Abstract
This study has used proton transfer reaction-mass spectrometry (PTR-MS) for direct air analyses of volatile products resulting from the reactions of ozone with human skin lipids. An initial series of small-scale in vitro and in vivo experiments were followed by experiments conducted with human subjects in a simulated office. The latter were conducted using realistic ozone mixing ratios (≈15 ppb with occupants present). Detected products included mono- and bifunctional compounds that contain carbonyl, carboxyl, or α-hydroxy ketone groups. Among these, three previously unreported dicarbonyls have been identified, and two previously unreported α-hydroxy ketones have been tentatively identified. The compounds detected in this study (excepting acetone) have been overlooked in surveys of indoor pollutants, reflecting the limitations of the analytical methods routinely used to monitor indoor air. The results are fully consistent with the Criegee mechanism for ozone reacting with squalene, the single most abundant unsaturated constituent of skin lipids, and several unsaturated fatty acid moieties in their free or esterified forms. Quantitative product analysis confirms that squalene is the major scavenger of ozone at the interface between room air and the human envelope. Reactions between ozone and human skin lipids reduce the mixing ratio of ozone in indoor air, but concomitantly increase the mixing ratios of volatile products and, presumably, skin surface concentrations of less volatile products. Some of the volatile products, especially the dicarbonyls, may be respiratory irritants. Some of the less volatile products may be skin irritants.
Keywords: Criegee mechanism, indoor chemistry, PTR-MS, squalene, surface chemistry
Ozone is transported from outdoors to indoors via ventilation and infiltration and can be emitted indoors by devices such as photocopiers, electrostatic precipitators, ozone generators and ionizers (1–3). Numerous studies have examined the products of ozone-initiated chemistry in actual or simulated indoor settings (1, refs. 4–6 and references therein). However, these studies have focused on materials and consumer products, and have been conducted in the absence of human occupants. Recent investigations in a simulated aircraft cabin (7–9) have shown that humans are significant sinks for ozone; this is a consequence of skin lipids that react with ozone to produce characteristic oxidation products. Related investigations have observed reactions between ozone and skin lipids on human hair (10) and on soiled clothing fabrics (11). Collectively these studies benefited from earlier work by Fruekilde et al. (12), which demonstrated that ozone reacted with human skin lipids to produce acetone, 6-methyl-5-hepten-2-one (6-MHO), geranyl acetone and 4-oxo-pentanal (4-OPA) and that squalene was the major precursor for these oxidation products.
As reported by Nicolaides (13), the lipids on the outermost layer of exposed skin consist of wax esters (22%), triacyl glycerols (25%), di- and monoacyl glycerols (10%), and unesterified fatty acids (25%). About half of these wax esters, glycerols, and fatty acids contain unsaturated carbon-carbon bonds that react rapidly with ozone. The most abundant unsaturated fatty acids are cis-hexadec-6-enoic acid (5.4%), cis-octadec-8-enoic acid (2.2%), and cis-15-methylpentadec-6-enoic acid (1.0%). The single most abundant ozone-reactive constituent is squalene [10 to 12% of the surface lipids (13)]. Other “antioxidants” present in the stratum corneum can react with ozone, including ascorbic acid (vitamin C), tocopherols and tocotrienols (vitamin E), cholesterol, ubiquinones (coenzyme Q), glutathione, and uric acid (14–16). However, these are much less abundant than squalene and unsaturated fatty acid moieties in their free or esterified forms (17).
In the present study we have used proton-transfer-reaction mass spectrometry (PTR-MS) to identify and monitor in real time the products formed when ozone reacts with constituents of skin oil in a series of “benchtop” experiments. We have also conducted studies in which ozone reacts with human subjects in a simulated office setting under commonly occurring conditions. Although we can infer surface retained ozonolysis products, the focus of this article is gas phase products that are volatilized from the human skin. The use of PTR-MS facilitates detection of volatile organic compounds (VOCs) that are missed by methods routinely applied to the analysis of indoor air (7, 9) and sidesteps artifacts that occur when VOCs are sampled in the presence of ozone (18–20). A number of the identified products have not been previously reported, and the majority of these commonly occurring oxidation products have been overlooked in surveys of indoor air pollutants (e.g., see refs. 21–25).
Results and Discussion
Volatile Products Derived from Reactions of Ozone with Skin Oils.
In a series of experiments clean, silanized glass wool was rubbed between the fingers and across other skin surfaces of human volunteers. A soiled specimen was placed in a Teflon PFA tube and VOC-free air containing no ozone or 100 ppb of ozone was then passed through the tube directly into the PTR-MS. The mass spectrum (Fig. S1) was much more complex with ozone than without ozone. Count rates for the larger peaks were equivalent to gas-phase mixing ratios in the hundreds of ppt’s to tens of ppb’s. Mass spectra of skin oils from different males and females were similar and highly reproducible.
