Abstract
Siphoviridae is the most abundant viral family on earth which infects bacteria as well as archaea. All known siphophages infecting gram+ Lactococcus lactis possess a baseplate at the tip of their tail involved in host recognition and attachment. Here, we report analysis of the p2 phage baseplate structure by X-ray crystallography and electron microscopy and propose a mechanism for the baseplate activation during attachment to the host cell. This ∼1 MDa, Escherichia coli-expressed baseplate is composed of three protein species, including six trimers of the receptor-binding protein (RBP). RBPs host-recognition domains point upwards, towards the capsid, in agreement with the electron-microscopy map of the free virion. In the presence of Ca2+, a cation mandatory for infection, the RBPs rotated 200° downwards, presenting their binding sites to the host, and a channel opens at the bottom of the baseplate for DNA passage. These conformational changes reveal a novel siphophage activation and host-recognition mechanism leading ultimately to DNA ejection.
Keywords: crystal structure, electon microscopy, Lactococcus lactis, Siphoviridae, bacteriophage
Lactococcus lactis is a gram-positive bacterium extensively used as starter cultures for the industrial production of an array of milk fermented products, including cheeses (1). Virulent lactococcal phages are ubiquitous in dairy environments and their lytic cycle leads to bacterial cell lysis, thereby slowing the milk fermentation process and lowering the overall quality of the manufactured products. Hundreds of virulent L. lactis phages have been characterized worldwide and the vast majority of them belong to the Siphoviridae family (2).
Phages of the Siphoviridae family (Caudovirales order) possess a proteinacious capsid, containing a double-stranded DNA genome, connected to a long noncontractile tail. The host-recognition and adsorption device is located at the tip of the tail and is used to start the phage infection process (3). Contrary to what is observed in most Siphoviridae phages, such as coliphage T5 (4) and Bacillus phage SPP1 (5), the adsorption device of most lactococcal phages is a large organelle of 1–2 MDa with a typical diameter of 20–30 nm called baseplate (6–9). Still, the host-recognition process by phages is poorly understood in gram-positive bacteria and the mechanistic details are only beginning to be unraveled.
In contrast, the proteinacious baseplates are common in phages of the Myoviridae family (with contractile tail) and that of coliphage T4 has been extensively studied. T4 baseplate is a remarkable nano-machine able to perform movements of several hundreds of Å (10, 11). These conformational changes trigger the tail contraction, leading to the ejection of the DNA from the capsid through the tail tube into the host (12). This organelle is much larger in Escherichia coli myophage T4 than in lactococcal phages. Moreover, such large movements of baseplate proteins have never been documented in phages with noncontractile tails, such as those of the Siphoviridae family.
Within the baseplate are located, among other proteins, several copies of the phage receptor-binding proteins (RBPs) which are necessary to specifically recognize the receptors at the host cell surface (6–9, 13). Recently, the 3D structures of RBPs from three lactococcal phages (p2, bIL170, TP901-1) have been solved (14–17). These homotrimeric proteins are composed of three domains named shoulders, neck, and head, the latter domain bearing the receptor-binding area. These structures made it possible to identify a sugar/glycerol binding site in the RBP head domain and led to postulate that they may recognize lipoteichoic acids (LTAs), which are phospho-glycerol polymers. This hypothesis is still waiting experimental confirmation. Here, we have structurally and functionally characterized the baseplate of a siphophage. We have chosen the virulent phage p2, a representative of the 936 group, which is the most prominent group of lactococcal phages isolated from dairy samples worldwide.
Results
Baseplate Composition and Overall Structure.
The genome of phage p2 consists of a linear, double-stranded, 27 595 bp DNA molecule containing 49 orfs and is very similar to the lactococcal phage sk1 genome (18). Twelve structural proteins (ORF4–ORF11, ORF14, ORF15, ORF16, ORF18) were identified by liquid chromatography coupled mass spectrometry (LCMS/MS) analysis using the whole purified phage p2 virions as well as from bands cut from a preparation of p2 migrated on SDS-PAGE gels (Fig. S1).
Sequence analysis as well as its size (999 aa) indicated that the ORF14 is the tape measure protein while the ORF19 (holin) and ORF20 (endolysin) are involved in cell lysis. Based on their genomic location, we hypothesized that the four genes downstream of orf14, namely, orfs 15, 16, 17, and 18 (RBP) encoded baseplate-related proteins. ORF16 and ORF17 are highly conserved among lactococcal phages whereas ORF15 and ORF18 show some diversity.
The contiguous cluster of four genes was cloned and expressed in E. coli (19). We then purified the resulting macromolecular complex of ∼1 MDa by affinity chromatography (ORF15 was His6-tagged at the N-terminus) and gel filtration. We could determine that the ensemble produced is the phage p2 baseplate, formed by ORFs 15, 16 and 18. ORF17 could not be detected in this ensemble, which is in agreement with its absence in virion particles.
