Abstract
The Wnt pathway regulates multiple biological and pathological processes including angiogenesis and inflammation. Here we identified a unique inhibitor of the Wnt pathway, SERPINA3K, a serine proteinase inhibitor with anti-inflammatory and angiogenic activities. SERPINA3K blocked the Wnt pathway activation induced by a Wnt ligand and by diabetes. Coprecipitation and ligand binding assay showed that SERPINA3K binds to low-density lipoprotein receptor-like protein 6 (LRP6) with a Kd of 10 nM, in the range of its physiological concentration in the retina. Under the same conditions, SERPINA3K did not bind to the frizzled (Fz) receptor or low-density lipoprotein receptor. Further, SERPINA3K bound to LRP6 at the extracellular domain and blocked its dimerization with the Fz receptor induced by a Wnt ligand. The antagonizing activity of SERPINA3K to LRP6 was further confirmed by Xenopus axis duplication assay. These results suggest that SERPINA3K is a high-affinity, endogenous antagonist of LRP6. The blockade of Wnt signaling may represent a unifying mechanism for the anti-inflammatory and anti-angiogenic effects of SERPINA3K.
Keywords: angiogenesis, β-catenin, lipoprotein receptor-related protein 6, vascular endothelial growth factor
Wnts are a group of secreted, cysteine-rich glycoproteins that bind to the frizzled (Fz) receptors or to a coreceptor complex of Fz and low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6) (1). In the absence of Wnt ligands, transcription factor β-catenin, a downstream effector of the canonical Wnt pathway, is phosphorylated by a protein complex containing GSK-3 (2). The phosphorylated β-catenin is constantly degraded to prevent its accumulation (2, 3). Upon binding of certain Wnt ligands, the Fz receptor dimerizes with LRP5/6, forming a coreceptor complex, inhibiting phosphorylation of β-catenin (1). Subsequently, β-catenin is stabilized, leading to its accumulation (4, 5). β-catenin is then translocated into the nucleus, associates with T-cell factor (TCF) for DNA binding, and regulates expression of target genes, including proangiogenic factors and proinflammatory factors (6–9).
The Wnt pathway is known to play important roles in multiple physiological and pathological processes (10). It has been well studied in embryogenesis and carcinogenesis (10). Recent evidence suggests that the Wnt pathway is also important in the regulation of inflammatory response and angiogenesis (11–13). Mutations in the Fz receptor and the LRP coreceptor have been shown to associate with vascular developmental defects (12). However, an endogenous antiangiogenic factor endostatin, a fragment of collagen, was found to inhibit the Wnt pathway (14).
Angiogenesis is known to be regulated by two counterbalancing systems, proangiogenic factors and antiangiogenic factors (15). Vascular endothelial growth factor (VEGF) has been shown to be a major proangiogenic and proinflammatory factor (16, 17). However, a number of natural antiangiogenic factors or angiogenic inhibitors have been identified (18). The identified angiogenic inhibitors include several serine proteinase inhibitors (SERPIN), such as pigment epithelium-derived factor (PEDF), α1-antitrypsin (AAT), maspin, SERPINA3K, and headpin (19–25).
SERPINA3K was first identified as a serpin family member with specific inhibitory effect on tissue kallikrein, and thus named kallikrein-binding protein (26). It is expressed in the liver, kidney, pancreas, and retina (26–28). Our previous studies have shown decreased retinal levels SERPINA3K in a diabetic retinopathy animal model (29), and the reduced vitreous levels of kallistatin from patients with diabetic retinopathy (30). Our recent studies showed that SERPINA3K attenuates inflammation in the retina with retinopathy (31). SERPINA3K has been found to inhibit ischemia-induced retinal neovascularization (25). Further, the antiangiogenic effect of SERPINA3K has been shown to be independent of its interactions with the kallikrein-kinin system (25). The molecular mechanism for the serpin angiogenic inhibitors remains uncertain.
In the present study, we have investigated the interactions of SERPINA3K with the Wnt pathway and determined if the beneficial effects of SERPINA3K in diabetic retinopathy are at least partially through inhibition of Wnt signaling.
Results
Inhibitory Effects of SERPINA3K on Retinal Inflammation and Wnt Pathway Activation in Diabetic Rats.
Two months after the onset of diabetes, streptozotocin (STZ)-induced diabetic rats received an intravitreal injection of purified SERPINA3K. Retinal vascular permeability assay at 48 h following the injection showed that the retinal vascular leakage in the diabetic rats was significantly reduced by SERPINA3K, compared with the contralateral retinas in rats injected with BSA and the control retinas in the diabetic rats without injection (Fig. 1A).
Fig. 1.
SERPINA3K blocked Wnt signaling in the retina with diabetic retinopathy and in high-glucose-treated cells. (A) STZ-diabetic rats received an intravitreal injection of 10 μg/eye SERPINA3K and the same amount of BSA into the contralateral eye. Untreated diabetic rats were used as control (DM). Retinal vascular permeability was measured using Evans blue as tracer 48 h after the injection of SERPINA3K, normalized by retinal protein concentrations, and expressed as a percentage of the permeability increase over that of nondiabetic rats (mean ± SD, n = 5). (B) The diabetic rats received an intravitreal injection of Ad-SA3K, with Ad-LacZ as control. Four weeks after the injection, levels of SERPINA3A, VEGF, and cytosolic β-catenin in the retina were measured by Western blot analysis using 50 μg of retinal proteins from each rat sample. (C) The Western blots shown in B were representatives of three independent experiments, and the results were quantified by densitometry, normalized by β-actin levels, and expressed as a percentage of that in nondiabetic control (mean ± SD, n = 6). (D–G) ARPE19 cells (D and E) and primary HRCEC (F and G) were exposed to low-glucose (LG; 5 mM D-glucose + 25 mM L-glucose) and high-glucose (HG; 30 mM D-glucose) media with different concentrations of SERPINA3K for 8 h (D), 24 h (E and F), or 72 h (G). (D and F) Cytosolic β-catenin levels were measured by Western blot analysis. (E) VEGF secreted into the medium was measured using an ELISA kit (R&D Systems) (mean ± SD, n = 3). (G) Cell migration of HRCEC was measured using Transwell migration assay (mean ± SD, n = 10). *P < 0.05.
