Abstract
The key to understanding amyloid disease is the characterization of oligomeric species formed during the early stages of fibril assembly. Here we have used electrospray ionisation-ion mobility spectrometry-mass spectrometry to identify and structurally characterize the oligomers formed during amyloid assembly from β2-microglobulin (β2m). β2m oligomers are shown to have collision cross-sections consistent with monomeric units arranged in elongated assemblies prior to fibril formation. Direct observation, separation, and quantification of transient oligomeric species reveals that monomers to tetramers are populated through the lag phase with no evidence for the significant population of larger oligomeric species under the conditions employed. The dynamics of each oligomeric species were monitored directly within the ensemble at concentrations commensurate with amyloid formation by observing the subunit exchange of 14N- and 15N- labeled oligomers. Analysis of the data revealed a decrease in oligomer dynamics concomitant with increasing oligomer size and the copopulation of dynamic dimeric and trimeric species with more stable trimeric and tetrameric species. The results presented map the events occurring during the lag phase of fibril formation and give a clear insight into the structural characteristics and dynamic nature of the β2m oligomers, demonstrating the existence of elongated assemblies arising from an intact amyloidogenic protein during fibril formation.
Dialysis-related amyloidosis is a serious complication of long-term haemodialysis in which β2-microglobulin (β2m) has been identified as the major component of the amyloid fibrils (1). Despite the importance of amyloid disorders in human health, our knowledge of the structural molecular mechanism of amyloid formation remains limited, primarily because of the heterogeneity of the intermediates in the assembly process, as well as in the final fibrillar products themselves. Techniques such as atomic force microscopy (AFM) (2), NMR (3) and single molecule fluorescence (4) have been used to observe ensembles of protein oligomers formed during amyloid formation. However, electrospray ionisation-mass spectrometry (ESI-MS) offers further potential for the individual characterization of these copopulated, transient species. ESI-MS is ideally suited to the characterization of macromolecular protein complexes (5–8), and ion mobility spectrometry (IMS) coupled to ESI-MS (ESI-IMS-MS) has enabled the separation of copopulated protein conformers (9–13) and calculation of their individual collision cross-sections (Ωs) in a single experiment (14 and 15). In the context of amyloid fibril formation, ESI-MS has been applied to monitor monomer depletion during fibril assembly (16–18) and to identify transient oligomeric species (11, 16, 19, 20) for a number of amyloidogenic proteins, whereas ESI-IMS-MS has been employed to measure the Ωs of transient intermediates populated during the assembly of Aβ40 and Aβ42 (11).
In vitro β2m assembles rapidly into amyloid-like fibrils akin to their ex vivo counterparts via a nucleation-dependent mechanism at pH < 3.0 (2 and 21). The population of oligomers formed during β2m fibril formation under acidic conditions has been investigated by ESI-MS with monomers through to tetramers, but no higher order species, being observed during the lag time of assembly (16). This lack of higher order oligomers suggests that nucleation-dependent assembly occurs via one or more high-energy oligomeric species greater in size than tetramer, or that conformational rearrangement of one or more of the observed oligomers constitutes nucleation (21). Here we have used real-time ESI-MS and ESI-IMS-MS to probe the population, structural properties and dynamics of transient oligomers within highly heterogeneous solutions formed in the lag time of β2m amyloid fibril assembly. Ωs have been calculated for these species and compared with proteins of known structure, revealing that, by contrast with the ring-like arrangement of oligomers formed during Aβ assembly (11), the oligomers of β2m are arranged in an elongated manner. Combined with measurements of oligomer dynamics using 14N/15N subunit exchange, the data indicate that the initial stages of β2m amyloid formation proceed via a pool of rapidly interconverting, elongated oligomers which become less dynamic with increasing numbers of monomer subunits and, for some oligomers, with time. Subsequent formation of the amyloid nucleus results in rapid fibril formation without the obvious population of larger intermediates.
Results
Separation and Structural Characterization of β2m Oligomers.