Crystals of the isolated phage p2 baseplate were obtained but showed no or poor diffraction (∼20 Å). We therefore mixed the p2 baseplate with an excess of the camelid antibody heavy-chain variable fragment (VHH5), which has been previously shown to bind ORF18/RBP and to inhibit phage adsorption (6, 16, 20). We obtained rhombohedric crystals and collected several datasets eventually reaching 2.60 Å resolution. The structure was solved by molecular replacement using a template model built from the ORF18/RBP trimeric structure and its complex with VHH5 (16) (Table 1).
Table 1.
Data collection and refinement statistics
| Data collection | Form 1 (rest) | Form 2 (active) | Form 3 (active) |
| Molecular content (asymmetric unit) | 1/3 baseplate | 2 baseplates | 1 baseplate |
| Space group | R 3 2 (H 3 2) | P21 | C2 |
| Cell dimensions a,b,c (Å), ß(°) | 202.9,202.9, 760.5 | 219.5,219.3, 392.4 β = 90° | 300.3,239.5, 274.8, β = 124.4° |
| Beam line, wavelength (Å) | ESRF, ID23-1, 0.9708 | Soleil, Proxima 1, 0.980 | Soleil, Proxima 1, 0.980 |
| Resolution (Å) | 48.7–2.6 (2.7–2.6) | 44.6–5.5 (5.8–5.5) | 120–3.9 (4.1–3.9) |
| Rsym (last shell) | 0.130 (0.476) | 0.073 (0.458) | 0.160 (0.483) |
| Mean I/σI | 8.1 (2.6) | 12.3 (2.8) | 5.4 (2.3) |
| Completeness (%) | 99.3 (99.9) | 91.4 (87.2) | 97.0 (97.5) |
| Redundancy | 5.0 (5.0) | 4.2 (3.9) | 3.0 (3.0) |
| No. of reflections | 184,640 | 112,351 (15,601) | 141,413 |
| Refinement | |||
| Resolution (Å) | 38.1–2.60 (2.67–2.60) | 44.56–5.46 (5.66–5.46) | 39.31–3.90 (4.00–3.90) |
| No. of reflections | 183,300 (13268) | 112,191 (9789) | 141,150 (10511) |
| Rwork; Rfree | 17.8; 20.9 (25.9; 29.2) | 29.1; 29.7 (34.7; 35.9) | 22.9; 24.2 (25.3; 26.0) |
| No. of protein atoms/water | 25,638/2009 | 119,226/6 Ca | 59,613/6 Sr |
| B-factors protein (Å2) | 67.7* | 205 | 80.0* |
| R.m.s. deviations Bonds (Å)/angles (°) | 0.009/1.15 | 0.01/1.67 | 0.007/1.03 |
Last shell data are in parenthesis.
*Residual B-factor after TLS refinement.
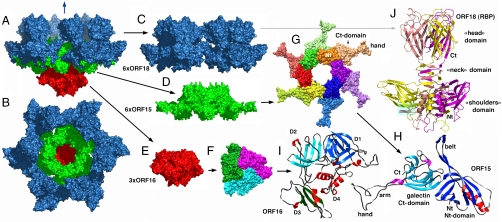
The baseplate-VHH5 (BP-VHH) structure is 230 Å wide and 160 Å high, displays a quasi hexagonal symmetry, and from bottom to top is formed of three ORF16, six ORF15, and six trimers of ORF18, as well as 18 VHH5 (Fig. 1 A–E and Fig. S2). Strict hexameric symmetry is not observed because ORF16 is trimeric. Furthermore, the symmetry of ORF16 disturbs the hexameric assemblies of ORF15 and ORF18. Notwithstanding, hexameric or quasihexameric symmetry seems to be a conserved feature of the baseplate of many lactococcal phages (7, 9).
Fig. 1.
Crystal structure of the phage p2 baseplate and its components. (A) View of the baseplate surface; ORF15 is in green, ORF16 in red, and ORF18 in blue. The blue arrow indicates the position of the quasi 6-fold axis and points toward the rest of the phage tail and the capsid. (B) The baseplate has been rotated by 90° around the horizontal axis. The central channel formed by ORF15 hexamer is closed by the ORF16 trimeric dome. (C) The arrangement of ORF18 as six trimers. (D) Hexameric ORF15. (E) Trimeric ORF16. (F) View of ORF16 trimer rotated 180° relative to baseplate (B) with a different color for each subunit. (G) ORF15 hexamer is viewed in the same orientation as in (B). Each subsubunit, as well as the N- and C-terminus domains, have a different color. The central channel is ∼40 Å wide. (H) Ribbon view of ORF15 subunit. The N- and C-terminus domains have their β-strands colored dark and light blue, respectively; helices are colored red and violet, respectively; and coiled sections are gray. The striking features of the structure, the arm (which helps hexamerization) and the extension (which grips ORF18 trimer) have been identified. Note the closure of the surface at the center of the trimer. (I) Ribbon view of ORF16 subunit. The four domains have been identified by D 1 to 4 and different colors of the β-strands. Domain 4 is only helical. These domains correspond to those identified in gp27 from phage T4 (12). (J) Ribbon view of the receptor-binding protein ORF18 trimer, with a different color for each chain. The trimer domains of shoulders, neck, and head are represented. The VHHs are displayed in Fig. S2.