STZ-diabetic rats also received an intravitreal injection of adenovirus expressing SERPINA3K (Ad-SA3K, 5 × 107 pfu/eye). Retinal levels of SERPINA3K were decreased in the uninjected diabetic rats, compared with that in nondiabetic rats (Fig. 1 B and C). The Ad-SA3K injection resulted in overexpression of SERPINA3K in the retinas of the diabetic rats one month after the injection, compared with the eyes injected with control virus (Fig. 1 B and C). Retinal levels of VEGF, a major inflammatory and angiogenic factor, as well as a target gene of the Wnt pathway, were significantly increased in the retinas of untreated diabetic rats, compared with nondiabetic rats at the same age (Fig. 1 B and C). Cytosolic β-catenin levels in the retina were also significantly elevated in the retinas of diabetic rats. The injection of Ad-SA3K mitigated the overexpression of VEGF in the retinas and decreased retinal levels of cytosolic β-catenin in diabetic rats, compared with the Ad-LacZ injection control, suggesting an inhibition of the canonical Wnt pathway in diabetes (Fig. 1 B and C).
To evaluate the direct effect of SERPINA3K on the Wnt pathway induced by diabetes, ARPE19 cells were exposed to a medium containing 30 mM D-glucose. Cytosolic β-catenin levels and VEGF secretion were elevated by the high-glucose medium, when compared with the low-glucose control (5 mM D-glucose and 25 mM L-glucose), suggesting that high glucose is responsible for the activation of the Wnt pathway in the diabetic rat retina. SERPINA3K blocked the high-glucose-induced β-catenin accumulation and VEGF secretion in a dose-dependent manner (Fig. 1 D and E).
Endothelial cell migration can be up-regulated by high glucose (32) and Wnt ligand (33). Therefore, we investigated the effect of SERPINA3K on endothelial cell migration using primary human retinal capillary endothelial cells (HRCEC) and a cell line derived from rat endothelial cells, TR-iBRB (34). In both of the cells, the high glucose medium-induced β-catenin accumulation was blocked by SERPINA3K (Fig. 1F and Fig. S1B). The Transwell migration of the endothelial cells was up-regulated by high glucose and can be inhibited by SERPINA3K (Fig. 1G and Fig. S1N). In TR-iBRB cells, the increase in scratch wound closure induced by high glucose was also attenuated by SERPINA3K (Fig. S1 C–G and M ).
It has been reported that phosphorylation of LRP6 is a critical step in the Wnt pathway activation (35, 36). As shown by Western blot analysis using an antibody specific for phosphorylated LRP6 (p-LRP6), phosphorylation of the endogenous LRP6 was induced in the cells exposed to 30 mM glucose for 6 h, compared with that in control cells exposed to low-glucose medium. SERPINA3K at 100 nM decreased the p-LRP6 to a level similar to that in low-glucose control (Fig. 2A). In cultured HRCEC, the high-glucose-induced phosphorylation of LRP6 was also reduced by SERPINA3K (Fig. S1A).
Fig. 2.
SERPINA3K blocked Wnt signaling at the cell surface receptor level. (A) ARPE19 cells were exposed to HG or LG medium for 6 h, with 100 nM SERPINA3K or BSA. The same amount of total cellular proteins was blotted with an antibody specific for phosphorylated LRP6 (p-LRP6). (B) The cells were exposed to 50% Wnt3a conditioned medium with 500 nM SERPINA3K or BSA for 24 h. Control cells were treated with the 50% L cell conditioned medium. VEGF secreted into the culture medium was measured by ELISA and expressed as a percentage of control (mean ± SD, n = 3). (C) The cells were infected with an adenovirus expressing a constitutively active mutant of β-catenin (Ad-S37A) at MOI of 8, with 1,000 nM SERPINA3K or BSA; the same titer of the adenovirus expressing GFP (Ad-GFP) was used as control. (D and E) HEK293T cells were exposed to 20 mM LiCl for 1 h (D) or 24 h (E). Total β-catenin levels were measured by Western blot analysis (D). (E) The TCF/LEF promoter activity was measured by TOPFLASH luciferase assay. *P < 0.05; NS, P > 0.05.
To determine the effect of SERPINA3K on the Wnt pathway activation induced by the Wnt ligand, ARPE19 cells were exposed to a medium containing 50% Wnt3a conditioned medium, with the 50% L medium as control. SERPIA3K blocked the VEGF overexpression induced by Wnt3a (P < 0.05; Fig. 2B). Under the same condition, SERPINA3K also inhibited the Wnt3a-induced endothelial migration in TR-iBRB cells, as shown by Transwell assay and wound-healing assay (Fig. S1 H–N).