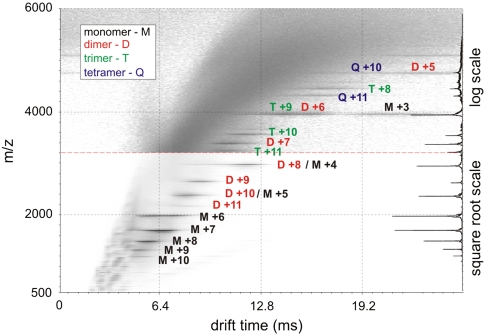
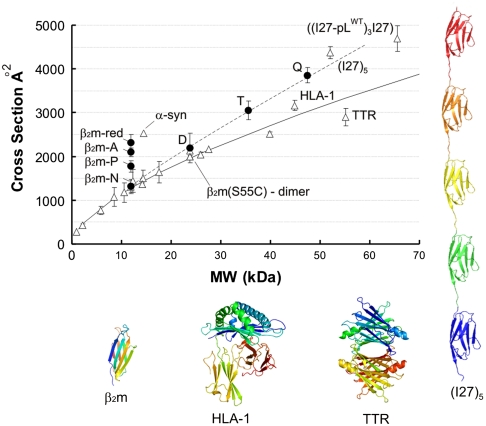
The transient nature of early intermediates of amyloid assembly, combined with their heterogeneity and instability, provide major challenges for their structural characterization. Here we use the powerful separation capability of ESI-IMS-MS to identify and characterize individual species copopulated during the self-assembly of β2m into amyloid fibrils. Fig. 1 shows the separation of different oligomeric intermediates formed during the lag time of amyloid assembly from acid-unfolded β2m at pH 2.5. As reported previously, monomers, dimers, trimers, and tetramers are observed under these conditions by ESI-MS during the lag time of nucleated growth at this pH (16). Here the added dimension of ESI-IMS-MS has allowed copopulated species of the same m/z but different Ω to be resolved, e.g., m/z 3954.4 is copopulated by +9 trimer, +6 dimer, and +3 monomer ions and m/z 4745.1 is copopulated by +10 tetramer and +5 dimer ions. Thus, the contribution of each oligomer to a particular m/z value has been determined, paving the way for their individual quantification and structural characterization. To assess the structural nature of the β2m oligomers, their measured Ωs were compared with the Ωs of a range of globular proteins of known structure (Fig. 2 and Table S1). In addition, Ωs were calculated from molecular mass assuming a spherical structure (21). The average density over all the globular protein standards measured was found to be 0.44 Da/Å3 under the conditions employed (Fig. 2), a value similar to the 0.47 Da/Å3 reported previously (8). Using these data it is possible to estimate whether a protein of unknown structure adopts a globular conformation by comparison of the observed Ω with the value predicted assuming a spherical structure of average density.
Fig. 1.
ESI-IMS-MS data for β2m (pH 2.5) one minute into the lag time of fibril assembly. M-monomer, D-dimer, T-trimer, and Q-tetramer; the number adjacent to each letter refers to the charge state of those ions. The scaling is adjusted to “square root” or “log” as appropriate to display the weaker ions clearly. The summed m/z spectrum is shown on the right.
Fig. 2.
Average collision cross-sections (Ω) over all charge states measured by ESI-IMS-MS for β2m monomer and oligomers, and a range of protein standards. Error bars are one standard deviation in Ω between charge states of a protein. Black circles represent the average Ω for each β2m species at pH 2.5: β2m-N is compact β2m monomer, β2m-P partially folded, and β2m-A acid-unfolded; three conformers copopulated at pH 2.5. β2m-red is acid-unfolded, reduced β2m at pH 2.5. D = dimer, T = trimer and Q = tetramer. Open triangles represent the Ωs of proteins analyzed at pH 6.5 (Table S1). Proteins of note: β2m(S55C)-dimer is a covalent dimer of two natively-folded β2m monomers bridged by a mutationally introduced intermolecular disulphide bond; α-synuclein is a natively-unfolded protein; transthyretin tetramer (TTR) and human leukocyte antigen complex I (HLA-I) are globular proteins of similar mass to the β2m tetramer; I275 is a linear polymer of five I27 domains; ((I27-pLWT)3I27) is a linear polymer of four I27 domains and three protein-L domains arranged alternately. Ribbon structures of native β2m at pH 6.5, HLA-I (3HLA), TTR (1ICT), and I275 (1TIT modified) are shown. Expected Ωs were calculated for a range molecular weights (MW) assuming a perfect sphere of density 0.44 Da/Å3 (—) (27).