Squalene is the most abundant unsaturated compound in human sebum (13, 26). In an accompanying experiment, air containing ozone passed over a film of pure squalene, and a fraction passed into the PTR-MS. With the exception of a few peaks, the resultant mass spectrum closely resembled the spectra obtained during ozonolysis of glass wool soiled with human skin oil (Fig. S1). Table 1 summarizes the volatile products that were common to both the skin oil and squalene ozonolysis experiments. The first column lists ion signals (m/z) that displayed elevated abundances; the second column lists the corresponding ozonolysis products inferred (or tentatively inferred) to be responsible for the signal; the third column lists the precursors for each of the identified products; the final column lists the original location in squalene of the double bond(s) cleaved by ozone in the course of producing the product in question. The precursors (third column) include squalene as well as first-generation products derived from squalene that are anticipated to accumulate on the ozone-aged specimen of soiled glass wool. The identified products, including those derived from secondary processes, were formed from surface reactions; the residence time in the tube containing the glass wool was too short (<0.6 s) for gas phase ozonolysis to contribute meaningfully to product formation. Each of the observed products can be explained in the context of ozone/squalene surface chemistry and the Criegee mechanism (27). After attack of a double bond by ozone, the resulting primary ozonide decomposes into a primary carbonyl (an aldehyde or a ketone depending on the degree of substitution at the target double bond) and a carbonyl-O-oxide (often termed a “Criegee intermediate”). In the condensed phase, the energy-rich carbonyl-O-oxide undergoes collisional relaxation followed by rearrangement to a stable isomer. Squalene ozonolysis generates two different types of carbonyl-O-oxides: R-CHOO and R-C(CH3)OO. Isomerization of R-CHOO yields a carboxylic acid terminal group; isomerization of R-C(CH3)OO generates an α-hydroxy ketone moiety (details below). Hence, squalene ozonolysis produces compounds that contain carbonyl, carboxyl, or α-hydroxy ketone functional groups.
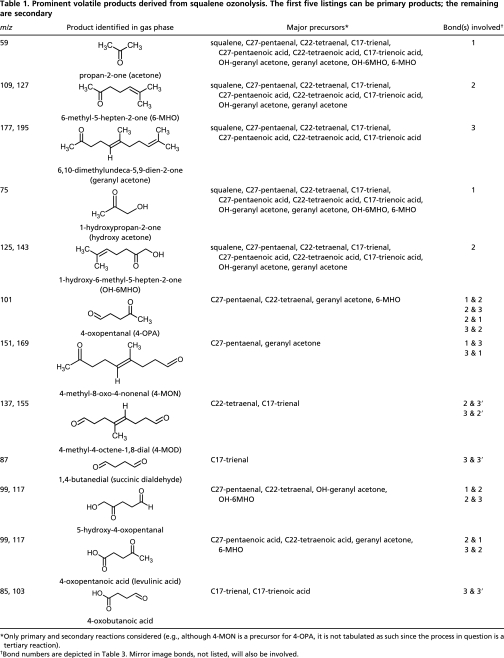 |
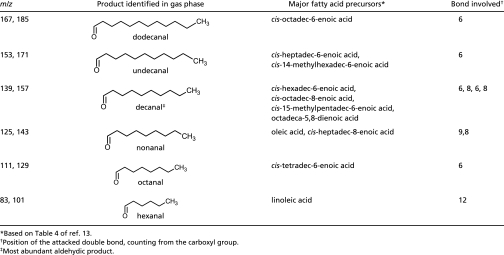
Squalene accounted for all of the observed volatile products in the skin oil experiments with the exception of the linear aldehydes (Table 2). Decanal was the major linear aldehyde and is formed from the most abundant unsaturated fatty acids in human skin lipids (13). Hexanal, octanal, undecanal, and dodecanal were also detected, but their fatty acid precursors are less abundant. Nonanal could not be unambiguously identified due to its mass spectral overlap with 1-hydroxy-6-methyl-5-hepten-2-one (produced from squalene ozonolysis).
Table 2.
Prominent volatile products derived from ozonolysis of unsaturated fatty acids; unsaturated acyl groups in wax esters and glycerols will lead to similar products
 |
Primary vs. Secondary Products.
The major volatile primary products produced from ozone reacting with squalene are acetone, 6-methyl-5-hepten-2-one (6-MHO) and 2,6-dimethyl-2,6-undecadien-10-one (geranyl acetone). These have been previously reported for the ozone/squalene system (12, 28). We infer that the complementary higher molecular weight aldehydes (the three polyunsaturated aldehydes shown in Table 3 and abbreviated as C27-pentaenal, C22-tetraenal and C17-trienal) remain surface-bound.
Table 3.
Structures for squalene and selected squalene products that serve as precursors for gas phase products
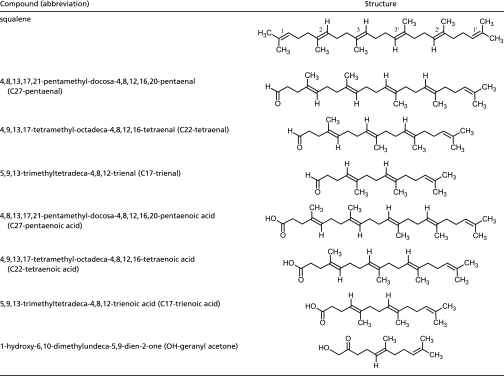 |
Two additional primary products were observed at relatively smaller mixing ratios and were presumably derived from isomerization of stabilized carbonyl-O-oxides. The first product has been tentatively identified as hydroxyacetone, presumably derived from the smaller of the carbonyl oxides [(CH3)2COO] that forms when ozone attacks at the 1 or 1′ bond. (CH3)2COO is thought to tautomerize to the vinyl hydroperoxide, which then isomerizes to hydroxyacetone (29–31). The second product has been tentatively identified as 1-hydroxy-6-methyl-5-hepten-2-one, formed in an analogous manner after ozone attack at the 2 or 2′ bond. We cannot exclude other compounds with the same m/z as these α-hydroxy ketones, but formed by alternate isomerization pathways. Regardless, we again infer that the complementary higher molecular weight products, hydroxy geranyl acetone and the three polyunsaturated fatty acids (Table 3), remain surface-bound.