To determine if SERPINA3K blocks Wnt signaling activated at the intracellular cascade, the cells were infected with an adenovirus expressing a constitutively active mutant of β-catenin (Ad-S37A), in which the phosphorylation site S37 in β-catenin was mutated. As shown by ELISA, VEGF secretion was induced significantly by Ad-S37A. SERPINA3K at 1,000 nM did not block the VEGF production induced by Ad-S37A (Fig. 2C). To determine whether SERPINA3K regulates the β-catenin degradation rate, LiCl, a GSK-3 inhibitor, was used to activate Wnt signaling at the GSK-3β level. LiCl induced the stabilization and accumulation of β-catenin (Fig. 2D) as well as the β-catenin-dependent reporter gene transcription as shown by the TOPFLASH activity assay, a reporter luciferase activity under the control of a promoter containing TCF binding sites (Fig. 2E). Neither the LiCl-induced β-catenin accumulation nor TOPFLASH activity was inhibited by SERPINA3K (Fig. 2 D and E). These findings suggest that the target of SERPINA3K may be at the Wnt receptor level.
SERPINA3K Binds to LRP6 with a High Specificity and Affinity.
To reveal the interactions of SERPINA3K with the cell-surface receptors of Wnts, ARPE19 cells were incubated with 100 nM His-tagged SERPINA3K (SA3K-HIS) for 1 h. Following three washes with PBS, the cells were lysed and the plasma membrane solubilized for coprecipitation assay by incubating the cell lysates with Ni resin. After thorough washes, Western blot analysis of the proteins pulled down by the Ni resin showed that LRP6 was coprecipitated with SERPINA3K-HIS (Fig. 3A). Under the same conditions, Fz4, a Frizzled receptor for Wnt, which is expressed in ARPE19 cells and is known to involve in angiogenesis (12), was not coprecipitated with SERPINA3K (Fig. 3A).
Fig. 3.
Specific binding of SERPINA3K with LRP6. (A) SERPINA3K-HIS (100 nM) was incubated with ARPE19 cells for 1 h. The cells were lysed following three washes, and SERPINA3K-HIS was precipitated using Ni-NTA resin. After thorough washes, the precipitates and input samples were immunoblotted separately with antibodies for LRP6, Fz4, and His-tag. (B) HEK293T cells were cotransfected with plasmids expressing SERPINA3K and that expressing LRP6-HIS (Left), or with that expressing LRP6 and SERPINA3K-HIS (Right). The His-tagged proteins were pulled down by Ni-NTA resin and immunoblotted with an anti-LRP6 or anti-SERPINA3K antibody. (C) Binding of the FITC conjugated SERPINA3K to COS cells expressing LRP6. COS cells were transfected with a LRP6 expression plasmid, with mock transfected cells as control. Twenty-four hours following the transfection, SERPINA3K was added to the culture medium to various concentrations and incubated with the cells for 1 h. After the unbound proteins were washed off, the cells were lysed, and fluorescence measured by fluorometer using a 485/530-nm filter. The fluorescence in the mock transfection control cells was considered as baseline and subtracted from that in the cells expressing LRP6 to calculate specific binding of SERPINA3K to LRP6 (mean ± SD, n = 8). (D) Scatchard analysis of the binding data shown in C. The estimated Kd for SERPINA3K binding with LRP6 is approximate 10 nM. (E) The cells expressing LRP6 were incubated with 100 nM FITC-SERPINA3K in the presence of excess amounts of unlabeled SERPINA3K for 1 h. After washes, the FITC-SERPINA3K remained bound to the cells were quantified (mean ± SEM, n = 8).
To further confirm the interactions between LRP6 and SERPINA3K, a His-tagged LRP6 (LRP6-HIS) and SERPINA3K (no His tag) were coexpressed in HEK293T cells using plasmid transfection. LRP6-HIS was precipitated using Ni resin. SERPINA3K was found to coprecipitate with LRP6-HIS (Fig. 3B). However, in the cells expressing LRP6 (no His tag) and SERPINA3K-HIS, LRP6 was also found to coprecipitate with SERPINA3K-HIS, which was pulled down by Ni resin (Fig. 3B). These findings suggested that SERPINA3K specifically binds to LRP6.
To determine the binding affinity of SERPINA3K to LRP6, COS cells were transfected with a plasmid expressing LRP6 and incubated with various concentrations of FITC-conjugated SERPINA3K. The same amount of FITC-SERPINA3K was incubated with the cells transfected with an empty plasmid as background control. The fluorescence intensity in the background control cells was used as nonspecific control and subtracted from the fluorescence in the LRP6-expressing cells. FITC-SERPINA3K displayed a concentration-dependent and saturable binding onto the cells expressing LRP6 (Fig. 3C). Scatchard plot analysis revealed a high binding affinity between SERPINA3K and LRP6 with a dissociate constant (Kd) of 10 nM (Fig. 3D). Further, binding of FITC-SERPINA3K onto the LRP6-tranfected cells was competed off by excess amounts of unlabeled SERPINA3K (Fig. 3E), suggesting that the binding is specific.
SERPINA3K Functions as an Antagonist of LRP6.
The LRP6-transfected HEK293T cells were treated with the 50% Wnt3a conditioned medium in the presence of various concentrations of SERPINA3K for 1 h. Western blot analysis of the cell lysate revealed that Wnt3a induced phosphorylation of LRP6. SERPINA3K blocked the Wnt3a-induced LRP6 phosphorylation in a concentration-dependent manner but did not alter total LRP6 levels (Fig. 4A). Likewise, cytosolic β-catenin levels were elevated by Wnt3a and decreased by SERPINA3K (Fig. 4B).