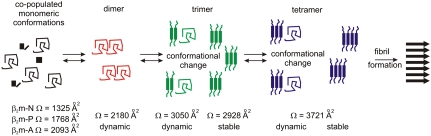
Monomeric β2m has been shown by ESI-IMS-MS to populate three distinct conformeric families in low ionic strength buffer at pH 2.5: acid-unfolded (β2m-A; 35%), partially folded (β2m-P; 55%), and native-like (β2m-N; 10%) conformations (Fig. 2) (10 and 15). The Ωs measured by ESI-IMS-MS for β2m-A, β2m-P, and β2m-N were 2,093, 1,768, and 1,325 Å2, respectively (10, 15, 22). The Ω of β2m-A can be compared with that of the intrinsically unfolded protein, α-synuclein, that at 14.5 kDa is of similar mass to β2m (11.8 kDa), but has a significantly more expanded shape (Ω = 2,530 Å2) (Fig. 2) (23). This comparison indicates that at pH 2.5 β2m-A retains residual structure, consistent with NMR analysis of this species (24 and 25). In accord with this, reduction of the single disulphide bond of β2m linking Cys25 and Cys80 (β2m-red) results in a more expanded species with increased Ω (2,401 Å2). The noncovalent dimer (D) of β2m detected at pH 2.5 has a Ω which is only slightly increased (2,180 Å) compared with β2m-A and β2m-P, suggesting that dimerization is accompanied by structural collapse or a restructuring event. To assess the nature of the dimer, the Ωs of various β2m dimers were measured by ESI-IMS-MS (Fig. S1). These included a noncovalent β2m dimer formed at pH 6.5 where β2m is natively structured, and two disulphide-linked dimers formed from natively-folded β2m engineered to contain a Cys at a solvent exposed site: S55C (forming an edge-to-edge dimer) or T73C (forming an end-to-end dimer). The Ω data for these species indicate that the β2m dimer present at pH 2.5 is 5%–10% more expanded than the S55C edge-to-edge dimer and the pH 6.5 dimer, but is more compact than predicted for a model of a highly expanded form of the protein dimer, whose Ω (2,500 Å2) was calculated in silico (15 and 22).
Comparison of the Ωs of the β2m oligomers with native, globular proteins of similar mass indicates that these assemblies are more expanded than may be expected for spherical constructs (Fig. 2). For example, the major β2m trimer (36 kDa) has a Ω of 2,928 Å2, which is larger than that of alcohol dehydrogenase (40 kDa; 2,490 Å2). Similarly, β2m tetramer (47 kDa) displays a Ω significantly larger (3,721 Å2) than that of the globular transthyretin tetramer (55 kDa; 2,900 Å2), but is more akin to a concatamer of five titin I27 Ig domains linked end-to-end (I275) (52 kDa; 4,297 Å2). The Ω measurements suggest that the β2m oligomers do not form spherical structures, since a spherical tetramer would require a very low density of ∼0.3 Da/Å3 to account for the Ω observed. Such a low density would require a highly unstructured conformation, which would be expected to yield a wide ESI-MS charge state distribution. By contrast, the tetramer populates predominantly the +11 and +10 ions, indicating some structurally defined elements. Moreover, limited pepsin proteolysis revealed that whereas the monomer shows indications of rapid degradation, higher order assemblies exhibit resistance to proteolysis (Fig. S2). Instead, the Ω data are more consistent with the β2m oligomers adopting an elongated structure. This arrangement could either be highly ordered throughout or held together by an element of local ordered structure (possibly involving residues in the region ∼60–70, which we have shown to play a pivotal role in determining the rate of fibril nucleation and elongation (25)), accompanied by less well ordered regions of the polypeptide chain.
The separative powers of ESI-IMS-MS allow all ions displayed in Fig. 1 to be assigned unambiguously to an oligomeric state and the relative populations of each to be monitored during self-assembly. Parallel experiments using negative stain EM confirmed that assembly resulted in the formation of long, straight fibrils typical of amyloid (Fig. S3). During the lag phase, the concentration of monomer and all oligomers decreased rapidly, with no evidence for an increase in oligomer population after their initial, rapid formation upon dissolution of lyophilised protein, and no species larger than tetramer were observed under the conditions used (other than occasional glimpses of minor ions consistent with pentamer but too weak for accurate analysis). By contrast, the formation of trimer and tetramer could be observed when fibril formation was initiated by exchange of the urea-unfolded protein monomer into fibrillogenic conditions (pH 2.5 lacking urea), some 30–60 min after initiation of fibril formation, confirming that they are indeed products of β2m self-assembly (Fig. S4).
Oligomers Are Dynamic Entities.
