Abstract
The envelope glycoprotein of vesicular stomatitis virus (VSV-G) enables viral entry into hosts as distant as insects and vertebrates. Because of its ability to support infection of most, if not all, human cell types VSV-G is used in viral vectors for gene therapy. However, neither the receptor nor any specific host factor for VSV-G has been identified. Here we demonstrate that infection with VSV and innate immunity via Toll-like receptors (TLRs) require a shared component, the endoplasmic reticulum chaperone gp96. Cells without gp96 or with catalytically inactive gp96 do not bind VSV-G. The ubiquitous expression of gp96 is therefore essential for the remarkably broad tropism of VSV-G. Cells deficient in gp96 also lack functional TLRs, which suggests that pathogen-driven pressure for TLR-mediated immunity maintains the broad host range of VSV-G by positively selecting for the ubiquitous expression of gp96.
Keywords: host factor, innate immunity, toll-like receptor, grp94
Vesicular stomatitis virus (VSV) is an enveloped negative-stranded RNA virus and the type member of the genus Vesiculovirus and the family Rhabdoviridae (1). The virus is enzootic in Central America, where it causes vesicular stomatitis in domestic animals, a disease with symptoms similar to foot-and-mouth disease. Regular outbreaks also occur in the southern United States (2). In humans, the disease is usually mild with only flu-like symptoms. Nevertheless, VSV carries significant biomedical potential because it is being developed into an oncolytic agent (3), and its envelope protein VSV-G is used to alter the tropism of (retro-) viral vectors for gene therapy (4, 5).
The tropism of VSV-G is one of the broadest of all viral envelope proteins because it enables the infection of most, if not all, human cell types and of organisms as distant as mammals, zebrafish, and Drosophila (1). Upon binding to an unknown receptor, VSV is taken up into target cells by clathrin-mediated endocytosis (6). The subsequent drop in endosomal pH induces a dramatic but reversible rearrangement in the structure of VSV-G that, by inserting the fusion domain of VSV-G into the target cell membrane, initiates the fusion reaction (7–9). It has long been assumed that the viral envelope would fuse directly to the limiting endosomal membrane, but recently it has been suggested that VSV-G targets the membrane of intraendosomal vesicles first (10). Only later, upon back-fusion of internal vesicles with the limiting membrane of multivesicular bodies under control of the cellular fusion machinery, would the viral capsid be released into the host cell cytoplasm. Although much is known about VSV-G and its intracellular trafficking, the mechanism underlying its efficient uptake and the selective force guaranteeing its broad host range have remained mysterious.
Toll-like receptors (TLRs) form a family of pattern recognition receptors that survey the extracellular environment for the presence of pathogens. Upon engagement with pathogen-derived ligands, TLRs trigger effector mechanisms of innate immunity and costimulate acquired immunity. The knockout of individual TLRs renders mice susceptible to infection with specific pathogens, whereas the functional ablation of multiple TLRs, for example by deletion of their shared adaptor protein MyD88, causes severe immunodeficiency (11). The export of TLRs from the endoplasmic reticulum to their final destination is tightly regulated; MD-2 (12), Unc93B1 (13), and Prat4A (14) control the export of limited TLR subsets, whereas gp96 (also known as grp94) is required for the functionality of multiple TLRs (15). gp96, a member of the hsp90 family of chaperones, is one of the most abundant ER resident proteins. However, despite its ubiquitous and high level expression, gp96 chaperones a very limited clientele with only TLRs, selected integrins, and insulin-like growth factor known to require its activity (15–17). Here we show that defects in gp96, besides rendering cells unresponsive to TLR agonists, also prevent their infection with viruses bearing VSV-G because such virions cannot bind the surface of gp96-deficient cells. We suggest that the selective pressure for TLR functionality exerted by exposure to a diverse range of pathogens upholds the ubiquitous expression of the otherwise nonessential protein gp96 and thereby maintains the broad host range of VSV-G.
Results and Discussion
Infection with VSV-G Bearing Viruses Requires a Gene Essential for Innate Immunity.