Fig. 4.
SERPINA3K inhibited the LRP6-mediated Wnt signaling. (A and B) HEK293T cells were transfected with the LRP6 expression vector or a control vector. Twenty-four hours after the transfection, the cells were exposed to the 50% Wnt3a conditioned medium in the presence of various concentrations of SERPINA3K or a control L cell medium for 1 h. Total protein concentrations in the culture medium was brought to the same by the addition of BSA. Total LRP6 and p-LRP6 were measured by Western blot analysis using 100 μg total proteins (A). (B) The cytosolic proteins (20 μg) were immunoblotted with an antibody for β-catenin and normalized by β-actin levels. (C) The cells were transfected with the LRP6 expression vector and the TOPFLASH vector, followed by exposure to the 50% Wnt3a conditioned medium containing various concentrations of SERPINA3K or control conditioned medium for 24 h. The TCF/β-catenin activity was measured using luciferase assay (mean ± SD, n = 3).
We also examined the effect of SERPINA3K on the Wnt3a-mediated gene transcription. HEK293T cells were transfected with the TOPFLASH construct. In the transfected HEK293T cells, LRP6 overexpression induced the TOPFLASH reporter (TCF/LEF) activity, which was further enhanced by the exposure to the Wnt3a conditioned medium (Fig. 4C). The TOPFLASH reporter activities induced by transfection of LRP6 and Wnt3a were inhibited by SERPINA3K in a concentration-dependent manner (Fig. 4C). These findings indicated that SERPINA3K is an antagonist of LRP6 and blocks the Wnt ligand-induced Wnt signaling.
SERPIA3K Inhibits Xenopus Axis Duplication Induced by Wnt Ligand.
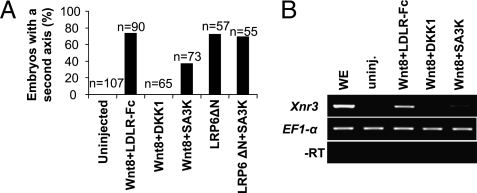
Wnt/β-catenin signaling induces dorsal axis formation in Xenopus embryos, and the axis duplication assay is a commonly used and reliable method to study the Wnt signaling pathway in vivo (37). Wnt8 and LRP6ΔN (a constitutively active mutant of LRP6 without the N-terminal extracellular domain) mRNAs were separately injected into the ventral marginal zone of a four-cell Xenopus embryos. The injections of Wnt8 and LRP6ΔN resulted in more than 70% embryos with duplicate axis formation (Fig. 5A). The SERPINA3K mRNA injection blocked the Wnt8 mRNA-induced axis duplication by ∼50% and did not show any effect on the axis duplication induced by the constitutively active LRP6ΔN (Fig. 5A). Dickkopf-1 (DKK1), a secreted head inducer and a high-affinity LRP6 antagonist (1) was used as a positive control, which induced a complete blockade of the Xenopus axis duplication (Fig. 5A).
Fig. 5.
SERPINA3K inhibited the secondary axis formation and Xnr3 expression induced by Wnt8. (A) Secondary axis was induced in 73.3% or 71.9% of embryos by injection of Wnt8 and LRP6ΔN, respectively. Wnt8 RNA was ventrally coinjected in four-cell stage Xenopus embryos with secreted LDL receptor (LDLR-Fc; as an RNA control), RNAs of DKK1, and SERPINA3K. SERPINA3K decreased 50% of the Wnt-induced axes, whereas DKK1 inhibited 100%. The LRP6ΔN-induced secondary axis was not inhibited by SERPINA3K. (B) RT-PCR showing Xnr3 message induced by XWnt8 in animal cap (AC) explants. SERPINA3K decreased the Wnt8-induced Xnr3 message, whereas DKK1 totally blocked Wnt8-induced Xnr3 expression. EF1-α was used as loading control. (Bottom) PCR without reverse transcriptase (−RT). WE, whole embryo.
To further confirm the antagonizing effect of SERPINA3K on Wnt signaling, we examined the XWnt8-mediated induction of Xenopus nodal-related 3 (Xnr3), a directly downstream target of Wnt signaling (37) using RT-PCR. The XWnt8-induced Xnr3 transcription was attenuated by DKK1 and SERPINA3K (Fig. 5B). Taken together, these findings suggest that SERPINA3K inhibits Wnt signaling in the Xenopus embryo model, and the N-terminal extracellular domain of LRP6 is necessary for the SERPINA3K inhibitory function.
SERPINA3K Binds to the Extracellular Domain of LRP6.
To define the SERPINA3K-binding region in LRP6, the extracellular portion of LRP6 (E1-E4) fused with a Myc-tag (LRP6N-Myc) was expressed and incubated with the recombinant SERPINA3K-HIS or a control protein, His-tagged cellular retinol-binding protein (CRBP-HIS). After LRP6N-Myc was immunoprecipitated with the anti-Myc antibody, SERPINA3K-HIS, but not CRBP-HIS, was coprecipitated with LRP6N-Myc (Fig. 6A). Furthermore, SERPINA3K-HIS was incubated with LRP6N-Myc or the extracellular region of a control receptor, the N-terminal portion of low-density lipoprotein receptor fused with the Myc-tag (LDLRN-Myc), and pulled down by Ni resin. LRP6N-Myc, but not LDLRN-Myc, was specifically coprecipitated with SERPINA3K-HIS, as shown by Western blot analysis (Fig. 6B). These observations suggest that SERPINA3K specifically binds to the extracellular domain of LRP6.