Structure of the Baseplate Components and Their Assembly.
ORF16 is a four domain protein (Fig. 1I and Fig. S3A) of 398 residues that adopts a fold comparable to that of gp27 of myophage T4, the latter being located within the central part of the baseplate (12) [DALI (21) Z score = 11.3; Fig. S3B]. In contrast with gp27, the trimeric association ORF16 forms a dome at the terminus of the baseplate of the siphophage p2, thereby closing its central channel (Fig. 1F).
ORF15 is composed of two domains. The N-terminal domain (“ring domain” 1–132) shows a split barrel-like fold similar to that found in FMN-binding proteins (SCOP:50475; DALI Z score = 6.6). The most similar Protein Data Bank (PDB) entry found by secondary structure matching when using this domain was the PDB entry 3eaa, a Type-VI secretion system (T6SS) protein (EvpC) from the enterobacteria Edwardsiella tarda. A long kinked extension (the “belt”) of four β-strands embraces the next ORF15 molecule in the hexameric ring (Fig. 1 G and H and Fig. S4A). The N-terminal domains form a tight ring with two layers of β-strands. This ring is ∼35 Å high and frames a channel of 40 Å diameter, largely sufficient for dsDNA passage. Of note, the enterobacterial T6SS protein EvpC forms hexamers very similar to those formed by ORF15 of lactococcal phage p2. The C-terminal domains (residues 137–275) are located at the ring periphery and do not contact each other (Fig. 1G). They display a galectin fold (DALI Z score = 6.0), except for a long extension (the “arm,” residues 147–188) which plays a critical role in the baseplate assembly (Fig. 1H and Fig. S4B). The arm extremity forms a three-digit hand that grips the N-terminal domain of the RBP (ORF18, see below).
The structure of ORF18/RBP (264 amino acids) was described previously (14, 16). As indicated above, ORF18 is a trimer (Fig. 1J) with three domains: an N-terminal β-sandwich domain (the “shoulder” residues 1–141), a central domain (the “neck” residues 142–163) forming an interlaced β-helix, and a C-terminal double greek-key domain (the “head” residues 164–264) harboring the receptor-binding site. The shoulder domain is relatively well conserved among RBPs of other lactococcal phages of the 936 group, whereas the neck and head domains are significantly diverse. Contrary to the structures of ORF18 crystallized alone, the N-terminal residues 2–17 of ORF18 in the baseplate structure are ordered and visible in the density. This ordering of the N-terminus is due to a tight interaction with the three-digit hand from the ORF15 galectin domain (Fig. S5). Furthermore, the most N-terminal residues of ORF18 (residues 2–7) protrude from each subunit, forming the first strand of the shoulder domain of the next subunit (Fig. 1J). Finally, each ORF18 trimer is coordinated by three VHH5s. The 18 VHH5 molecules together with the head domains of ORF18 build a large layer assembled through tight protein-protein contacts, which likely led to better diffracting crystals.
All in all, a total surface area (22) of 156,000 Å2 is buried in the X-ray structure of the baseplate (excluding the VHHs), compared to a total solvent-accessible surface of 285,000 Å2. A large part of this buried surface, however, is involved in ORF18 trimerisation (100,000 Å2) and therefore does not contribute directly to the baseplate stability. Each ORF15 contacts two ORF18s and two ORF16s. Notably, there are no contacts between ORF16 and ORF18. Therefore, the ORF15 hexamer plays the role of a central hub to which ORF16 and ORF18 are attached (Fig. S5).
One of the striking features of phage p2 baseplate is the grasp of the ORF15 hand on the ORF18 shoulder domain (Fig. S5 A and E). To adapt to the trimeric ORF18, the ORF15 hand adopts a quasi 3-fold symmetry. A bundle of six residues from ORF15 establishes strong hydrogen bonds with the three Asp23 residues from the ORF18 trimer: Lys158 and Tyr178 with Asp23h, Tyr 160 and 168 with Asp23i, and Tyr 170 and 176 with Asp23g (Fig. S5 A and E). Besides this strong and remarkable architecture, other hydrogen bonds are established at the periphery between main-chain atoms or main-chain/side-chain atoms.
Other useful information about the assembly of the p2 baseplate can be derived from the expression pattern of these proteins. We also cloned orf15 and orf16 in tandem and purified their complex readily. It is formed of six ORF15s and three ORF16s, as seen by multiangle light scattering/UV/refractive index (MALS/UV/RI) (9), in agreement with the complete baseplate structure. However, ORF15 degraded with time, leading to smaller fragments. Consistent with this observation, crystallization assays on ORF15/ORF16 complex samples yielded crystals of ORF16 alone. To our surprise, the ORF16 structure determined from the crystals formed after degradation of the ORF15/ORF16 complex did not display the ORF16 trimeric assembly observed in the baseplate, but was instead monomeric.