To gain further insights into the assembly mechanism, the dynamics of the oligomers were investigated by assessing the rate of subunit exchange by mixing 14N- and 15N-labeled β2m oligomers. Uniform 15N-labelling of β2m results in a mass increase of 140 Da/monomer. By incubating equal concentrations of 14N- or 15N-labeled β2m solutions separately under fibril growth conditions (pH 2.5, 20 °C) and then mixing the samples, the rate of subunit exchange of each oligomeric species was monitored. If the subunits of the oligomers are in rapid exchange, then the oligomers detected by MS will be formed stochastically from the protein pool. For the dimer, this would manifest as three peaks in the ratio 1∶2∶1 corresponding to 14N/14N, 14N/15N and 15N/15N dimers, for the trimer and tetramer, subunit exchange would result in four and five peaks respectively, with ratios of 1∶3∶3∶1 and 1∶4∶6∶4∶1 (Fig. S5). As a control to demonstrate that subunit exchange does not occur during the analysis, samples of 14N- and 15N-β2m were allowed to equilibrate separately at room temperature. To quench the dynamics and limit oligomer exchange the samples were then incubated on ice and mixed prior to ESI-MS analysis. Under these conditions the +10 to +8 trimer ions, and the +11 and +10 tetramer ions, gave signals resulting only from the 14N- or 15N-labeled protein with no mixed assemblies apparent, confirming that these oligomers are stable in solution at 4 °C and that oligomerisation is not induced by the ionisation process (Fig. S6). In contrast, the +11 trimer ions underwent rapid exchange at 4 °C giving rise to m/z values indicating a random mixture of 14N- and 15N-monomeric subunits. The two types of behavior displayed by the trimer ions of different charge states indicate the presence of two trimeric species: a more highly charged, faster exchanging conformer and a less charged, more stable conformer.
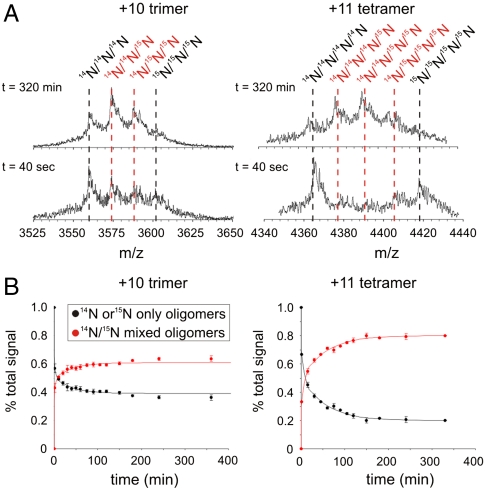
Further to this, the rates of subunit exchange of the dimer, trimer, and tetramer subunit exchange were monitored by forming oligomers from 14N- or 15N-labeled β2m under fibril growth conditions, mixing the two samples at 20 °C, and analyzing the resulting solution regularly over the next 320 min. The exchange profiles for the 14N- and 15N-labeled oligomers of the +10 trimer and +11 tetramer ions are shown in Fig. 3. As the experiment proceeds, peaks associated with 14N/15N mixed oligomers increase in intensity until equilibrium is reached. To determine the rate of exchange of each oligomer, the peak areas associated with homogenously labeled 14N- and 15N-oligomers (the reactants) and the peak areas associated with exchanged protein containing both 14N- and 15N-labeled molecules (reaction products) were summed individually and normalized to the total signal intensity. This analysis revealed that all of the dimer ions, and also the +11 trimer ions, are fully exchanged within the dead-time of the experiment (40 sec) and hence have a t50 of exchange of < 1 min. By contrast, the +10 trimer ions and the +11 tetramer ions show biphasic exchange kinetics over the time course of the experiment (Fig. 3). Although both displayed an initial time constant of exchange < 1 min, the amplitude of this phase in the case of the tetramer was much reduced compared with the trimer. The second phase for the +10 trimer ions occurs with t50 = 78 ± 15 min, while the +11 tetramer ions exchange with a similar time constant of t50 = 54 ± 7 min. Control experiments analyzing the +8 trimer ions show the same rates of exchange as the +10 ions of this species. Different Ωs were measured for the two trimers: The rapidly exchanging conformer had a Ω of 3,050 Å2 while the less dynamic conformer had a Ω of 2,928 Å2. Similar biphasic behavior is observed for the tetramer, although in this case only a single, narrow charge state distribution of ions was detected.
Fig. 3.
(A) 14N- and 15N-sub-unit exchange of the +10 trimer and +11 tetramer ions. Samples of 14N- and 15N-labeled preformed β2m oligomers at 20 °C were mixed together in solution in a 1∶1 ratio before ESI-MS analysis. m/z spectra at t = 40 sec and t = 320 min after mixing are shown. Black dashed lines show the 14N- or 15N-only β2m oligomers, red dashed lines show the 14N/15N mixed β2m oligomers. (B) Oligomer exchange dynamics. Individual peak areas were calculated and normalized to total signal at each time point. The data were summed so that the peak area for the reactants (14N- and 15N-only β2m oligomers; black), and the products (14N/15N mixed β2m oligomers; red), could be compared. The resulting datasets were fitted to a double exponential decay (solid line). (Fig. S5 for further details).