Fig. 6.
SERPINA3K bound to the extracellular domain of LRP6 and blocked Fz-LRP6 complex formation induced by a Wnt ligand. (A) The conditioned medium from HEK293T cells expressing the extracellular region of LRP6 with a Myc tag (LRP6N-Myc) was incubated with SERPINA3K-HIS (1 μg/mL) or a control protein CRBP-HIS overnight at 4 °C. LRP6N-Myc was then precipitated with the anti-Myc antibody-conjugated resin. The precipitated proteins and input proteins were separately blotted with antibodies for the His-tag and Myc-tag. (B) For a negative control, the conditioned medium containing the N-terminal region of the LDLR tagged with Myc (LDLRN-Myc) were harvested under the same conditions as that for LRP6N-Myc. SERPINA3K-His was precipitated with Ni-NTA resin. The precipitated and input proteins were blotted with antibodies for the Myc and His tags. (C) SERPINA3K prevented the Wnt1-induced Fz8CRD-LRP6N complex formation. The conditioned media containing LRP6N-Myc (0.2 mL) and Fz8CRD-IgG (0.1 mL) were mixed in the presence of 0.5 μg purified Wnt1 protein, with 1 and 10 μg of SERPINA3K. The precipitates and input samples were immunoblotted with antibodies for Myc, SERPINA3K, or IgG.
SERPINA3K Prevents the LRP6 and Fz Dimerization.
Because the dimerization between LRP6 and the Fz receptor is the first step in activation of the Wnt pathway (36), we determined whether SERPINA3K affects the complex formation between the extracellular domains of Fz8 and LRP6 induced by a Wnt ligand. The cysteine-rich domain (CRD) of the Fz8 receptor tagged with an Ig-γ Fc epitope (Fz8CRD-IgG) was expressed in HEK293 cells by transfection of an expression vector, and the conditioned medium was harvested 2 days following the transfection. LRP6N-Myc in the medium was incubated with Fz8CRD-IgG in the presence or absence of purified Wnt1. Fz8CRD-IgG showed only a basal level of dimerization with the LRP6N-Myc in the absence of the added Wnt1 (Fig. 6C). The addition of purified Wnt1 induced a significant dimerization of Fz8 and LRP6, as shown by coprecipitation of Fz8CRD-IgG and LRP6N (Fig. 6C). Inclusion of SERPINA3K in the incubation mixture effectively blocked such dimerization in a concentration-dependent manner (Fig. 6C).
Discussion
A number of serpin members have been identified as endogenous angiogenic inhibitors (20–23). Some of these serpin angiogenic inhibitors have also displayed anti-inflammatory activities (38). However, the receptors or mechanisms responsible for their antiangiogenic and anti-inflammatory activities have not been identified. In the present study, we have identified the first serpin angiogenic inhibitor, SERPINA3K, as an endogenous inhibitor of Wnt signaling and LRP6 antagonist. As Wnt signaling regulates angiogenesis and inflammation processes (11, 12), antagonizing LRP6 may represent a unifying mechanism responsible for the antiangiogenic and anti-inflammatory activities of serpin angiogenic inhibitors.
Wnt signaling is involved in angiogenesis process through regulation of the angiogenic factors such as VEGF (12, 39). Endothelial cell migration is an essential step in angiogenesis (40) and is regulated by Wnt signaling (33). In human retinal vascular diseases, mutations in the Fz4 (Wnt receptor) and LRP5 (Wnt coreceptor) genes have been found to associate with abnormal angiogenesis (12, 41). Our recent study showed that activation of the Wnt pathway also plays a pathogenic role in subretinal neovascularization in an animal model of wet AMD (42). Therefore, Wnts are considered a new class of angiogenic factors (12). Further, we reported that Wnt signaling is activated in the retinas of diabetic animal models and in human donor retinas with diabetic retinopathy. However, blockade of Wnt signaling by DKK1 attenuated retinal inflammation, vascular leakage in diabetic retinopathy models (43). These findings established a pathogenic role of Wnt signaling in diabetic retinopathy. Here, we found that β-catenin is stabilized in both diabetic retinas and in cultured cells exposed to high-glucose medium. Further, phosphorylated LRP6 levels were increased under high-glucose condition. Therefore, these observations further support the role of Wnt signaling in diabetic retinopathy.
There are 19 Wnt ligands, many of which function as agonists of Arrow/LRP5/LRP6 and the Fz receptors, and thus activate Wnt/β-catenin signaling (44). However, some natural inhibitors of the Wnt pathway, such as DKK family members and IGF1BP, have been identified (45–47). Here we showed that SERPINA3K blocks the Wnt pathway activation induced by high glucose and Wnt ligands, but not by LiCl or a constitutively active mutant of β-catenin, suggesting that SERPINA3K inhibits Wnt signaling, possibly at the Wnt receptor level. This assumption was supported by specific binding of SERPINA3K to LRP6. Further, the coimmunoprecipitation assay confirmed the physical association of SERPINA3K with LRP6. Coprecipitation assay of LRP6 and Fz8 showed that binding of SERPINA3K to LRP6 blocks the LRP6-Fz receptor complex formation, suggesting that SERPINA3K functions as an antagonist of LRP6 and an endogenous modulator of the Wnt pathway.