Secondary products derived from the ozone/squalene system result from a sequential process: initial attack of ozone at a squalene double bond to form an unsaturated surface-bound primary product, which is then subject to a second reaction with ozone. Given the high degree of unsaturation in squalene, tertiary and higher-order reaction products can also be generated, but will not be further discussed. Each of the volatile “primary products” derived from ozone reacting with squalene can also be “secondary products” derived from ozone reacting with unsaturated primary products. More importantly, sequential ozone attack generates secondary products that are bifunctional, containing carbonyl, carboxyl, or α-hydroxy ketone terminal groups. We have observed the full set of bifunctional volatile secondary products expected from Criegee-type ozonolysis of squalene. The major bifunctional products are the four dicarbonyls: 4-oxopentanal (4-OPA), 1,4-butanedial (succinic dialdehyde), 4-methyl-8-oxo-4-nonenal (4-MON), and 4-methyl-4-octene-1,8-dial (4-MOD) produced by attack at the bonds indicated in Table 1. The latter three compounds have not been previously reported in studies of the ozone/squalene system. Detected volatile secondary species also include 5-hydroxy-4-oxopentanal and/or its isomer 4-oxopentanoic acid (levulinic acid) and 4-oxobutanoic acid (succinic semialdehyde), a minor product. Other bifunctional ozonolysis products such as dicarboxylic acids (e.g., succinic acid) or hydroxyketoacids are expected to remain on the surface.
Besides isomerization, stabilized carbonyl-O-oxides may also undergo bimolecular reactions with water forming α-hydroxy-hydroperoxides or with primary carbonyls forming secondary ozonides. An ion was detected with an m/z matching that of protonated 2-hydroxypropyl hydroperoxide, which may be formed from the reaction of (CH3)2COO with water. However, this assignment is highly uncertain. No secondary ozonides were detected; they are anticipated to be relatively nonvolatile.
Time-Evolution of Products.
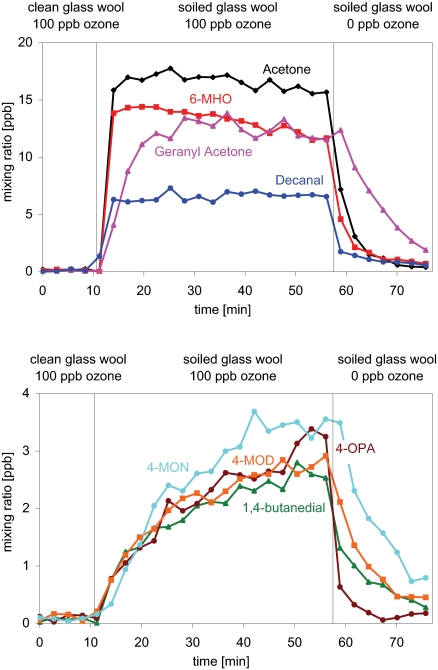
While it is currently difficult to observe such surface chemistry as it occurs (32), we have probed the kinetics of the system using real-time, continuous monitoring of volatilized products. The time evolution of the mixing ratios for primary (Fig. 1 Upper) and secondary (Fig. 1 Lower) products generated during ozonolysis experiments is quite different. The primary carbonyls quickly reached steady-state; geranyl acetone desorbed the slowest, consistent with its larger molecular weight. They then remained at steady-state levels until ozone was removed from the airstream, indicating that the skin oils on the glass wool were not meaningfully depleted during the 45 min of ozone exposure. In contrast to the behavior displayed by the primary products, the mixing ratios of the secondary products increased until ozone was removed from the airstream. This is consistent with a buildup, over the 45-min period, in the surface concentrations of the primary products from which the secondary products were derived. Notably, the secondary dicarbonyls were detected in significant amounts only after the soiled glass wool had been exposed to ozone for some time—duration of exposure is an important parameter when considering ozone/skin-oil chemistry.
Fig. 1.
Plots of mixing ratio as a function of time; 0–11 min: clean glass wool, 100 ppb O3; 12–57 min: glass wool soiled with skin oil, 100 ppb O3; 58–75 min: glass wool soiled with skin oil, no O3. (Upper) Mixing ratios of primary products. (Lower) Mixing ratios of secondary products.
Ozone/Human Skin Interaction.
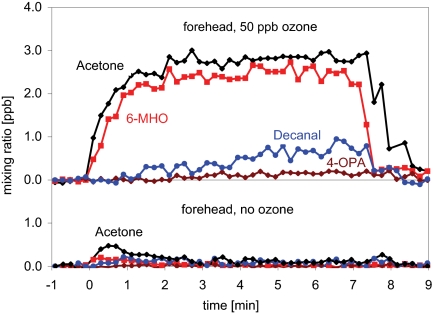
For in vivo monitoring of ozone-initiated reactions on human skin, we placed a small-volume enclosure on an investigator’s skin and passed air that contained ozone through the enclosure and into the PTR-MS (see Methods). Fig. 2 shows the results when the enclosure was placed on an individual’s forehead. Within three minutes, the mixing ratios of acetone and 6-MHO increased to steady-state levels of ≈2.5 ppb. Decanal and geranyl acetone (not plotted), although primary products, desorbed slowly from human skin; after 7 min the former and the latter had reached ≈0.7 and ≈0.15 ppb, respectively. The mixing ratio of 4-OPA also increased at a slow rate, reaching only ≈0.2 ppb after 7 min. This reflects its secondary production from primary oxidation products that remain on the skin (see Table 3). It is noteworthy that, in the absence of ozone, carbonyl emissions from human skin were negligible—a finding that challenges the common perception that carbonyls emitted from human skin are endogenously produced (e.g., ref. 33 and references therein).
Fig. 2.
Plots of mixing ratio as a function of time for acetone, 6-MHO, decanal, and 4-OPA in air that has passed over a subject’s forehead. (Upper) Air containing 50 ppb of O3. (Lower) Air containing no O3.