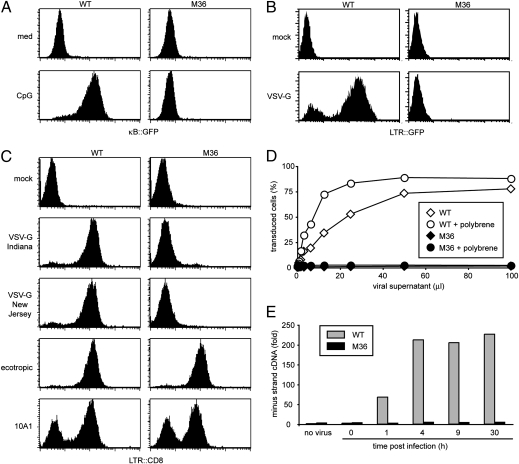
We have equipped the murine B cell line 70Z/3 with three NF-κB-dependent reporter genes to study NF-κB signaling by genetic means (18, 19). The resulting clone, GTPT3, carries NF-κB-controlled GFP and Thy1 for fluorescence-based readouts and a puromycin acetyltransferase: thymidine kinase fusion gene for metabolic selection. After mutagenesis of GTPT3 cells with ICR191, a frameshift mutagen, cells unresponsive to the TLR9 agonist CpG DNA were enriched by selection with gancyclovir, before individual clones were screened for lack of CpG DNA-induced GFP expression. M36, one of the mutants isolated in our screen, did not activate NF-κB in response to saturating amounts of CpG DNA as indicated by the absence of GFP expression (Fig 1A). While characterizing these cells we noted that, in contrast to parental GTPT3 cells, clone M36 resisted infection by HIV carrying the VSV-G envelope (Fig. 1B). MLV pseudotyped with VSV-G (MLVVSV-G) of either the Indiana or the New Jersey serotypes also failed to infect M36 cells, whereas no resistance to MLV bearing ecotropic or 10A1 envelope proteins was observed (Fig. 1C). Titration of MLVVSV-G revealed that M36 were at least 100-fold less susceptible to infection than parental cells (Fig. 1D). This defect was not ameliorated by polybrene, a polycation that enhances infection by neutralizing opposing charges on viral and cellular membranes. To further characterize the defect in M36, we performed quantitative PCR for newly synthesized viral DNA, which we found occurred at all time points in much smaller amounts in M36 than in parental GTPT3 cells (Fig. 1E). Resistance to infection is therefore manifested before reverse transcription completes.
Fig. 1.
Unresponsiveness to TLR9 stimulation and resistance to infection with VSV-G-bearing viruses. (A) Wild-type cells and M36, a clone derived by genome-wide chemical mutagenesis, were stimulated with CpG DNA for 24 h and analyzed for NF-κB-dependent GFP reporter activity. (B) Wild-type cells and clone M36 were infected with HIV bearing the VSV-G protein. Transduction was monitored after 2 days as LTR-dependent GFP expression. (C) Wild-type cells and clone M36 were infected with MLV bearing the indicated envelope proteins. Transduction was monitored after 2 days as LTR-dependent CD8 expression. (D) Wild type cells and clone M36 were infected in the presence or absence of polybrene with varying amounts of MLV bearing the VSV-G protein. Transduction was monitored after 2 days by flowcytometry as LTR-dependent GFP expression. (E) Wild-type cells and clone M36 were infected with MLV bearing the VSV-G protein for varying length of time. Minus strand viral DNA was measured by quantitative PCR.
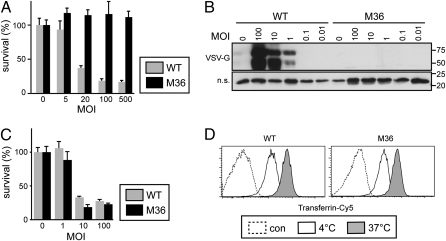
M36 was also resistant to infection with wild-type VSV as indicated by the absence of cytopathic effects (Fig. 2A) and failure to produce VSV-G protein (Fig. 2B). Taken together, our data show that in clone M36 a failure to support the function of VSV-G causes resistance to VSV and other VSV-G-bearing viruses.
Fig. 2.
Specific resistance to infection with VSV. (A) Wild-type cells and clone M36 were infected with VSV at the indicated multiplicity of infection (MOI). Twenty-four hours after infection cell survival was measured by MTT assay. Mean and standard deviations of triplicate infections are shown. (B) Total lysates of wild-type cells and clone M36 24 h after infection with VSV at the indicated MOI were separated by SDS/PAGE and immunoblotted for VSV-G. B Lower shows a nonspecific (n.s.) band from the same blot as a loading control. (C) Wild-type cells and clone M36 were infected with HSV-1 at the indicated MOI. Twenty-four hours after infection cell survival was measured by MTT assay. Mean and standard deviations of triplicate infections are shown. (D) Cells were incubated with transferrin-Cy5 for 20 min at 4 °C to allow binding before being shifted for 20 min to 37 °C to allow uptake. Unlabeled cells are shown as controls.