Despite their large interaction surfaces, neither ORF15 nor ORF16 were able to oligomerize on their own, and complexes of ORF15/ORF16 with “native” stoichiometry were formed only upon coexpression (19). It is likely that one molecule of ORF16 has to assemble to two molecules of ORF15 as an initial building block that will further trimerize to form the inner core of the baseplate. ORF18 would then be added to this core. It is noteworthy that a freshly prepared ORF15/ORF16 complex was capable to capture added ORF18 and to form a “native” baseplate, as assessed by gel filtration.
Structure of the p2 Baseplate by Electron Microscopy.
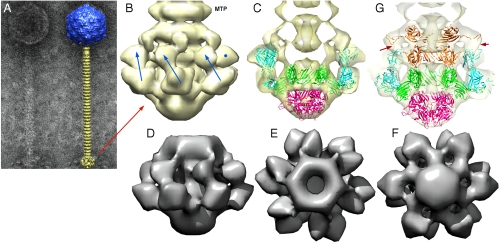
We have investigated the structure of the phage p2 virion by negative contrast electron microscopy. We report here the baseplate structure as seen in the virion (Fig. S6) and compare it with the expressed baseplate X-ray structure. The baseplate structure was easily recognizable at the end of the tail (Fig. 2 A and B). Its overall size was ∼220 Å wide by 180 Å high. We were able to fit as a block our X-ray structure, without the VHHs into the electron-microscopy (EM) structure using CHIMERA (23) and UROX (24). ORF15 and ORF16 could be fitted readily together, while small deviations appeared in the orientation of ORF18. We decided therefore to fit each of them separately. The fit was excellent and all the details of the structure were clearly visible, such as the position of the galectin domains and extensions, as well as the contacts between ORF15 and ORF18 (Fig. 2C and Table 2). However, a large electron density lump was left empty above ORF15 and below the last disk of the tail (Fig. 2C). This density consisted of two superposed rings of different diameters, the highest possessing six protrusions joining the ORF18 head domain.
Fig. 2.
EM structure of the baseplate of phage p2 and results of the fitting of the baseplate crystal structure. (A) EM micrograph of the virulent lactococcal phage p2. Overall EM structure of phage p2 (right) showing the capsid (blue, top), the tail, formed of rings of the major tail protein hexamers (gold), and the globular baseplate (gold, bottom). ( B) View of the EM structure of the baseplate with the first major tail protein (MTP) hexamer. The RBP positions are identified by blue arrows or a blue dot. The RBP head is pointing upwards. (C) View of the crystal structure of the baseplate fitted in the EM map. ORF15 is green, ORF16 red, and ORF18 blue. Each RBP, ORF15 hexamer, and ORF16 trimer have been fitted as rigid blocks. (D), (E), (F) Views of the cryoEM map of the expressed p2 baseplate, from side (D), up (E), and down (F), respectively. (G) Sliced view showing the fitting of a second ORF15 hexamer (brown) in the EM map, 180° rotated relative to that in the crystal structure. The ring formed by the N-terminus has been fitted as a block, and the rotated and translated galectin domains have been fitted as a second block. Note the extension touching the RBP head domain (red arrows).
Table 2.
Percentage of atoms and correlation coefficient resulting from the fitting of molecules or ensembles of the p2 baseplate in the EM map of the virion
| Molecule or complex | Atoms | Fit | % atoms | Correlation coeff. (20 Å) |
| ORF15 hexamer lower (all) | 14,554 | 13,454 | 92.5 | 0.52 |
| ORF16 trimer | 9,069 | 8,595 | 94.8 | 0.70 |
| ORF18 monomer * | 6,024 | 5,783 | 96.0 | 0.84 |
| ORF15 hexamer upper C-term † | 8,034 | 7,883 | 90.1 | 0.71 |
*Fitting has been done for the six monomers, yielding close values.
†Fitting is the best in this instance, because each galectin domain has been preliminarily positioned manually in the EM map to account for their conformational change.
We hypothesized that another hexamer of ORF15 might fill this orphan density. To verify this hypothesis, we determined the cryoEM structure of the expressed baseplate. As expected, the baseplate possess the same two extra rings at its top, as seen in the phage structure (Fig. 2 D, E, and F and Fig. S7). ORF16 and ORF18 could not account for this density, since their shapes were not compatible with it, while the shape of ORF15 was found to be compatible with the EM density.