Results similar to those shown in Fig. 2 were obtained in replicate forehead experiments. Other locations that were probed included the cheek and forearm. Absolute mixing ratios of acetone and 6-MHO were larger when the enclosure was on the forehead compared to these other locations (forehead:cheek:forearm ≈1:0.75:0.65). This is consistent with larger squalene levels on the forehead compared with other regions of the body (26). At a given skin location, the steady-state mixing ratios of acetone, 6-MHO and hydroxyacetone scaled linearly with the ozone mixing ratio (either 25 or 50 ppb).
Ozone Reactions with Occupants in a Simulated Office.
The products identified in the experiments outlined above are anticipated to form whenever humans encounter ozone. To further explore ozone reacting with human surfaces (exposed skin, hair, and clothing) in indoor environments, we performed a series of experiments in a 28.5 m3 chamber configured to resemble a typical office. We examined two commonly occurring, but quite different scenarios. In the first scenario, two subjects entered an empty office that already contained ozone; in the second scenario, the subjects occupied an ozone-free office and after several hours, ozone was introduced with the ventilation air.
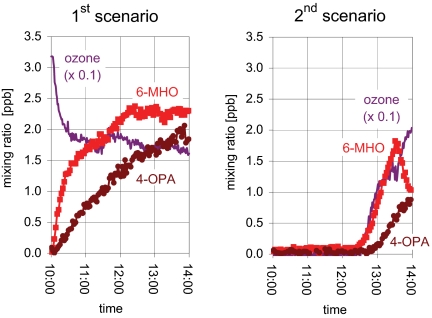
First scenario: the room was continuously ventilated (1 h−1) with air containing a constant concentration of ozone. From 08:00 until 10:00, the room was empty and the ozone level was 32–33 ppb. Two subjects entered the room at 10:00 and remained there for the next 5 h. The major gas phase oxidation products derived from the subjects were acetone, 6-MHO, geranyl acetone, decanal, 4-OPA, and 1,4-butanedial. Fig. 3 Left shows the evolution of ozone, 6-MHO, and 4-OPA during the experiment. The ozone concentration started to decrease as soon as the subjects entered the room, dropping to 18 ppb by 11:00; during this same period, 6-MHO increased from <0.1 ppb to 1.6 ppb and 4-OPA increased from <0.1 ppb to 0.75 ppb. By 14:00, the concentration of ozone had decreased to 16 ppb, while 6-MHO and 4-OPA had increased to 2.3 ppb and 2.0 ppb, respectively. The level of geranyl acetone evolved in a manner similar to that of 6-MHO, but attenuated roughly a factor of eight; that of 1,4-butanedial evolved in a manner similar to 4-OPA, but attenuated by roughly a factor of five. This experiment was repeated on another day with similar results.
Fig. 3.
Mixing ratios of O3 (values plotted are 1/10 measured values), 6-MHO, and 4-OPA in the simulated office. (Left) First scenario: two subjects entered at 10:00 and remained in the room until the end of the experiment. (Right) Second scenario: two subjects entered the simulated room at 09:00; at 12:00 the ozone generators were turned on; at 13:30 the subjects left the room, and the ozone generators remained on.
In addition to the products shown in Fig. 3, we detected hydroxyacetone and 1-hydroxy-6-methyl-5-hepten-2-one (each tentatively identified) at sub ppb levels. Signals corresponding to other volatile ozonolysis products of squalene were below or at the detection threshold of the instrument. A reanalysis of experiments conducted at higher occupant density and higher ozone levels (9) also revealed the presence of these compounds (ppt levels).
Second scenario: the room was initially ventilated (1 h−1) with ozone-free air. Two subjects entered the room at 09:00. Beginning at 12:00, the room was ventilated with air containing a constant concentration of ozone. As in the first scenario, the major resulting oxidation products were acetone, 6-MHO, gera-nyl acetone, decanal, 4-OPA, and 1,4-butanedial. At 13:30, the subjects left the room, which remained ventilated with air that contained ozone. Fig. 3 Right shows the evolution of ozone, 6-MHO, and 4-OPA during the experiment. When the ozone generator was on and the subjects were present (12:00–13:30), the ozone concentration increased and then leveled off at ≈14 ppb, 6-MHO increased to 1.7 ppb, and 4-OPA increased to 0.5 ppb. After the subjects left, ozone further increased, reaching 20 ppb by 14:00; 6-MHO rapidly decreased; and 4-OPA continued to increase, reaching ≈0.8 ppb.
Fig. 3 illustrates that in both scenarios the mixing ratio of 4-OPA increased more slowly than that of 6-MHO; furthermore, the mixing ratio of 4-OPA continued to grow even after that of 6-MHO leveled off or began to decrease. More generally, in both scenarios the mixing ratios of secondary products increased at much slower rates than those of primary products. This indicates that the duration of ozone/human interactions is an important consideration when evaluating potential inhalation of volatile secondary products derived from this chemistry.
Gas phase reactions are anticipated to contribute to some of the products observed in both scenarios. However, kinetic calculations using the reported second order rate constants for the reactions of ozone with 6-MHO and geranyl acetone (34, 20), indicate that ≈90% of the 4-OPA found in the gas phase during these experiments was derived from surface reactions (see SI Text).
None of the volatile products of ozone/skin-oil chemistry (with the exception of acetone) has been reported in previous measurements of organic compounds found within home, school, or office air (e.g., refs. 21–25), although some have been measured in outdoor settings (12, 35, 36). This reflects the fact that the analytical methods routinely applied to indoor air are not suitable for the detection of such compounds.
Ozone Removal in Indoor Settings by Human Occupants.