Because VSV-G mediates, besides binding of virions to cells and their subsequent fusion, also the clathrin-dependent uptake of VSV, we tested whether clathrin-dependent processes might be disturbed in M36. However, neither the infection with HSV-1 (Fig. 2C) nor the uptake of transferrin (Fig. 2D) were defective in M36. This result further proves that M36 are rendered specifically resistant to VSV-G bearing viruses. We conclude from the dual phenotype of M36, i.e., its unresponsiveness to CpG DNA and its resistance to VSV, that a gene required for innate immunity also encodes an essential host factor for the VSV-G-mediated entry of virions.
Defects in gp96 Prevent Infection with VSV-G Bearing Virus.
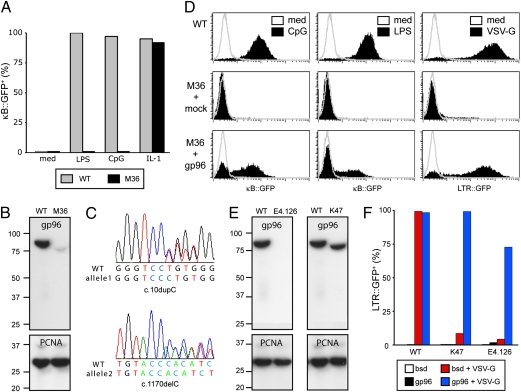
Further characterization of the NF-κB defect in M36 revealed that the cells also failed to signal in response to LPS, a TLR4 agonist, while remaining sensitive to interleukin 1 (IL-1) (Fig. 3A). The only known gene that, if deficient, impairs signaling from multiple TLRs without affecting IL-1 is gp96, a chaperone essential for TLR maturation in the endoplasmic reticulum (15). We therefore tested whether M36 carried a defect in gp96. Western blotting for gp96 in M36 lysates revealed the absence of wild-type sized protein (Fig. 3B). We detected two frameshifting mutations in the ORF of gp96 in M36, c.[10dupC(+)1170delC], consistent with a compound heterozygote genotype and explaining the absence of wild-type protein (Fig. 3C). The size of the truncated gp96 protein occurring in small amounts in M36 is consistent with utilization of the next in-frame start codon downstream of c.10dupC, which would render the protein nonfunctional because of the lack of a leader peptide. Complementation of M36 with gp96 but not with a control gene restored the NF-κB response to LPS and CpG DNA (Fig. 3D). We then tested whether the defect in gp96 also prevented infection with MLVVSV-G and found that complementation of M36 with gp96 restored the ability of MLVVSV-G to infect the cells. We conclude that defects in gp96 simultaneously prevent infection with VSV-G-bearing viruses and incapacitate TLRs.
Fig. 3.
gp96 is required for infection with VSV-G bearing viruses and signaling from TLRs. (A) Wild-type cells and clone M36 were stimulated with the indicated agonists for 24 h and analyzed by flowcytometry for NF-κB dependent GFP expression. (B) Total lysates of wild-type cells and clone M36 were probed with antibodies against gp96 (Upper) and PCNA (Lower). (C) Sequencing chromatograms of gp96 derived from PCR-amplified genomic DNA of clone M36. (D) Using ecotropic MLV, cells were mock transduced or transduced with gp96. Two days later, cells were infected with MLV bearing VSV-G or were stimulated with LPS and CpG DNA as indicated. Infection with MLVVSV-G was monitored by LTR-dependent GFP expression. NF-κB-dependent reporter activity was assessed by flowcytometry. (E) Total lysates of wild-type cells and clones E4.126 and K47 were probed with antibodies against gp96 (Upper) and PCNA (Lower). (F) Using ecotropic MLV, wild-type cells and clones E4.126 and K47 were transduced with gp96 or a control gene (bsd). Two days later, cells were infected with MLV bearing VSV-G. Infection was monitored by flowcytometry as LTR-dependent GFP expression.