It has been shown that SERPINA3K levels are decreased in the retina, correlating with activation of the Wnt pathway in the retina of a diabetic retinopathy model (28, 43). Further, intravitreal injection of SERPINA3K or injection of adenovirus expressing SERPINA3K attenuated the Wnt pathway activation in the retina with diabetic retinopathy. To further prove the hypothesis that the decreased SERPINA3K levels may contribute to the pathological Wnt pathway activation, we performed siRNA knockdown of SERPINA3K in cultured RPE and endothelial cells. In both of the cell lines, cytosolic β-catenin levels were up-regulated by knockdown of SERPINA3K alone (Fig. S2 A and B). Further, VEGF secretion level in cultured RPE cells and migration of endothelial cells were both increased by knockdown of SERPINA3K (Fig. S2 C and D). Next, we measured transcript levels of several known Wnt target genes, including VEGF, connective tissue growth factor (CTGF), Cyclin D1, and alkaline phosphatase (AP), to reflect the Wnt pathway activation (Fig. S3). Our real-time RT-PCR results showed that knockdown of SERPINA3K alone resulted in up-regulation of mRNA levels of all of these Wnt target genes (Fig. S3). Consistently, the real-time RT-PCR results showed the increased levels of all of these four Wnt target genes in the diabetic rat retinas, providing another support for the Wnt signaling activation in the diabetic retinopathy (Fig. S3). These findings provide further evidence suggesting that decreased SERPINA3K levels contribute, at least in part, to the Wnt pathway activation in diabetic retinopathy.
Xenopus axis duplication is a commonly used model to study Wnt signaling, as the Wnt pathway is known to play a key role in the axis formation (37). In the Xenopus axis duplication assay, SERPINA3K blocked axis duplication induced by Wnt8, which confirmed the inhibitory effect of SERPINA3K on Wnt signaling. This finding suggests that SERPINA3K can antagonize LRP6 in broad cell types. In the Xenopus embryos experiment, SERPINA3K has no effect on Wnt signaling induced by the constitutively active mutant of LRP6 (LRP6ΔN), which is consistent with the observation that SERPINA3K interrupts dimerization between the extracellular domains of LRP6 and the Fz receptor, suggesting that the inhibitory effect of SERPINA3K is dependent on the extracellular domain of LRP6, similar to that of DKK1. SERPINA3K inhibited the functions of Wnt3a (Fig. 4), Wnt8 (Fig. 5), and Wnt1 (Fig. 6), all of which are ligands of LRP6 (1). These findings provide further evidence that LRP6 is the molecular target of SERPINA3K. However, it remains to be investigated which domain in LRP6 the SERPINA3K binds to, and whether SERPINA3K plays a role in embryo development.
Similar to many other serpins, SERPINA3K has high levels in the circulation (27). Previous studies showed that plasma SERPINA3K levels are approximately 0.4 μg/mL (8 nM) in rats (48). The total SERPINA3K concentration in the serum is close to 10 μg/mL, as shown in our Western blotting result (Fig. S4C). The discrepancy could be due to that SERPINA3K may be bound by a partner in the serum, which blocks the antibody binding site in ELISA. To dissociate SERPINA3K from its binding partner, we pretreated the sample with urea, and, consequently, the ELISA result showed that the serum concentration was 6.9 μg/mL (138 nM), significantly higher than that without urea treatment. However, in the retina and vitreous samples, the effect of urea was not as substantial as in the serum. In the retina, the concentration of SERPINA3K was 565 ng/mg (wet weight; Fig. S4D). In the vitreous, the concentration of SERPINA3K was 94 ng/mg (wet weight; Fig. S4D). These findings showed approximate concentrations of SERPINA3K to be 11 nM in the retina and 2 nM in the vitreous. The retinal concentration is close to the Kd (10 nM) for SERPINA3K to bind with LRP6. In the retina, SERPINA3K is expressed in various cell types, with highest levels in ganglion cells (Fig. S4). Adenovirus injection induced overexpression of SERPINA3K in ganglion cells (Fig. S5). Because SERPINA3K is a secreted protein, overexpressed SERPINA3K can diffuse into other retinal layers to exert its functions.
SERPINA3K is known as a specific inhibitor of tissue kallikrein (27). Our previous studies have suggested that it is also a potent angiogenic inhibitor (25). The present study has established a unique anti-inflammatory activity of SERPINA3K in diabetic retinopathy, as it down-regulated inflammatory cytokines and reduced retinal vascular leakage in diabetic rats. In vitro assays demonstrated the inhibitory effect of SERPINA3K on retinal endothelial cell migration, another evidence for its antiangiogenic effect. However, SERPINA3K did not prevent pericyte loss in the retina of diabetic rats (Fig. S6). These findings, together with previous studies showing the SERPINA3K inhibits retinal neovascularization and ameliorates retinal inflammation (25, 31), suggest that SERPINA3K has therapeutic potential for diabetic retinopathy
Materials and Methods
Proteins, Plasmids, Adenovirus, Conditioned Medium, Transfection, and Reporter Assay.
Expression and purification of SERPINA3K-HIS and CRBP-HIS from E. coli were performed as described previously (49). SERPINA3K-HIS and LRP6-HIS were cloned into the pTriEx1.1 vector (Novagen). SERPINA3K and LRP6 (no His tag) were cloned into the pCDNA3 vector. LRP6N-Myc, LDLRN-Myc, and Fz8CRD-IgG were expressed in pCS2+ vectors, and the TOPFLASH vector was constructed as described (50). Fugene 6 (Roche Applied Science) was used for transfection following manufacturer's protocol.