The present results support recent studies (8–11, 37) demonstrating or inferring that humans are major sinks for ozone. The reaction probabilities reported for human hair (10) and clothing soiled with skin oil (11) are high, ranging from 0.5 × 10−4 to 4 × 10−4. These are reasonable, considering that reaction probabilities with squalene and unsaturated fatty acids range from 5 × 10−4 to 2 × 10−3 (28, 38, 39). In Scenario 1, the two occupants removed ozone with a first-order rate constant of 2.0 h−1, and in Scenario 2, two different occupants removed ozone with a first-order rate constant of 1.7 h−1. Given the volume of the office (28.5 m3) and assuming a surface area of 1.7 m2/person, the rate constants for ozone removal by occupants correspond to deposition velocities between 0.4 and 0.5 cm s−1. Such values are similar to values for 3-h average deposition velocities (0.37 to 0.46 cm s−1) recently reported for reactions between ozone and pieces of cotton, wool, and polyester fabrics soiled with skin oils (11). They are somewhat larger than values (0.20 and 0.23 cm s−1) reported for ozone and passengers in a simulated aircraft cabin (8), values (≈0.25 cm s−1) derived for ozone and human hair (10), and values (0.29 to 0.44 cm s−1) calculated from results presented in (37) at air exchange rates between 0.5 to 2.0 h−1. The deposition velocity of ozone to the surface of the human envelope is sensitive to air movement around the body (10). Additionally, gas phase chemistry contributes to the overall ozone removal associated with human occupants. Regardless, in a 30 m3 room, a single occupant may contribute between 10 and 25% to the overall ozone removal (i.e., the sum of the first-order rate constants for removal by air exchange, room surfaces, gas phase chemistry, and a single occupant, where the latter is ≈0.9 h−1). Only a small fraction of human removal is due to respiration (see SI Text).
Implications for Human Health.
The lipids in human skin oils, especially squalene and unsaturated acyl groups (13, 26), function as the first line of dermal defense against oxidizing agents in the air. Furthermore, reactions of ozone on human surfaces are predicted to reduce ozone levels in the breathing zone (37). The major scavenger of ozone on human surfaces is squalene, producing a cascade of carbonyls, dicarbonyls, and other mono- and bifunctional compounds. This conclusion is supported by the product analysis reported above (further details, including estimated yields, are in SI Text) and stands in contrast to earlier reports that have focused on vitamin E and other less abundant antioxidants as ozone scavengers (14–16) and malondialdehyde as a prominent ozonolysis product (14).
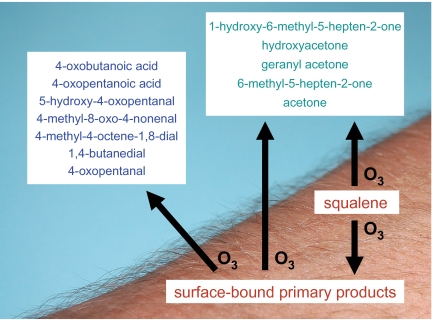
In indoor environments, in addition to being present on skin, hair, and worn clothing, skin oils may also be present on soiled bedding, stored clothing, and surfaces such as keyboards, chairs, and carpets that have been in contact with occupants. To some extent, skin oils also redistribute to all exposed indoor surfaces (40). Despite the ubiquitous presence of skin oils and the common indoor occurrence of ozone, human exposure to products derived from their resultant chemistry has been overlooked. Taken together, the experiments conducted in this study demonstrate that ozone reacts with skin oils to produce both saturated and unsaturated oxidation products of varying volatility (Fig. 4). A large fraction of the less volatile products remain on the surface, where the unsaturated species further react with ozone, producing still other products of varying volatility. At the same time, volatile unsaturated products, which have desorbed into the gas phase, react with ozone to produce additional gas phase products. When ozone is present, the larger the occupant density and the lower the air exchange rate, the larger the resulting exposure to oxidation products. The volatile products of ozone/skin-oil chemistry can be inhaled, while the less volatile oxidation products can remain on the skin. Some of the inhaled volatile products, especially the dicarbonyls, may be respiratory irritants (41–43). Some of the less volatile products may be skin irritants (14–16, 44, 45). Additionally, breathing zone levels of products derived from reactions occurring on the body envelope are predicted to be 1.2 to 2.5 times greater than room levels at typical air exchange rates (37).
Fig. 4.
Schematic of ozone reacting with squalene on exposed skin. The initial reaction produces both gas phase and surface-bound primary products. Ozone further reacts with surface bound primary products (see Table 3) to produce additional gas-phase products.
Implications for Indoor and Outdoor Atmospheres.
Skin oils, on occupants and on other indoor surfaces to which they have migrated, influence indoor mixing ratios of oxidizing agents and oxidation products. This is true not only for ozone and ozone-derived products, but also for hydroxyl radicals, nitrate radicals, and their oxidation products. The current study shows that a single human occupant removes 10 to 25% of the ozone in a typical 30 m3 room. This removal is matched by emissions into the air of volatile skin-oil oxidation products. The unsaturated volatile products (e.g., 6-MHO, geranyl acetone, 4-MOD), in addition to reacting with ozone, can rapidly consume hydroxyl and nitrate radicals, which may be present due to ozone/alkene (46–49) and ozone/nitrogen dioxide reactions (49, 50). In effect, humans substantially alter the mix and mixing ratio of oxidizing and oxidized species in the indoor environments that they occupy.