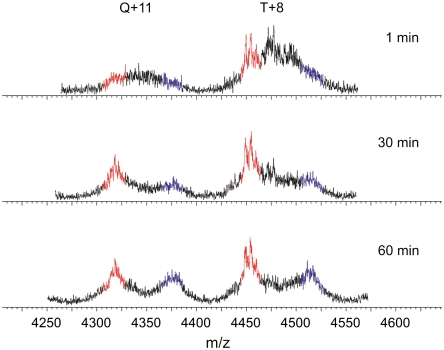
A similar subunit exchange experiment was performed whereby 14N- and 15N-labeled β2m were diluted independently into pH 2.5 buffer from urea to allow rapidly formed oligomers to be characterized (Fig. 4). At different times (1, 30, and 60 min) after initiating fibrillogenesis in this manner, 14N- and 15N-labeled β2m were mixed and spectra acquired. Strikingly, the trimer and tetramer ions both show rapid exchange kinetics at 1 min after urea exchange and then slow exchange kinetics later (> 30 min). These data suggest that fibrillogenesis of β2m occurs via the initial formation of dimers and trimers, before a conformational change takes place that allows stable trimers and tetramers to form.
Fig. 4.
Subunit exchange investigated in oligomers formed early in fibrillogenesis initiated by urea exchange. 14N- and 15N-labeled β2m were each unfolded in 8 M urea and pH 2.5 fibrillogenesis initiated by urea exchange. The solutions were incubated for 1, 30, and 60 min prior to mixing (1∶1 v/v) and immediate ESI-MS analysis. 100% 14N-β2m and 100% 15N-β2m are shown in red and blue, respectively. The mixed 14N/15N signals are shown in black. Both sets of ions undergo rapid subunit exchange at the earliest time point, resulting in oligomers with mixed labels. After 30 min, the +11 tetramer and +8 trimer ions are less dynamic, as depicted by the lower intensity of oligomers with mixed labels and the higher intensity of all 14N- and all 15N-labeled β2m peaks.
Discussion
Characterizing the conformation and dynamics of prefibrillar oligomers is key to understanding the aberrant aggregation of misfolding proteins and likely to provide vital insights into the design of potential therapies. Single molecule experiments examining the aggregation of the SH3 domain of PI3 kinase have shown that although the size distribution of the SH3 oligomers remains constant (38 ± 10 subunits) during aggregation, oligomer stability increases substantially during fibril growth (3). An earlier ESI-MS study into the formation of β2m oligomers during amyloid assembly at low pH revealed the population of monomers, dimers, trimers, and tetramers, although detailed structural and stability characterization was not performed (16). Consistent with these results, global analysis of the concentration-dependence of the kinetics of β2m fibril assembly has demonstrated that fibril formation at pH 2.5 is likely to proceed via monomer addition, populating dimers, trimers, and tetramers, until a structural nucleus approximately the size of a hexamer develops (26).
Here, using ESI-IMS-MS, we have measured the Ωs of β2m oligomers populated in the lag time of fibril assembly at pH 2.5 and compared these values with Ωs measured for a range of globular proteins (Fig. 2). The globular proteins were used to calculate an average protein density of 0.44 Da/Å3. Comparison of the Ωs revealed a β2m monomer population at acidic pH that is expanded compared with the native and partially-folded structured forms, but nonetheless retains nonrandom structure stabilized by the disulphide bond, consistent with previous results (10, 15, 24). Structural collapse of the monomer occurs concomitantly with dimer formation resulting in a dimer with a Ω only 5% larger than the unfolded monomer (β2m-A). Comparison of the dimer Ω measurement with the calculated Ω of a modeled, extended dimer and measured Ωs of disulphide-bridged, covalent β2m dimers of native structure in different orientation, indicated that the β2m dimer populated during the lag phase of fibril growth is compact with a Ω similar to that measured for a native dimer and smaller than would be expected for an extended structure. Whether the monomer collapses to a species with a native-like Ω and then associates, or collapses upon dimerisation, cannot be determined from these data. β2m trimers and tetramers observed during the lag phase of assembly appear structured as they are protected from limited proteolysis and possess narrow charge state distributions (+11 to +8 trimer; +11 to +10 tetramer ions) (27). The Ωs of these oligomers, however, are larger than would be expected for globular proteins of a similar mass and are thus consistent with elongated arrangements. This subunit architecture is in contrast to the oligomers formed during the assembly of Aβ42 which constitute a hexameric ring and a dodecamer of two stacked hexameric rings (11).