The second hexamer of ORF15 was modeled in the EM maps (virion and expressed baseplate) by placing it back to back to the hexamer observed in the crystal structure (Fig. 2G, Fig. S7, and Table 2). The fit of the N-terminal ring was excellent, while the galectin domains and extensions were almost totally out of density. However, the shape of the density suggested that it could account for galectin domains and extensions from ORF15, provided that they undertook a rigid-body rearrangement. Indeed, after rigid body fitting of each of the six ORF15 galectin domains, we could obtain a satisfactory structure with these domains fitted in the second orphan ring, and their corresponding arms and hands filling in the map extensions towards ORF18 (Fig. 2G and Table 2). In contrast with their position in the lower ring, the galectin domains of the second ORF15 ring model are in contact with each other and form a continuous volume. Their arm and hand extensions contact the head domain of ORF18. This contact is not made over the 3-fold axis, but laterally. This arrangement implies that the way these extensions interact with ORF18 is very different from that of the lower ORF15 extensions, a feature that we did not model at the atomic level. All these data taken altogether, the native baseplate of the virulent lactococcal siphophage p2 is composed of 2 × 6 ORF15s, 3 ORF16s, and 6 × 3 ORF18s (Fig. 2G).
An “Activated” Form of the p2 Baseplate.
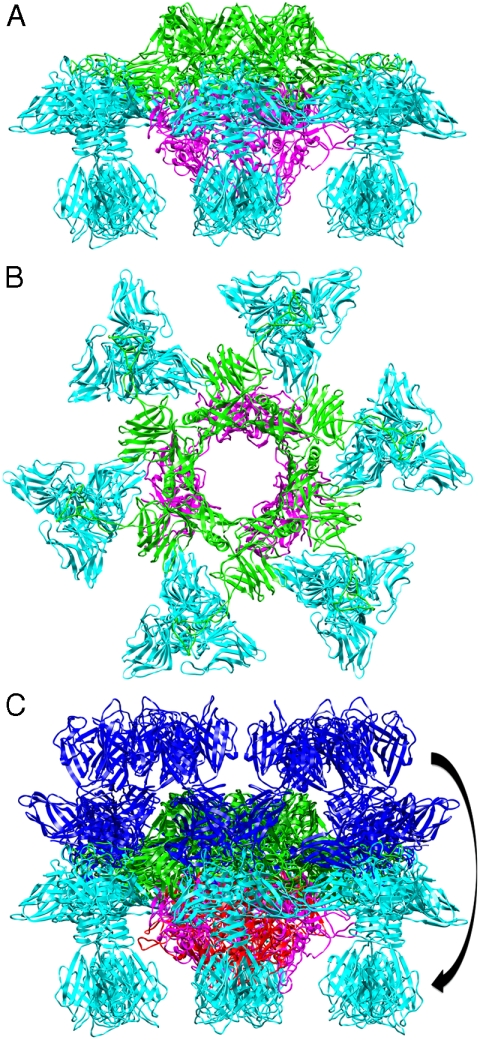
The structure of the baseplate reported above exhibited an unexpected conformation. Indeed, one would have expected the head domains of the RBPs (ORF18), which harbor the receptor-binding site, to point “downwards,” i.e. in the direction of the host cell surface as determined by the position of the tip protein ORF16. Instead, the RBPs were observed in a “heads-up” conformation.
Additional explanations came from two other crystal forms of the baseplate of phage p2 that were obtained in the presence of Ca2+ (sg P21) or Sr2+ (sg C2) and in the absence of VHH5 (Table 1). Calcium has been shown to be essential for lactococcal phages infection (25). The new structures were determined at 5.5 and 3.9 Å resolution, respectively, by molecular replacement using successively ORF18, ORF15, and ORF16 as search models (Table 1). The two structures are identical, taking into account their resolution. Because the structure obtained in the presence of Sr2+ is better defined, it will be used further in the description of this conformation of the baseplate. The structure is composed of six ORF15s, three ORF16s, and 3 × 6 ORF18s (Fig. 3 A and B). The most striking feature of this baseplate structure is a “downwards” rotation of the ORF18 trimers by ∼200°, leading to a “heads-down” conformation (Fig. 3C). The ring formed by the N-terminal domain of ORF15 superposed well in the two structures (r.m.s. deviation of 0.52 Å), but the galectin, arm, and hand domains had moved significantly (Fig. S8 and Movie S1). The Sr2+ ion (or Ca2+ ion) is located at the interface between the N-terminal and the galectin domains of ORF15 and is coordinated by side chains of residues Asn10, Asp12, Asn241, Asp246 and main-chain carbonyl of Leu11. The hand domains have rotated so that they are oriented in opposite directions as compared to the “heads-up” structure (Fig. S8 and Movie S1). The ORF16 trimer was strongly affected, resulting in the opening of the dome with the concomitant formation of a channel of ∼32 Å diameter (Fig. 3B and Movies S1 and S2), large enough for dsDNA passage. This opening results from an outwards rotation of ORF16 cores with respect to the channel axis, and the opening of a crevice between domains 1, 2, 4, and 3 in an iris-like movement. Domain 3 remains in close contact with the next ORF16 in the trimer (Movie S2). Noteworthy is the large contact area between ORF16s and ORF18s, a feature absent in the BP-VHH structure. In fact, these contacts lock the ORF18s in their “heads-down” conformation, giving to ORF16 the role played by the VHH5 molecules in the BP-VHH complex or by the second ORF15 hexamer in the native virion EM structure.
Fig. 3.