These findings have relevance for ozone-initiated processes that occur outdoors. Unsaturated organic species such as squalene, related terpenoids, and unsaturated fatty acids are common on various outdoor surfaces, including plant surfaces, soil with plant litter, airborne particles, sea surface layers, and man-made structures. The chemistry that we have elucidated in the present experiments is anticipated to occur on such surfaces. To a large extent, the resultant products are likely to have been overlooked in surveys of outdoor pollutants for the same reasons that they have been overlooked in surveys of indoor pollutants—analytical methods have been used that were not suited to the detection of easily oxidized or highly functionalized compounds. Some of these ozone-derived products may play meaningful roles in atmospheric processes, influencing the budgets of OH radicals and ozone (refs. 51 and 52 and references therein).
Finally, as previously cautioned by Fruekilde et al. (12), ozone/skin oil interactions may be an important source of artifacts in both indoor and outdoor measurements of atmospheric oxidants and VOCs. Whenever sampling lines or surfaces in analytical instruments are contaminated with human skin lipids, there is a significant surface ozone sink and a meaningful source of VOCs that are highly reactive toward common atmospheric oxidants.
Methods
Small Scale Experiments.
For in vitro investigations of ozone/skin-oil interactions, silanized glass wool (Supelco) was rubbed between the fingers and across the forehead and nose-bridge of human volunteers. The soiled specimen was immediately placed in a Teflon PFA tube (length: 7.5 cm, OD: 6.4 mm, ID: 4 mm) and VOC-free air containing no ozone or 100 ppb of ozone was then passed through the tube (flow rate: 115 sccm, total residence time: 0.6 s) directly into the PTR-MS. In vivo measurements of ozone-initiated reactions on human skin were performed using a hollow cylindrical Teflon PFA enclosure (length: 13 mm, ID: 13 mm) with a dual inlet top end and an open bottom end (≈133 mm2). The enclosure was manually pressed against the skin (forehead, cheek, and forearm). The enclosure was flushed with VOC-free air (flow rate: 115 sccm; total residence time: 0.9 s) containing 0, 25, or 50 ppb of ozone, a portion of which flowed directly into the PTR-MS. A similar arrangement was used to study ozone-initiated reactions with a film of pure squalene (>98%, Sigma-Aldrich) on an inert substrate.
Experiments in Simulated Office.
Ethical review boards in Denmark and the U.S. approved the use of human subjects for the present experiments. These studies were conducted within a carpeted 28.5 m3 chamber that contained two small stainless-steel tables, two chairs, two flat screen LCD monitors, two headsets, one walkie-talkie, one small mixing fan, a few books, two laptops, and two bottles of water. The chamber temperature was 23 °C, the relative humidity was 20–25%, and the outdoor air exchange rate was 0.9–1.1 h−1.
Generation and Measurement of Ozone.
For the small scale experiments, ozone was produced using VOC-free air from a zero air generator (model 75–83, Parker-Balston) that passed through a Teflon PFA line externally irradiated with a UV lamp (Jelight). No volatile organic by-products or artifacts (e.g., aldehydes) were produced during ozone generation. For the simulated office experiments, flow-meters controlled the delivery of high purity O2 (hydrocarbons <1 ppm) from a compressed gas cylinder through one or two Jelight UV ozone generators into a 4 m3 stainless-steel chamber. Here, the O2/O3 mixed with outdoor air that had passed through particle and charcoal filters. This air, in turn, was introduced into the “office.” In both small scale and office experiments, ozone levels were continuously measured using photometric analyzers operating at 254 nm (Dasibi 1003-AH).
PTR-MS Measurements.
Organic compounds in the gas phase were measured using PTR-MS, a chemical ionization technique based on proton transfer reactions from H3O+ to gaseous analytes with a higher proton affinity than water. The instrument was calibrated using dynamically diluted gas standards containing ≈1 ppm (±5% accuracy) of saturated linear C2-C10 aldehydes, acrolein, acetone, and 2-butanone, respectively. Calibration factors were very similar for all investigated carbonyls. The instrumental response to acetone was used as a proxy to derive mixing ratios of uncalibrated carbonyls with an estimated accuracy of ±20%. Additional experimental details are reported in (9). Given that this method does not require preconcentration on a sorbent or in a canister, a significant source of artifacts (ozone reacting with captured compounds) can be ruled out.
Supplementary Material
Acknowledgments
We thank Peter Strøm-Tejsen for assistance with the simulated office experiments, and Armin Hansel and Tilmann D. Märk for their continued support. This work has been partially supported by the Danish Technical Research Council (STVF) through the International Centre for Indoor Environment and Energy at the Technical University of Denmark.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904498106/DCSupplemental.
References
- 1.Weschler CJ. Ozone in indoor environments: Concentration and chemistry. Indoor Air. 2000;10:269–288. doi: 10.1034/j.1600-0668.2000.010004269.x. [DOI] [PubMed] [Google Scholar]
- 2.Britigan N, Alshawa A, Nizkorodov SA. Quantification of ozone levels in indoor environments generated by ionization and ozonolysis air purifiers. J Air Waste Manage Assoc. 2006;56:601–610. doi: 10.1080/10473289.2006.10464467. [DOI] [PubMed] [Google Scholar]
- 3.Jacober C, Phillips T. Evaluation of Ozone Emissions from Portable Indoor Air Cleaners: Electrostatic Precipitators and Ionizers.Staff Technical Report to the California Air Resources Board, February 2008. 2008. Availabe at http://www.arb.ca.gov/research/indoor/esp_report .pdf. Accessed August 2, 2009.