To investigate the dynamic nature of the oligomers detected during β2m assembly the rate of subunit exchange was monitored using 14N/15N-labelling. The data revealed that all dimer ions undergo rapid exchange within the dead-time of the experiment. Detailed analysis of the trimer provided evidence for two separate conformers. As it is unlikely that the trimer forms by the spontaneous interaction of three monomeric subunits, this suggests a model whereby a monomer interacts with a collapsed dimer to form an unstable trimer which then reorganizes into a more stable structure (Fig. 5). Such a model would also explain the two phases in the kinetics of subunit exchange, a theory strengthened by the data presented in Figs. 3 and 4. The tetramer also displays biphasic exchange behavior and so may form via a similar mechanism. However, as the two forms of the tetramer are unresolved by charge state distribution or drift time under the conditions used, this suggests they have similar (within < 5%) Ωs (10 and 22).
Fig. 5.
Model of the events occurring during the onset of β2m fibril formation under acidic conditions (pH 2.5, 100 mM ammonium formate). Monomeric β2m copopulates three distinct states at pH 2.5: native-like compact, partially folded, and acid-unfolded (10 and 15); oligomer formation occurs in < 2 min and results in the detection of monomers to tetramers in the lag phase. The dimer (red) is a highly dynamic species with Ω only 5% larger than that of the acid-unfolded monomer. Further association proceeds by stacking monomeric subunits with Ωs consistent with an elongated structure. Initial association of the monomer to the dimer results in a dynamic trimer that undergoes structural change giving rise to a longer-lived, less dynamic species (green). The tetramer (blue) evolves in a similar manner, first forming with a dynamic structure that subsequently becomes more stable as revealed by biphasic oligomer exchange kinetics.
Together, using the unique separative and analytical powers of ESI-MS in conjunction with IMS, this set of experiments has highlighted unique insights into the mass, the shape, and arrangement of subunits (cross-sectional area) and physical properties (both stability and subunit exchange) of the copopulated ensembles of oligomers present during the early stages of β2m fibril formation (Fig. 5). The elongated oligomers, formed via monomer addition, have been found to become more stable and deviate further from globular dimensions as the oligomer size increases. Further monomer association to pentamer or higher order oligomers presumably results in the formation of the amyloid nucleus as predicted by kinetic analysis (21), leading to spontaneous amyloid formation without measurable population of larger oligomeric intermediates. Further questions regarding the finer structural detail of the oligomers and the proposed conformational change upon monomer docking remain to be resolved. Nevertheless, these data provide a valuable framework outlining the events occurring during the lag phase of fibril formation and allow the dynamic nature of oligomers during assembly to be characterized. Moreover, the results provide unique insights into the very early stages of fibrillogenesis of a large, 99-residue protein that occurs by the formation of elongated assemblies with distinct structural and physical properties.
Materials and Methods
Reagents.
The Escherichia coli strain BL21 (DE3) was obtained from Promega. Dithiothreitol, Q-Sepharose, des-Arg bradykinin and other reagents were purchased from Sigma-Aldrich. Spectrapore membrane (molecular mass cut-off 3,500 Da) was obtained from Spectrum Laboratories Inc.. Superdex 75 was purchased from Amersham Biosciences. Carbenicillin was from Melford Chemicals. Oligonucleotides were purchased from MWG Biotech. 14N- and 15N-recombinant β2m was prepared as described previously (2 and 28). Im9 was provided by Dr. G. Morgan (University of Leeds); the HLA-I complex was provided by Dr. T. R. Jahn (University of Cambridge); I275 and ((I27-pLWT)3I27) were provided by Dr. D. J. Brockwell (University of Leeds). Solvents were purchased from Fisher Scientific.
Fibril Formation.
Formation of amyloid fibrils from β2m at pH 2.5 and quantification of monomer concentration was carried out as described (16).
14N/15N Oligomer Exchange.
Samples of 14N- and 15N-β2m (0.4 mg/mL) in 100 mM ammonium formate were preincubated at 4 or 20 °C for 20 min. 200 μL of each sample were then mixed and the products of subunit exchange analyzed by ESI-MS.
Initiation of Fibrillogenesis by Urea Exchange.
β2m (0.7 mg/mL) unfolded in 8 M urea (50 mM ammonium acetate, pH 6.5, 18 h) was buffer exchanged (100 mM ammonium formate, pH 2.5) using a HiTrap desalting column (GE Healthcare) to initiate fibril formation.
Electron Microscopy.
Colloidon-coated copper EM grids were coated with protein samples as described (2). Images were taken using a Philips CM10 electron microscope operating at 80 keV.
ESI-(IMS)-MS.