The crystal structure of the “heads-down” conformations of p2 baseplate. (A) Side view in ribbon representation of the “heads-down” conformations of p2 baseplate. (B) View from top (ORF15, green; ORF16, pink; ORF18, blue). (C) Superposition of the rings formed by the N-terminal domains of ORF15. ORF18 trimers have undergone a 200° rotation downwards.
Discussion
Host recognition by bacterial viruses is often a two-step process (26, 27). Coliphage T4 (Myoviridae) recognizes first its host saccharidic receptors via its long tail fibers. This first interaction induces a conformational change of the baseplate that exposes the short tail fibers and ensures a definitive docking of the phage onto the host cell wall (10). In parallel, the tail contracts and the dsDNA is ejected. For noncontractile-tailed siphophages, such as E. coli T5, or Bacillus subtilis SPP1, saccharidic chains are recognized nonspecifically by phage components in a first reversible step, followed by the irreversible specific docking of the tail fiber protein onto the host receptor, the FhuA porin (4) or the extramembrane domain of YueB (28), respectively. In contrast with the latter phages, and despite using several different strategies, no proteinaceous receptor has been identified for the lactococcal phages belonging to the 936 group.
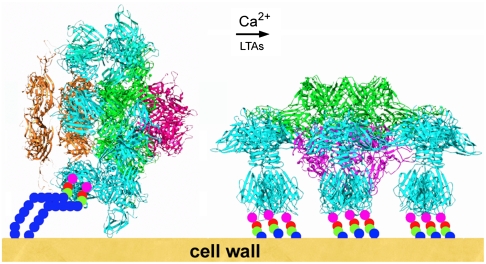
The conformational difference observed with the ORF18/RBP in our two crystal forms of the baseplate suggests also that, as for phage T4, host recognition could be based on a two-step mechanism. When free in the environment, lactococcal phage p2 has its RBPs in a “heads-up” conformation, forming the baseplate “rest form.” In this conformation, the RBP trimers could still recognize saccharidic receptors laterally, but with less affinity, because they use a limited number of their binding sites. This recognition might be sufficient to destabilize the baseplate and provoke the RBPs rotation (Fig. 4). In this context, it is noteworthy that due to the presence of numerous phosphate groups, LTAs are well known as “Ca2+ sponges” (25). Thus, it is possible that the binding of the first LTA molecules to the RBPs may help the release of Ca2+ cations from the phosphate groups, which in turn might facilitate the conformational change to the “activated form” of the base plate. In a subsequent step, the 18 receptor-binding sites of the “heads-down” activated form would become available to firmly anchor the phage to the receptors and to place it in a more favorable position for DNA ejection. Furthermore, this large movement might represent a firing signal, which, transmitted through the tail to the phage portal protein would trigger portal opening and dsDNA release and its passage through the tail and the open ORF16 trimer in the baseplate.
Fig. 4.
Schematic representation of the putative adsorption mechanism of phage p2 to its host. The rest form (left) of the baseplate is activated by Ca2+ cations and by the traction of lipoteichoic acids bound to a few receptor-binding sites which destabilize the RBPs. They subsequently rotate by 200° and become available for a complete binding. Concomitantly, the tip of the baseplate opens, giving way to dsDNA.
Although functional similarities have been underlined between coliphage T4 and lactococcal phage p2, the Myoviridae baseplate is notably more complex than the one found in Siphoviridae. Aside from their distinct tail morphology and some structural similarity in some of their components, they have completely different mechanisms for cell-wall puncturing and DNA ejection. The above-mentioned similarities should therefore be the result of convergent evolution towards an efficient system of adsorption, instead of a reminiscence of a common ancient adsorption mechanism.
Materials and Methods
Purification and Crystallization.
The baseplate orfs 15–18 of the lactococcal phage p2 were cloned into the Gateway pDEST147 vector (His6-tag at N-terminal of ORF15), overexpressed in E. coli and purified by Ni affinity column and gel filtration (19). Crystallization and structure determination are reported in Table 1 and SI Text.
X-Ray Structure Determination and Refinement.
Several datasets were collected at Swiss Light Source (SLS) (Villigen, Switzerland), Soleil (Saint Aubin, France), and European Synchrotron Radiation Facility (ESRF) (Grenoble, France). Data were integrated and reduced with XDS and SCALA (29). The structures were solved by molecular replacement using an ORF18 trimer (RBP) as a search model with the program PHASER (29). Model building was performed with COOT (30), and refinement was begun with REFMAC5 (31) and completed with AutoBuster v 1.7.2 (32). Data are presented in Table 1 and SI Text.
Electron Microscopy.
Virion structure.
Samples were adsorbed onto a freshly glow-discharged carbon-coated grid and stained with 2% uranyl acetate. About 1,000 CCD images were recorded on a CM200 operating at 200 kV and 38 K magnification. The digitized images were then coarsened by 2 × 2 pixel averaging, thereby resulting in a pixel size of 2.32 Å/pixel (Fig. S6A). Images were collected at different defoci in the range of 500–1,500 nm (Fig. S8A) and contrast transfer function (CTF) corrected using IMAGIC-5 FINDCTF2D program.