The recombinant adenovirus was generated using the human adenovirus serotype 5 (Ad5) vectors and the AdEasy XL adenoviral vector system from Stratagene.
The TOPFLASH and renilla luciferase pRL-TK vectors were cotransfected into the cells. TOPFLASH activity was measured using dual luciferase reporter system (Promega) and normalized by renilla luciferase activity.
Experimental Animals.
Care, use, and treatment of all of the animals in this study were in strict agreement with the Statement for the Use of Animals in Ophthalmic and Vision Research. The experimental diabetes was induced as described previously (51). The retinal vascular permeability was quantified using the Evans blue-albumin leakage method as described previously (51).
Western Blot Analysis and Immunoprecipitation.
Wesern blot analysis was performed as described previously (31). Antibodies for VEGF, LRP6, and β-catenin were purchased from Santa Cruz Biotechnology and used at 1:1,000, 1:500, and 1:3,000 dilutions, respectively. Antibodies for His-tag (1:1,000) and for β-actin (1:3,000) were purchased from Invitrogen. Antibody for phosphorylated LRP6 was purchased from Cell Signaling Technology and used at 1:500 dilution. The monoclonal antibody for SERPINA3K (1:1,000) was generated using the recombinant SERPINA3K through the service with Proteintech Group.
For immunoprecipitation, recombinant proteins, supernatant of cell lysate, and conditioned media were mixed. For coprecipitation assay, the Protein G agarose resin (Roche Applied Science), Ni-NTA resin (Novagen), or the Myc-tag antibody-conjugated resin (Pierce) was added to the cell lysates or conditioned medium following the manufacturer's protocol. Briefly, after overnight incubation on a nutator at 4 °C, the resins were precipitated and washed six times with washing buffer [PBS for Protein G resin, PBS with 20 mM Tris (pH 8.0), for Ni resin, and TBS-T for the Myc-tag antibody conjugated resin]. The precipitated resins with proteins were then boiled for 1 min in 50 μL SDS-loading buffer before Western blot analysis.
Xenopus Embryo RNA Injection and Animal Explants.
Embryo manipulations and RT-PCR were performed as described previously (52). Capped RNAs were synthesized using the mMessage mMachine in vitro transcription kit (Ambion). For secondary axis induction, RNAs were injected into the ventral marginal region of Xenopus embryo at the four-cell stage. The following amounts of RNA were used: 1.6 pg Wnt8, 1 ng secreted LDL receptor (LDLR-Fc), 45 pg DKK1, 80 pg LRP6ΔN, and 1 ng SERPINA3K. The presence of a secondary axis was scored at tail bud stage (stage 30). For animal pole explant assays, synthetic RNAs were injected into both blastomeres at the two-cell stage in the animal-pole region, and animal caps were isolated at the blastula stage (stage 9) and cultured to equivalent stage 10 (gastrula stage) and processed for RT-PCR.
Statistical Analysis.
Student's t test was used in all statistical analyses, and statistical significance was accepted when the P value was less than 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Vijay Sarthy for the generous gift of rMC-1 cells and Dr. K Hosoya and Dr. T Terasaki for kindly providing the TR-iBRB cell line. We also thank the technical help from Dr. John Ash for the retinal trypsin digestion assay and Dr. Krysten Farjo for the CRBP recombinant protein. This study was supported by National Institutes of Health (NIH) Grants EY012231, EY019309, EY015650, and P20RR024215 from the National Center for Research Resources, grants from the Oklahoma Center for the Advancement of Science and Technology and American Diabetes Association (to J-x.M.), and NIH Grant GM057603 (to X.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906764107/DCSupplemental.
References
- 1.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 2.Polakis P. Casein kinase 1: A Wnt'er of disconnect. Curr Biol. 2002;12:R499–R501. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 4.Dale TC. Signal transduction by the Wnt family of ligands. Biochem J. 1998;329:209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orsulic S, Peifer M. Cell-cell signalling: Wingless lands at last. Curr Biol. 1996;6:1363–1367. doi: 10.1016/s0960-9822(96)00731-2. [DOI] [PubMed] [Google Scholar]
- 6.Ojesina AI. The role of beta-catenin in regulating angiogenesis in Wilms tumor. J Pediatr Surg. 2004;39:1446–1447; author reply 1447. doi: 10.1016/j.jpedsurg.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Howe LR, Subbaramaiah K, Chung WJ, Dannenberg AJ, Brown AM. Transcriptional activation of cyclooxygenase-2 in Wnt-1-transformed mouse mammary epithelial cells. Cancer Res. 1999;59:1572–1577. [PubMed] [Google Scholar]
- 8.Tachikawa K, Schröder O, Frey G, Briggs SP, Sera T. Regulation of the endogenous VEGF-A gene by exogenous designed regulatory proteins. Proc Natl Acad Sci USA. 2004;101:15225–15230. doi: 10.1073/pnas.0406473101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Easwaran V, et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–3153. [PubMed] [Google Scholar]
- 10.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 11.George SJ. Wnt pathway: A new role in regulation of inflammation. Arterioscler Thromb Vasc Biol. 2008;28:400–402. doi: 10.1161/ATVBAHA.107.160952. [DOI] [PubMed] [Google Scholar]
- 12.Masckauchán TN, Kitajewski J. Wnt/Frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology (Bethesda) 2006;21:181–188. doi: 10.1152/physiol.00058.2005. [DOI] [PubMed] [Google Scholar]
- 13.You J, Nguyen AV, Albers CG, Lin F, Holcombe RF. Wnt pathway-related gene expression in inflammatory bowel disease. Dig Dis Sci. 2008;53:1013–1019. doi: 10.1007/s10620-007-9973-3. [DOI] [PubMed] [Google Scholar]