Experiments were performed on a Synapt HDMS (Waters United Kingdom Ltd.) quadrupole-travelling wave IMS-oaTOF mass spectrometer, equipped with a Triversa (Advion Biosciences) automated nano-ESI interface. Positive ESI was used with a capillary voltage of 1.7 kV and a nitrogen nebulising gas pressure of 0.7 p.s.i. For the 14N/15N-oligomer exchange experiments, the sample chamber of the Triversa was set at either 4 °C or 20 °C as appropriate. A sampling cone voltage of 175 V and a backing pressure of 3.8 mbar were set for the observation of β2m oligomers. For the 14N/15N-oligomer exchange experiments, the quadrupole analyzer was operated in resolving mode to transmit m/z 3,559.0–3,601.0 and 4,313.7–4,366.6. Otherwise, data were acquired over the range m/z 500–6,000 for 5 min. Mass calibration was performed by a separate infusion of CsI cluster ions. The ion accelerating voltages into the trap and transfer T-wave devices were set at 4 V and 40 V, respectively, ensuring that solvent clusters, but not the oligomers, were dissociated to improve the signal to noise ratio. The raw data were processed using MassLynx v.4.1 software (Waters United Kingdom Ltd.).
For IMS-MS, the wave height was ramped from 2–25 V at a speed of 300 ms-1. Drift times were corrected for both mass dependent and mass independent times as reported previously (22). The drift time cross-section function was calibrated as described (15). Computer-based cross-sectional area calculations were made from Protein Data Bank structures (Fig. S1) using an in-house algorithm described previously (15 and 22).
Where appropriate, data from the 14N/15N-labelling experiments were imported into Origin Pro 7.5 (Originlab), where plots for either the +10 trimer ions or +11 tetramer ions were fitted to a series of Gaussian distributions. The peak centers were fixed at: m/z 3,559.0, 3,573.0, 3,587.0, and 3,601.0 for the trimer +10 ions and m/z 4,313.7, 4,326.5, 4,339.2, 4,351.9, and 4,364.6 for the tetramer +11 ions. Reported data are the average of four separate experiments.
Supplementary Material
Acknowledgments.
We thank members of the Ashcroft and Radford groups for helpful discussions. D.P.S. was supported by the Biotechnology and Biological Sciences Research Council [Grant Number BB/D010284/1]. The Synapt HDMS was purchased with funds from the Biotechnology and Biological Sciences Research Council’s Research Equipment Initiative [Grant Number BB/E012558/1].
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913046107/-/DCSupplemental.
References
- 1.Gejyo F, et al. A new form of amyloid protein associated with chronic-hemodialysis was identified as beta 2-microglobulin. Biochem Biophys Res Commun. 1985;129:701–706. doi: 10.1016/0006-291x(85)91948-5. [DOI] [PubMed] [Google Scholar]
- 2.Kad NM, et al. Hierarchical assembly of beta 2-microglobulin amyloid in vitro revealed by atomic force microscopy. J Mol Biol. 2003;330:785–797. doi: 10.1016/s0022-2836(03)00583-7. [DOI] [PubMed] [Google Scholar]
- 3.Carulla N, et al. Experimental characterization of disordered and ordered aggregates populated during the process of amyloid fibril formation. Proc Natl Acad Sci USA. 2009;106:7828–7833. doi: 10.1073/pnas.0812227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orte A, et al. Direct characterization of amyloidogenic oligomers by single-molecule fluorescence. Proc Natl Acad Sci USA. 2008;105:14424–14429. doi: 10.1073/pnas.0803086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilag LL, et al. Heptameric (L12)6/L10 rather than canonical pentameric complexes are found by tandem MS of intact ribosomes from thermophilic bacteria. Proc Natl Acad Sci USA. 2005;102:8192–8197. doi: 10.1073/pnas.0502193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rostom AA, Robinson CV. Detection of the intact GroEL chaperonin assembly by mass spectrometry. J Am Chem Soc. 1999;121:4718–4719. [Google Scholar]
- 7.Tito MA, Tars K, Valegard K, Hajdu J, Robinson CV. Electrospray time-of-flight mass spectrometry of the intact MS2 virus capsid. J Am Chem Soc. 2000;122:3550–3551. [Google Scholar]
- 8.Lorenzen K, Olia AS, Uetrecht C, Cingolani G, Heck AJ. Determination of stoichiometry and conformational changes in the first step of the P22 tail assembly. J Mol Biol. 2008;379:385–396. doi: 10.1016/j.jmb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruotolo BT, et al. Evidence for macromolecular protein rings in the absence of bulk water. Science. 2005;310:1658–1661. doi: 10.1126/science.1120177. [DOI] [PubMed] [Google Scholar]
