. S., Ketner G. Deletion mutants of adenovirus 2: isolation and initial characterization of virus carrying mutations near the right end of the viral genome. Virology. 1981 Oct 15;114(1):196–209. doi: 10.1016/0042-6822(81)90265-8. [DOI] [PubMed] [Google Scholar]
- Dewar R. L., Natarajan V., Vasudevachari M. B., Salzman N. P. Synthesis and processing of human immunodeficiency virus type 1 envelope proteins encoded by a recombinant human adenovirus. J Virol. 1989 Jan;63(1):129–136. doi: 10.1128/jvi.63.1.129-136.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson W. P., Purcell R. H., Gundelfinger B. F., Love J. W., Ludwig W., Chanock R. M. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue culture. II. specific protective effect against epidemic disease. JAMA. 1966 Feb 7;195(6):453–459. [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S. Identification and classification of adenoviruses. Virology. 1962 Oct;18:312–319. doi: 10.1016/0042-6822(62)90018-1. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S., Mackey J. K., Rigden P. Analysis of human tonsil and cancer DNAs and RNAs for DNA sequences of group C (serotypes 1, 2, 5, and 6) human adenoviruses. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6606–6610. doi: 10.1073/pnas.76.12.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Kloszewski E. D., Anderson R. W., Thompson D. L., Tevethia S. S. Effect of herpes simplex virus types 1 and 2 on surface expression of class I major histocompatibility complex antigens on infected cells. J Virol. 1985 Dec;56(3):757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J Virol. 1973 Sep;12(3):643–652. doi: 10.1128/jvi.12.3.643-652.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin J. E., Lubeck M. D., Barton J. E., Conley A. J., Davis A. R., Hung P. P. Recombinant adenovirus induces antibody response to hepatitis B virus surface antigen in hamsters. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4626–4630. doi: 10.1073/pnas.84.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini D. L., Dubovi E. J., Clyde W. A., Jr A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984 Jul;150(1):92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- Persson H., Jansson M., Philipson L. Synthesis and genomic site for an adenovirus type 2 early glycoprotein. J Mol Biol. 1980 Feb 5;136(4):375–394. doi: 10.1016/0022-2836(80)90396-4. [DOI] [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HUEBNER R. J., GILMORE L. K., PARROTT R. H., WARD T. G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953 Dec;84(3):570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- Ross S., Levine A. J. The genomic map position of the adenovirus type 2 glycoprotein. Virology. 1979 Dec;99(2):427–430. doi: 10.1016/0042-6822(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Severinsson L., Martens I., Peterson P. A. Differential association between two human MHC class I antigens and an adenoviral glycoprotein. J Immunol. 1986 Aug 1;137(3):1003–1009. [PubMed] [Google Scholar]
- Severinsson L., Peterson P. A. Abrogation of cell surface expression of human class I transplantation antigens by an adenovirus protein in Xenopus laevis oocytes. J Cell Biol. 1985 Aug;101(2):540–547. doi: 10.1083/jcb.101.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Tevethia S. S. Differential effect of adenovirus 2 E3/19K glycoprotein on the expression of H-2Kb and H-2Db class I antigens and H-2Kb- and H-2Db-restricted SV40-specific CTL-mediated lysis. Virology. 1988 Aug;165(2):357–366. doi: 10.1016/0042-6822(88)90580-6. [DOI] [PubMed] [Google Scholar]
- Tollefson A. E., Wold W. S. Identification and gene mapping of a 14,700-molecular-weight protein encoded by region E3 of group C adenoviruses. J Virol. 1988 Jan;62(1):33–39. doi: 10.1128/jvi.62.1.33-39.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top F. H., Jr, Grossman R. A., Bartelloni P. J., Segal H. E., Dudding B. A., Russell P. K., Buescher E. L. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J Infect Dis. 1971 Aug;124(2):148–154. doi: 10.1093/infdis/124.2.148. [DOI] [PubMed] [Google Scholar]
- Wang E. W., Scott M. O., Ricciardi R. P. An adenovirus mRNA which encodes a 14,700-Mr protein that maps to the last open reading frame of region E3 is expressed during infection. J Virol. 1988 Apr;62(4):1456–1459. doi: 10.1128/jvi.62.4.1456-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Cladaras C., Magie S. C., Yacoub N. Mapping a new gene that encodes an 11,600-molecular-weight protein in the E3 transcription unit of adenovirus 2. J Virol. 1984 Nov;52(2):307–313. doi: 10.1128/jvi.52.2.307-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Deutscher S. L., Takemori N., Bhat B. M., Magie S. C. Evidence that AGUAUAUGA and CCAAGAUGA initiate translation in the same mRNA region E3 of adenovirus. Virology. 1986 Jan 15;148(1):168–180. doi: 10.1016/0042-6822(86)90412-5. [DOI] [PubMed] [Google Scholar]