Our collection of mutants unresponsive to TLR agonists contains two additional clones with defects in gp96, E4.126 and K47. E4.126 harbors two frameshifting mutations in gp96, c.[1146dupC(+)1840dupG] (15), whereas sequencing of K47 revealed that it carried a frameshifting mutation and a substitution in a splice donor site (c.[452dupG(+)2262+2T > G]). Consequently, E4.126 and K47 did not contain wild-type gp96 protein as judged by Western blot (Fig. 3E). However, K47 cells expressed a truncated form of gp96 consistent in size with an alternative splice product caused by the splice donor mutation. Like M36, E4.126 and K47 were also not infected by MLVVSV-G and failed to signal in response to LPS or CpG DNA (Fig. 3F). Complementation of either mutant with gp96 but not with a control gene restored their phenotype to wild-type. These data confirm our initial conclusion that gp96 is required for TLR responsiveness and infection with VSV-G-bearing viruses.
ATPase Activity of gp96 Is Required for Its Host Factor Function.
The role of gp96 as a host factor for VSV-G-bearing viruses could be either indirect, i.e., mediated via a client protein of gp96, or may involve a direct interaction of gp96 and VSV-G. To address this question, we compared a panel of gp96 mutants (15) for their ability to support infection by MLVVSV-G and to chaperone known gp96 client proteins (Fig. 4). The ability of gp96 to complement M36 was lost if either the N-domain or the C-domain were deleted or substituted with the corresponding domains of hsp90. In contrast, deleting the charged domain or replacing it with that of hsp90 still allowed gp96 to function; neither was the endoplasmic reticulum retention signal KDEL required for gp96 function. However, gp96D149N, which is deficient in binding ATP and client proteins (15), failed to complement gp96-deficient cells. Thus, in all our mutants the requirement for gp96 to chaperone client proteins and to permit infection with MLVVSV-G correlated perfectly, suggesting that gp96 is obligatory for infection with VSV-G-bearing viruses because it chaperones a cellular protein essential for viral entry.
Fig. 4.
Chaperoning activity of gp96 is required for infection with VSV-G-bearing virus. The domain structure of gp96, hsp90 (light and dark blue, respectively), and their chimera are shown in Left. Using ecotropic MLV, clone E4.126 was complemented with the indicated constructs. (Right) Two days later, cells were stimulated with LPS and CpG DNA. Alternatively, cells were infected with MLV bearing VSV-G. NF-κB dependent reporter activity and infection with VSV-G-bearing virus were assessed by flowcytometry.
gp96 Is Essential for Binding of VSV-G Bearing Virions to Cells.
Consistent with the broad tropism of VSV-G, phosphatidylserine, a ubiquitous membrane lipid, has been suggested to act as its receptor (20). Although the evidence was indirect and has been disputed later (21), we tested whether deficiency in gp96 would influence the amount of phosphatidylserine present in cells (Fig. 5A). However, binding of annexin V, a specific ligand for phosphatidylserine, to wild-type and gp96-deficient cells was indistinguishable, suggesting that the resistance of gp96-deficient cells to infection with VSV-G-bearing virus is not caused by a lack of phosphatidylserine.
Fig. 5.
gp96 is essential for binding of VSV-G-bearing virions to cells. (A) Fixed and permeabilized cells were stained with or without PE-labeled annexinV in the absence or presence of an excess of unlabeled annexinV. (B) Using ecotropic MLV, wild-type cells and clone E4.126 were transduced with gp96 or were mock transduced. Two days later, cells were incubated at 4 °C with supernatants containing MLV-bearing His-tagged or untagged VSV-G. Cells were stained with anti-His antibody, followed by a PE-labeled secondary antiserum.