- 14.Hanai J, et al. Endostatin is a potential inhibitor of Wnt signaling. J Cell Biol. 2002;158:529–539. doi: 10.1083/jcb.200203064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollina EA, et al. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7:2056–2070. doi: 10.4161/cc.7.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaji-Kegan K, Su Q, Angelini DJ, Champion HC, Johns RA. Hypoxia-induced mitogenic factor has proangiogenic and proinflammatory effects in the lung via VEGF and VEGF receptor-2. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1159–L1168. doi: 10.1152/ajplung.00168.2006. [DOI] [PubMed] [Google Scholar]
- 17.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: Vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172:4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 19.Miao RQ, Agata J, Chao L, Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100:3245–3252. doi: 10.1182/blood-2002-01-0185. [DOI] [PubMed] [Google Scholar]
- 20.Yamagishi S, Matsui T, Nakamura K, Takeuchi M, Imaizumi T. Pigment epithelium-derived factor (PEDF) prevents diabetes- or advanced glycation end products (AGE)-elicited retinal leukostasis. Microvasc Res. 2006;72:86–90. doi: 10.1016/j.mvr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Huang H, et al. Alpha1-antitrypsin inhibits angiogenesis and tumor growth. Int J Cancer. 2004;112:1042–1048. doi: 10.1002/ijc.20494. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 23.Shellenberger TD, et al. Headpin: A serpin with endogenous and exogenous suppression of angiogenesis. Cancer Res. 2005;65:11501–11509. doi: 10.1158/0008-5472.CAN-05-2262. [DOI] [PubMed] [Google Scholar]
- 24.Dawson DW, et al. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 25.Gao G, et al. Kallikrein-binding protein inhibits retinal neovascularization and decreases vascular leakage. Diabetologia. 2003;46:689–698. doi: 10.1007/s00125-003-1085-9. [DOI] [PubMed] [Google Scholar]
- 26.Chao J, Tillman DM, Wang MY, Margolius HS, Chao L. Identification of a new tissue-kallikrein-binding protein. Biochem J. 1986;239:325–331. doi: 10.1042/bj2390325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao J, et al. Tissue kallikrein-binding protein is a serpin. I. Purification, characterization, and distribution in normotensive and spontaneously hypertensive rats. J Biol Chem. 1990;265:16394–16401. [PubMed] [Google Scholar]
- 28.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 29.Hatcher HC, Ma JX, Chao J, Chao L, Ottlecz A. Kallikrein-binding protein levels are reduced in the retinas of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 1997;38:658–664. [PubMed] [Google Scholar]
- 30.Ma JX, et al. Kallistatin in human ocular tissues: Reduced levels in vitreous fluids from patients with diabetic retinopathy. Curr Eye Res. 1996;15:1117–1123. doi: 10.3109/02713689608995143. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B, Hu Y, Ma JX. Anti-inflammatory and antioxidant effects of SERPINA3K in the retina. Invest Ophthalmol Vis Sci. 2009;50:3943–3952. doi: 10.1167/iovs.08-2954. [DOI] [PubMed] [Google Scholar]
- 32.Huang Q, Sheibani N. High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol. 2008;295:C1647–C1657. doi: 10.1152/ajpcell.00322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samarzija I, Sini P, Schlange T, Macdonald G, Hynes NE. Wnt3a regulates proliferation and migration of HUVEC via canonical and non-canonical Wnt signaling pathways. Biochem Biophys Res Commun. 2009;386:449–454. doi: 10.1016/j.bbrc.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 34.Hosoya K, et al. Conditionally immortalized retinal capillary endothelial cell lines (TR-iBRB) expressing differentiated endothelial cell functions derived from a transgenic rat. Exp Eye Res. 2001;72:163–172. doi: 10.1006/exer.2000.0941. [DOI] [PubMed] [Google Scholar]
- 35.Tamai K, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 36.Zeng X, et al. Initiation of Wnt signaling: Control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 38.Zhang SX, et al. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006;20:323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–6054. [PubMed] [Google Scholar]
- 40.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 41.Ye X, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem. 2007;282:34420–34428. doi: 10.1074/jbc.M611289200. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, et al. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol. 2009;175:2676–2685. doi: 10.2353/ajpath.2009.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 45.Semënov MV, et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 46.Mao B, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 47.Zhu W, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature. 2008;454:345–349. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
- 48.Hatcher HC, Wright NM, Chao J, Chao L, Ma JX. Kallikrein-binding protein is induced by growth hormone in the dwarf rat. FASEB J. 1999;13:1839–1844. doi: 10.1096/fasebj.13.13.1839. [DOI] [PubMed] [Google Scholar]
- 49.Zhang B, Ma JX. SERPINA3K prevents oxidative stress induced necrotic cell death by inhibiting calcium overload. PLoS One. 2008;3:e4077. doi: 10.1371/journal.pone.0004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semënov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 51.Zhang SX, et al. Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol. 2005;166:313–321. doi: 10.1016/S0002-9440(10)62255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamai K, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.