- 4.Liu XY, Mason M, Krebs K, Sparks L. Full-scale chamber investigation and simulation of air freshener emissions in the presence of ozone. Environ Sci Technol. 2004;38:2802–2812. doi: 10.1021/es030544b. [DOI] [PubMed] [Google Scholar]
- 5.Singer BC, et al. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos Environ. 2006;40:6696–6710. doi: 10.1021/es052198z. [DOI] [PubMed] [Google Scholar]
- 6.Sarwar G, Corsi R. The effects of ozone/limonene reactions on indoor secondary organic aerosols. Atmos Environ. 2007;41:959–973. [Google Scholar]
- 7.Wisthaler A, et al. Products of ozone-initiated chemistry in a simulated aircraft environment. Environ Sci Technol. 2005;39:4823–4832. doi: 10.1021/es047992j. [DOI] [PubMed] [Google Scholar]
- 8.Tamas G, Weschler CJ, Bako-Biro Z, Wyon DP, Strom-Tejsen P. Factors affecting ozone removal rates in a simulated aircraft cabin environment. Atmos Environ. 2006;40:6122–6133. [Google Scholar]
- 9.Weschler CJ, et al. Ozone-initiated chemistry in an occupied simulated aircraft cabin. Environ Sci Technol. 2007;41:6177–6184. doi: 10.1021/es0708520. [DOI] [PubMed] [Google Scholar]
- 10.Pandrangi LS, Morrison GC. Ozone interactions with human hair: Ozone uptake rates and product formation. Atmos Environ. 2008;42:5079–5089. [Google Scholar]
- 11.Coleman BK, Destaillats H, Hodgson AT, Nazaroff WW. Ozone consumption and volatile byproduct formation from surface reactions with aircraft cabin materials and clothing fabrics. Atmos Environ. 2008;42:642–654. [Google Scholar]
- 12.Fruekilde P, Hjorth J, Jensen NR, Kotzias D, Larsen B. Ozonolysis at vegetation surfaces: A source of acetone, 4-oxopentanal, 6-methyl-5-hepten-2-one, and geranyl acetone in the troposphere. Atmos Environ. 1998;32:1893–1902. [Google Scholar]
- 13.Nicolaides N. Skin lipids: Their biochemical uniqueness. Science. 1974;186:19–26. doi: 10.1126/science.186.4158.19. [DOI] [PubMed] [Google Scholar]
- 14.Thiele JJ, Traber MG, Polefka TG, Cross CE, Packer L. Ozone-exposure depletes vitamin E and induces lipid peroxidation in murine stratum corneum. J Invest Dermatol. 1997;108:753–757. doi: 10.1111/1523-1747.ep12292144. [DOI] [PubMed] [Google Scholar]
- 15.Thiele JJ, Podda M, Packer L. Tropospheric ozone: An emerging environmental stress to skin. Biol Chem. 1997;378:1299–1305. doi: 10.1515/bchm.1997.378.11.1299. [DOI] [PubMed] [Google Scholar]
- 16.Weber SU, Thiele JJ, Cross CE, Packer L. Vitamin C, uric acid, and glutathione gradients in murine stratum corneum and their susceptibility to ozone exposure. J Invest Dermatol. 1999;113:1128–1132. doi: 10.1046/j.1523-1747.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- 17.Passi S, De Pita O, Puddu P, Littarru GP. Lipophilic antioxidants in human sebum and aging. Free Radic Res. 2002;36:471–477. doi: 10.1080/10715760290021342. [DOI] [PubMed] [Google Scholar]
- 18.Helmig D. Ozone removal techniques in the sampling of atmospheric volatile organic trace gases. Atmos Environ. 1997;31:3635–3651. [Google Scholar]
- 19.Fick J, Pommer L, Andersson B, Nilsson C. Ozone removal in the sampling of parts per billion levels of terpenoid compounds: An evaluation of different scrubber materials. Environ Sci Technol. 2001;35:1458–1462. doi: 10.1021/es0001456. [DOI] [PubMed] [Google Scholar]
- 20.Pollmann J, Ortega J, Helmig D. Analysis of atmospheric sesquiterpenes: Sampling losses and mitigation of ozone interferences. Environ Sci Technol. 2005;39:9620–9629. doi: 10.1021/es050440w. [DOI] [PubMed] [Google Scholar]
- 21.United States Environmental Protection Agency. The Total Exposure Assessment Methodology (TEAM) Study: Summary and Analysis: Volume I. Washington, DC: U.S. EPA; 1987. EPA/600/6–87/002a. [Google Scholar]
- 22.Daisey JM, Hodgson AT, Fisk WJ, Mendell MJ, Ten Brinke J. Volatile organic compounds in twelve California office buildings: Classes, concentrations and sources. Atmos Environ. 1994;28:3557–3562. [Google Scholar]
- 23.Shields HC, Fleischer DM, Weschler CJ. Comparisons among VOCs measured in three types of U.S. commercial buildings with different occupant densities. Indoor Air. 1996;6:2–17. [Google Scholar]
- 24.Weisel C, et al. Collection Methods and Descriptive Analyses. Boston, MA: Health Effects Institute; 2005. Relationships of Indoor, Outdoor, and Personal Air (RIOPA): Part 1; p. 127. [PubMed] [Google Scholar]
- 25.United States Environmental Protection Agency. Building Assessment Survey and Evaluation (BASE) Study. Washington, DC: U.S. EPA; 2006. EPA 402-C-06–002. Available at http://www.epa.gov/iaq/base/. Accessed March 22, 2009. [Google Scholar]
- 26.Nikkari T, Schreibman PH, Ahrens EH., Jr In vivo studies of sterol and squalene secretion by human skin. J Lipid Res. 1974;15:563–573. [PubMed] [Google Scholar]
- 27.Criegee R. Mechanisms of ozonolysis. Angew Chem Int Ed Engl. 1975;14:745–752. [Google Scholar]