Although VSV-G-mediated membrane fusion is enhanced in the presence of phosphatidylserine, no additional proteins are required as demonstrated by the ability of VSV-G to fuse artificial membranes in vitro (22). In medium of low pH, i.e., under conditions VSV would otherwise encounter only inside endosomes, virions even fuse to spheroplasts of Saccharomyces cerevisiae, a species that does not encode gp96 (23). Such ability to experimentally bypass the gp96-dependent step in infection suggests that neither gp96 nor its chaperone function are required for the fusion event per se. We therefore tested whether gp96 was required for a step preceding fusion, i.e., the expression of functional VSV-G receptors on the cell surface, by measuring the binding of MLVVSV-G to cells (Fig 5B). We found that in contrast to wild-type cells those deficient in gp96 were unable to bind MLVVSV-G. Complementation with gp96 fully restored binding in gp96-deficient cells, which demonstrates that gp96 is essential for the occurrence of functional VSV-G receptors at the cell surface. Because we found that infection with VSV-G-bearing viruses depends on the ATPase activity of gp96, we conclude that the receptor, if proteinaceous in nature, is likely to be a gp96 client. Alternatively, given the widely held belief in a membrane lipid acting as the receptor for VSV-G (24–26), one must consider the possibility that gp96 may not interact directly with the receptor but rather that a gp96 client protein is required for the generation of functional VSV-G receptors. Enzymes of the endoplasmic reticulum or Golgi apparatus, for example those involved in the modification of carbohydrates, are attractive candidates. We also conclude that lack of the VSV-G receptor from the cell surface, despite its ubiquitous expression, is compatible with cellular viability. Although we can only speculate on the identity of the gp96 client protein required for infection with VSV, it seems reasonable to assume that a genetic defect in the client would be as compatible with cellular viability as the indirect defect caused by gp96 deficiency. We therefore believe that the identification of the gp96 client required for infection with VSV-G-bearing viruses using somatic cell genetics may be feasible.
The remarkably broad tropism of VSV-G suggests its receptor and all other essential host factors are ubiquitously expressed. The expression pattern of gp96 matches this requirement. One may also expect that only a small number of additional essential host factors exists. It therefore seems surprising that an RNAi screen of the human kinome has identified ≈100 kinases contributing to VSV infection (27). However, some of the kinases may be involved in steps of the VSV life cycle other than VSV-G-mediated entry and some, although promoting infection, will not be essential. A final prediction for host factors essential to VSV-G-mediated entry is that they fulfill essential functions at the cellular level. Otherwise, the negative selective pressure exerted by the virus should have caused resistance in at least some cell lineages. However, gp96 expression is not essential for cellular viability; neither in vitro nor in vivo, and not even under conditions of ER stress (15–17). Given these facts, why do all cells express gp96 and why has no resistance to VSV-G been selected for? We suggest the evolutionary interesting scenario that pathogen-driven pressure for TLR functionality upholds the ubiquitous expression of the otherwise nonessential protein gp96 and, thereby, maintains the broad host range of VSV-G.
Materials and Methods
Reagents.
Antibodies were from BD-Pharmingen (CD8, ratThy1), Stressgen (gp96), Santa Cruz (PCNA), Sigma (VSV-G, V4888), Qiagen (penta-His), Jackson ImmunoResearch Laboratories (goat-anti-mouse-PE), and Dabco (HRP-conjugated reagents), polybrene, LPS (Escherichia coli O127:B8), and phosphothioate CpG DNA (ODN1668 TCCATGACGTTCCTGATGCT) were from Sigma, and Interleukin 1 from R&D Systems.
Plasmids.
pCL-eco and pCL-10A1 were from Imgenex, and pMD-OGP and pMD-VSVG (serotype Indiana) were kind gifts of Richard Mulligan (Harvard Medical School, Department of Genetics, Boston, MA). The ORF for VSV-G serotype New Jersey was synthesized by GeneArt and cloned into Peak8CD5L. PeakMMP-gp96 and M5P-GFP have been described (15, 28). The reference sequence for murine gp96 used in this study is NM_011631.
Cell Culture and Mutagenesis.
GTPT3 reporter cells have been described (18); they carry three NF-κB-dependent reporter genes (GFP, Thy1, and puromycin acetyltransferase fused to thymidine kinase). Mutagenesis and selection were performed as described (15, 19). After recovery, cells were stimulated with 1 μM CpG DNA and selected with 0.5 μM ganciclovir (Sigma). Mutant clones were identified by absence of GFP and Thy1 expression upon stimulation with CpG DNA.
Production of Retroviral Supernatants.
Recombinant retroviruses were produced by transient transfection of 293ET cells with retroviral vectors derived from MLV (M5P, PeakMMP; refs. 15, 28, and 29) or HIV (pHRsinUBEm; ref. 30)). Helper plasmids were cotransfected: pCL-eco to produce ecotropic MLV, pCL-10A1 for MLV carrying the 10A1 envelope, pMD-OGP and pMD-VSVG (serotype Indiana) or Peak8CD5L-VSVG (serotype New Jersey) for VSV-G pseudotyped MLV, and pCMVR8.91 and pMD-VSVG for VSV-G pseudotyped HIV. Target cells were spin-infected (2 h, 540 g, room temperature) in the absence of polybrene unless indicated otherwise.