Approximately 10,000 particles were manually selected from CTF-corrected images and processed using IMAGIC-5 (33). The particles were windowed into 100 × 100 pixels, band-pass filtered, and subjected to multivariate statistical analysis (34) and classified with approximately 10 images per class. A class average was selected and aligned to have the 6-fold axis aligned to the z axis. An initial three-dimensional model was calculated from the aligned class averages imposing 6-fold symmetry. The final model was calculated to a resolution of 22 Å determined by Fourier shell correlation with the ½ bit correlation criterion (Fig. S6C) (35).
CryoEM of the expressed baseplate.
Expressed p2 baseplates were cross-linked by glutaraldehyde, followed by freezing in liquid ethane, and imaged using a JEOL 2200 FS electron microscope. Micrographs were digitized using a Nikon Coolscan 9000 ED; 10,732 individual views of baseplates were semiautomatically extracted and submitted to image processing using IMAGIC V package (Fig. S7).
Models fitting.
All models fittings in the EM maps were done in two steps, with Chimera (23) followed by UROX (24).
Accession Codes.
The genome sequence of phage p2 has been deposited at GenBank (GQ979703). The structures of the baseplate in complex with VHH form and the Ca2+ and Sr2+ activated forms have been submitted to the Protein Data Bank with accession codes 2WZP, 2X54 + 2X5A, and 2X53, respectively. The virion baseplate EM map has been deposited at Electron Microscopy Data Bank, with accession code EMD-1699. The expressed baseplate EM map has been deposited at EMDB, with accession code EMD-1706.
Supplementary Material
Acknowledgments.
We would like to thank Adeline Goulet for help with the cryoEM studies. We would like to thank the synchrotrons ESRF, Soleil, and SLS for providing us with beam time access. This work was supported, in part, by grants from the Marseille-Nice Génopole (to J.L.), the Centre National de la Recherche Scientifique, the Natural Sciences and Engineering Research Council of Canada (to S.M.), the novel alternatives to antibiotics research initiative of the Canadian Institutes of Health Research (to S.M.), and the Agence Nationale de la Recherche (Grant ANR-07-BLAN-0095, “siphophages”). Molecular graphics images were produced using the University of California, San Francisco (UCSF) Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by National Institutes of Health P41 RR-01081).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000232107/DCSupplemental.
References
- 1.Rousseau GM, Moineau S. Evolution of Lactococcus lactis phages within a cheese factory. Appl Environ Microbiol. 2009;75:5336–5344. doi: 10.1128/AEM.00761-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deveau H, Labrie SJ, Chopin MC, Moineau S. Biodiversity and classification of lactococcal phages. Appl Environ Microbiol. 2006;72:4338–4346. doi: 10.1128/AEM.02517-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maniloff J, Ackermann HW. Taxonomy of bacterial viruses: Establishment of tailed virus genera and the order Caudovirales. Arch Virol. 1998;143:2051–2063. doi: 10.1007/s007050050442. [DOI] [PubMed] [Google Scholar]
- 4.Boulanger P, et al. Phage T5 straight tail fiber is a multifunctional protein acting as a tape measure and carrying fusogenic and muralytic activities. J Biol Chem. 2008;283:13556–13564. doi: 10.1074/jbc.M800052200. [DOI] [PubMed] [Google Scholar]
- 5.Plisson C, et al. Structure of bacteriophage SPP1 tail reveals trigger for DNA ejection. Embo J. 2007;26:3720–3728. doi: 10.1038/sj.emboj.7601786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Haard HJ, et al. Llama antibodies against a lactococcal protein located at the tip of the phage tail prevent phage infection. J Bacteriol. 2005;187:4531–4541. doi: 10.1128/JB.187.13.4531-4541.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrath S, et al. Anatomy of a lactococcal phage tail. J Bacteriol. 2006;188:3972–3982. doi: 10.1128/JB.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vegge CS, et al. Identification of the lower baseplate protein as the antireceptor of the temperate lactococcal bacteriophages TP901-1 and Tuc2009. J Bacteriol. 2006;188:55–63. doi: 10.1128/JB.188.1.55-63.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciara G, et al. A topological model of the baseplate of lactococcal phage Tuc2009. J Biol Chem. 2008;283:2716–2723. doi: 10.1074/jbc.M707533200. [DOI] [PubMed] [Google Scholar]
- 10.Leiman PG, Chipman PR, Kostyuchenko VA, Mesyanzhinov VV, Rossmann MG. Three-dimensional rearrangement of proteins in the tail of bacteriophage T4 on infection of its host. Cell. 2004;118:419–429. doi: 10.1016/j.cell.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Rossmann MG, Mesyanzhinov VV, Arisaka F, Leiman PG. The bacteriophage T4 DNA injection machine. Curr Opin Struct Biol. 2004;14:171–180. doi: 10.1016/j.sbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Kanamaru S, et al. Structure of the cell-puncturing device of bacteriophage T4. Nature. 2002;415:553–557. doi: 10.1038/415553a. [DOI] [PubMed] [Google Scholar]