- 28.Wells JR, Morrison GC, Coleman BK. Kinetics and reaction products of ozone and surface-bound squalene. J ASTM Int. 2008;5(7):1–12. [Google Scholar]
- 29.Story PR, Burgess JR. Ozonolysis. Evidence for carbonyl oxide tautomerization and for 1,3-dipolar addition to olefins. J Am Chem Soc. 1967;89:5726–5727. [Google Scholar]
- 30.Barton M, Ebdon JR, Foster AB, Rimmer S. Ozonolysis of tetramethylethylene: Characterization of cyclic and open-chain oligoperoxidic products. J Org Chem. 2004;69:6967–6973. doi: 10.1021/jo035624c. [DOI] [PubMed] [Google Scholar]
- 31.Epstein SA, Donahue NM. The kinetics of tetramethylethene ozonolysis: Decomposition of the primary ozonide and subsequent product formation in the condensed phase. J Phys Chem A. 2008;112:13535–13541. doi: 10.1021/jp807682y. [DOI] [PubMed] [Google Scholar]
- 32.Segal-Rosenheimer M, Dubowski Y. Heterogeneous ozonolysis of cypermethrin using real-time monitoring FTIR techniques. J Phys Chem C. 2007;111:11682–11691. [Google Scholar]
- 33.Gallagher M, et al. Analyses of volatile organic compounds from human skin. Brit J Dermatol. 2008;159:780–791. doi: 10.1111/j.1365-2133.2008.08748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grosjean E, Grosjean D, Seinfeld JH. Gas-phase reaction of ozone with trans-2-hexenal, trans-2-hexenyl acetate, ethylvinyl ketone, and 6-methyl-5-hepten-2-one. Int J Chem Kinet. 1996;28:373–382. [Google Scholar]
- 35.Ciccioli P, Brancaleoni E, Frattoni M, Cecinato A, Brachetti A. Ubiquitous occur-rence of semivolatile carbonyl compounds in tropospheric samples and their possible sources. Atmos Environ Part A. 1993;27:1891–1901. [Google Scholar]
- 36.Matsunaga S, Mochida M, Kawamura K. High abundance of gaseous and particulate 4-oxopentanal in the forestal atmosphere. Chemosphere. 2004;55:1143–1147. doi: 10.1016/j.chemosphere.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Rim D, Novoselec A, Morrison GC. The influence of chemical interactions at the human surface on the breathing-zone levels of reactants and products. Indoor Air. 2009;19:324–334. doi: 10.1111/j.1600-0668.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- 38.Thornberry T, Abbatt JPD. Heterogeneous reaction of ozone with liquid unsatu-rated fatty acids: Detailed kinetics and gas-phase product studies. Phys Chem Chem Phys. 2004;6:84–93. [Google Scholar]
- 39.Moise T, Rudich Y. Reactive uptake of ozone by aerosol-associated unsaturated fatty acids: Kinetics, mechanism, and products. J Phys Chem A. 2002;106:6469–6476. [Google Scholar]
- 40.Weschler CJ, Nazaroff WW. Semivolatile organic compounds in indoor environments. Atmos Environ. 2008;42:9018–9040. [Google Scholar]
- 41.Azadi S, Klink KJ, Meade BJ. Divergent immunological responses following glutaraldehyde exposure. Toxicol Appl Pharmacol. 2004;197:1–8. doi: 10.1016/j.taap.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 42.Jarvis J, Seed MJ, Elton R, Sawyer L, Agius R. Relationship between chemical structure and the occupational asthma hazard of low molecular weight organic compounds. Occup Environ Med. 2005;62:243–250. doi: 10.1136/oem.2004.016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson SE, et al. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol Sci. 2007;97:355–363. doi: 10.1093/toxsci/kfm043. [DOI] [PubMed] [Google Scholar]
- 44.Schultz-Larsen F. Atopic dermatitis: A genetic-epidemiologic study in a population based twin sample. J Am Acad Dermatol. 1993;28:719–723. doi: 10.1016/0190-9622(93)70099-f. [DOI] [PubMed] [Google Scholar]
- 45.Podda M, Fuchs J. Influence of environmental polluting ozone on the skin. Der Hautarzt. 2004;55:1120–1124. doi: 10.1007/s00105-004-0842-0. in German. [DOI] [PubMed] [Google Scholar]
- 46.Weschler CJ, Shields HC. Production of the hydroxyl radical in indoor air. Environ Sci Technol. 1996;30:3250–3258. [Google Scholar]
- 47.Weschler CJ, Shields HC. Measurements of the hydroxyl radical in a manipulated but realistic indoor environment. Environ Sci Technol. 1997;31:3719–3722. [Google Scholar]
- 48.Sarwar G, Corsi R, Kimura Y, Allen D, Weschler CJ. Hydroxyl radicals in indoor environments. Atmos Environ. 2002;36:3973–3988. [Google Scholar]
- 49.Carslaw N. A new detailed chemical model for indoor air pollution. Atmos Environ. 2007;41:1164–1179. [Google Scholar]
- 50.Weschler CJ, Brauer M, Koutrakis P. Indoor ozone and nitrogen dioxide—A potential pathway to the generation of nitrate radicals, dinitrogen pentaoxide, and nitric-acid Indoors. Environ Sci Technol. 1992;26:179–184. [Google Scholar]
- 51.Di Carlo P, et al. Missing OH reactivity in a forest: Evidence for unknown reactive biogenic VOCs. Science. 2004;304:722–725. doi: 10.1126/science.1094392. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein AH, Galbally IE. Known and unexplored organic constituents in the earth’s atmosphere. Environ Sci Technol. 2007;41:1514–1521. doi: 10.1021/es072476p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.