- 10.Smith DP, Giles K, Bateman RH, Radford SE, Ashcroft AE. Monitoring copopulated conformational states during protein folding events using electrospray ionization-ion mobility spectrometry-mass spectrometry. Journal of the American Society for Mass Spectrometry. 2007;18:2180–2190. doi: 10.1016/j.jasms.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein SL, et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemmer DE, Hudgins RR, Jarrold MF. Naked protein conformations—cytochrome-c in the gas-phase. J Am Chem Soc. 1995;117:10141–10142. [Google Scholar]
- 13.Guharay SK, Dwivedi P, Hill HH. Ion mobility spectrometry: Ion source development and applications in physical and biological sciences. IEEE T Plasma Sci. 2008;36:1458–1470. [Google Scholar]
- 14.Ruotolo BT, Benesch JL, Sandercock AM, Hyung SJ, Robinson CV. Ion mobility-mass spectrometry analysis of large protein complexes. Nat Protoc. 2008;3:1139–1152. doi: 10.1038/nprot.2008.78. [DOI] [PubMed] [Google Scholar]
- 15.Smith DP, et al. Deciphering drift time measurements from travelling wave ion mobility spectrometry—mass spectrometry studies. Eur J Mass Spectrom. 2009;15:113–130. doi: 10.1255/ejms.947. [DOI] [PubMed] [Google Scholar]
- 16.Smith AM, Jahn TR, Ashcroft AE, Radford SE. Direct observation of oligomeric species formed in the early stages of amyloid fibril formation using electrospray ionisation mass spectrometry. J Mol Biol. 2006;364:9–19. doi: 10.1016/j.jmb.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 17.Nettleton EJ, et al. Characterization of the oligomeric states of insulin in self-assembly and amyloid fibril formation by mass spectrometry. Biophys J. 2000;79:1053–1065. doi: 10.1016/S0006-3495(00)76359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson JL, Ko E, Miranker AD. Direct measurement of islet amyloid polypeptide fibrillogenesis by mass spectrometry. Protein Sci. 2000;9:427–431. doi: 10.1110/ps.9.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baskakov IV, Legname G, Baldwin MA, Prusiner SB, Cohen FE. Pathway complexity of prion protein assembly into amyloid. J Biol Chem. 2002;277:21140–21148. doi: 10.1074/jbc.M111402200. [DOI] [PubMed] [Google Scholar]
- 20.Jablonowska A, Bakun M, Kupniewska-Kozak A, Dadlez M. Alzheimer’s disease Abeta peptide fragment 10–30 forms a spectrum of metastable oligomers with marked preference for N to N and C to C monomer termini proximity. J Mol Biol. 2004;344:1037–1049. doi: 10.1016/j.jmb.2004.09.083. [DOI] [PubMed] [Google Scholar]
- 21.Xue WF, Homans SW, Radford SE. Systematic analysis of nucleation-dependent polymerization reveals new insights into the mechanism of amyloid self-assembly. Proc Natl Acad Sci USA. 2008;105:8926–8931. doi: 10.1073/pnas.0711664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapman TW, Berryman JT, Campuzano I, Harris SA, Ashcroft AE. Considerations in experimental and theoretical collision cross-section measurements of small molecules using travelling wave ion mobility spectrometry—mass spectrometry. Int J Mass Spectrom. 2009 doi: 10.1255/ejms.947. in press: 10.1016/j.ijms.2009.1009.1011. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein SL, et al. Alpha-synuclein: stable compact and extended monomeric structures and pH dependence of dimer formation. Journal of the American Society for Mass Spectrometry. 2004;15:1435–1443. doi: 10.1016/j.jasms.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Platt GW, McParland VJ, Kalverda AP, Homans SW, Radford SE. Dynamics in the unfolded state of beta 2-microglobulin studied by NMR. J Mol Biol. 2005;346:279–294. doi: 10.1016/j.jmb.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 25.Routledge KE, Tartaglia GG, Platt GW, Vendruscolo M, Radford SE. Competition between intramolecular and intermolecular interactions in an amyloid-forming protein. J Mol Biol. 2009;389:776–786. doi: 10.1016/j.jmb.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue WF, Homans SW, Radford SE. Systematic analysis of nucleation-dependent polymerization reveals new insights into the mechanism of amyloid self-assembly. Proc Natl Acad Sci USA. 2008;105:8926–8931. doi: 10.1073/pnas.0711664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benesch JL, Ruotolo BT, Simmons DA, Robinson CV. Protein complexes in the gas phase: technology for structural genomics and proteomics. Chem Rev. 2007;107:3544–3567. doi: 10.1021/cr068289b. [DOI] [PubMed] [Google Scholar]
- 28.McParland VJ, Kalverda AP, Homans SW, Radford SE. Structural properties of an amyloid precursor of beta 2-microglobulin. Nat Struct Biol. 2002;9:326–331. doi: 10.1038/nsb791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.