- 13.Dupont K, Vogensen FK, Neve H, Bresciani J, Josephsen J. Identification of the receptor-binding protein in 936-species lactococcal bacteriophages. Appl Environ Microbiol. 2004;70:5818–5824. doi: 10.1128/AEM.70.10.5818-5824.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tremblay DM, et al. Receptor-binding protein of Lactococcus lactis phages: Identification and characterization of the saccharide receptor-binding site. J Bacteriol. 2006;188:2400–2410. doi: 10.1128/JB.188.7.2400-2410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinelli S, et al. Modular structure of the receptor binding proteins of Lactococcus lactis phages. The RBP structure of the temperate phage TP901-1. J Biol Chem. 2006;281:14256–14262. doi: 10.1074/jbc.M600666200. [DOI] [PubMed] [Google Scholar]
- 16.Spinelli S, et al. Lactococcal bacteriophage p2 receptor-binding protein structure suggests a common ancestor gene with bacterial and mammalian viruses. Nat Struct Mol Biol. 2006;13:85–89. doi: 10.1038/nsmb1029. [DOI] [PubMed] [Google Scholar]
- 17.Ricagno S, et al. Crystal structure of the receptor-binding protein head domain from Lactococcus lactis phage bIL170. J Virol. 2006;80:9331–9335. doi: 10.1128/JVI.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandry PS, Moore SC, Boyce JD, Davidson BE, Hillier AJ. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol Microbiol. 1997;26:49–64. doi: 10.1046/j.1365-2958.1997.5491926.x. [DOI] [PubMed] [Google Scholar]
- 19.Campanacci V, et al. Solution and electron-microscopy characterization of lactococcal phage baseplates expressed in E. coli. J Struct Biol. 2010 doi: 10.1016/j.jsb.2010.02.007. in press. [DOI] [PubMed] [Google Scholar]
- 20.Ledeboer AM, et al. Preventing phage lysis of Lactococcus lactis in cheese production using a neutralizing heavy-chain antibody fragment from llama. J Dairy Sci. 2002;85:1376–1382. doi: 10.3168/jds.s0022-0302(02)74204-5. [DOI] [PubMed] [Google Scholar]
- 21.Holm L, Kaariainen S, Rosenstrom P, Schenkel A. Searching protein structure databases with DaliLite v.3. Bioinformatics. 2008;24:2780–2781. doi: 10.1093/bioinformatics/btn507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 24.Siebert X, Navaza J. UROX 2.0: An interactive tool for fitting atomic models into electron-microscopy reconstructions. Acta Crystallogr D. 2009;65:651–658. doi: 10.1107/S0907444909008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geller BL, Ngo HT, Mooney DT, Su P, Dunn N. Lactococcal 936-species phage attachment to surface of Lactococcus lactis. J Dairy Sci. 2005;88:900–907. doi: 10.3168/jds.S0022-0302(05)72756-9. [DOI] [PubMed] [Google Scholar]
- 26.Kostyuchenko VA, et al. The tail structure of bacteriophage T4 and its mechanism of contraction. Nat Struct Mol Biol. 2005;12:810–813. doi: 10.1038/nsmb975. [DOI] [PubMed] [Google Scholar]
- 27.Baptista C, Santos MA, Sao-Jose C. Phage SPP1 reversible adsorption to Bacillus subtilis cell wall teichoic acids accelerates virus recognition of membrane receptor YueB. J Bacteriol. 2008;190:4989–4996. doi: 10.1128/JB.00349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sao-Jose C, et al. The ectodomain of the viral receptor YueB forms a fiber that triggers ejection of bacteriophage SPP1 DNA. J Biol Chem. 2006;281:11464–11470. doi: 10.1074/jbc.M513625200. [DOI] [PubMed] [Google Scholar]
- 29.CCP4 CCPN. The CCP4 suite: Programs for crystallography. Acta Crystallogr D. 1994;50:760–766. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 30.Emsley P, Cowtan K. COOT: Model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 31.Murshudov G, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the Maximum-likelihood method. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 32.Blanc E, et al. Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr D. 2004;60:2210–2221. doi: 10.1107/S0907444904016427. [DOI] [PubMed] [Google Scholar]
- 33.van Heel M, Harauz G, Orlova EV, Schmidt R, Schatz M. A new generation of the IMAGIC image processing system. J Struct Biol. 1996;116:17–24. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 34.van Heel M. Multivariate statistical classification of noisy images (randomly oriented biological macromolecules) Ultramicroscopy. 1984;13:165–183. doi: 10.1016/0304-3991(84)90066-4. [DOI] [PubMed] [Google Scholar]
- 35.van Heel M, Schatz M. Fourier shell correlation threshold criteria. J Struct Biol. 2005;151:250–262. doi: 10.1016/j.jsb.2005.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.