Infection with VSV and HSV-1.
For survival assays cells were seeded in triplicates in a 96-well format and spin-infected (1.5 h, 540 g, 37 °C) with VSV (Indiana) or HSV-1 (KOS strain). After growth for 24 h at 37 °C in a CO2 incubator, cells were incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (5 mg/mL) for 4 h. Formazan crystals were dissolved overnight at 37 °C in 10% SDS/0.01 M HCl and absorbance (A) was measured at 595 and 655 nm using a spectrophotometer. The percentage of cell survival was calculated as follows: (A595/655 infected cells/A595/655 control cells) × 100. For protein assays cells were infected in 24-well plates.
Transferrin Uptake.
Cells were incubated with or without 150 ng of transferrin-Cy5 for 20 min on ice, followed by another 20 min either on ice or at 37 °C. All tubes were washed three times with PBS and analyzed by flowcytometry.
Binding of Virions to Cells.
Cells were spun (2 h, 540 × g, 4 °C) with supernatants produced by 293ET cells containing virions pseudotyped with VSV-G or His-tagged VSV-G. Cells were stained with anti-His monoclonal antibody (Qiagen), followed by goat-anti-mouse-PE antiserum.
Annexin V staining.
Cells were stained with Annexin V-PE after having been permeabilized with Fix&Perm (Caltag Laboratories).
Quantitative PCR.
DNA was extracted using Puregene (Gentra Systems) from cells infected for varying length of time. SYBR Green PCRs (Applied Biosystems) were performed in a Taqman machine (Applied Biosystems). Primers to amplify viral minus strand DNA were 5′-ACATGGTCCTGCTGGAGTTC (GFP specific) and 5′-CCTACAGGTGGGGTCTTTCA (reverse, MLV specific). Primers to amplify murine genomic DNA were 5′-CTGTTCCTTGGTCAGGCAAT and 5′-ACTTGGGAAGTCAAGGCAGA.
Western Blot.
Total cell lysates were separated on 4–12% denaturing gels (Invitrogen) and visualized by immunoblotting using ECL detection reagents (Amersham Bioscience).
Acknowledgments
We thank Richard Mulligan for kindly providing plasmids, Katrin Rittinger and Paul Lehner for critical comments on the manuscript, and Ben Nichols for advice on transferrin endocytosis. J.M. is a PhD fellow with the “Institute for the promotion of Innovation by Science and Technology”. Work in R.B.’s laboratory is supported by research grants from the “Interuniversity Attraction Poles program” (IAP6/18), the Fonds Wetenschappelijk Onderzoek, the “Belgian Foundation against Cancer”, the “Strategic Basic Research program” of the IWT, the “Centrum voor Gezwelziekten” and the “Concerted Research Actions” of Ghent University.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Rose JK, Whitt MA. Rhabdoviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. Ed 4. Vol. 1. Philadelphia: Lippincott, Williams, & Williams; 2001. pp. 1221–1244. [Google Scholar]
- 2.Rodríguez LL. Emergence and re-emergence of vesicular stomatitis in the United States. Virus Res. 2002;85:211–219. doi: 10.1016/s0168-1702(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 3.Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 4.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: Concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannsdottir HK, Mancini R, Kartenbeck J, Amato L, Helenius A. Host cell factors and functions involved in vesicular stomatitis virus entry. J Virol. 2009;83:440–453. doi: 10.1128/JVI.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carneiro FA, Ferradosa AS, Da Poian AT. Low pH-induced conformational changes in vesicular stomatitis virus glycoprotein involve dramatic structure reorganization. J Biol Chem. 2001;276:62–67. doi: 10.1074/jbc.M008753200. [DOI] [PubMed] [Google Scholar]
- 8.Roche S, Bressanelli S, Rey FA, Gaudin Y. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science. 2006;313:187–191. doi: 10.1126/science.1127683. [DOI] [PubMed] [Google Scholar]
- 9.Roche S, Rey FA, Gaudin Y, Bressanelli S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein G. Science. 2007;315:843–848. doi: 10.1126/science.1135710. [DOI] [PubMed] [Google Scholar]
- 10.Le Blanc I, et al. Endosome-to-cytosol transport of viral nucleocapsids. Nat Cell Biol. 2005;7:653–664. doi: 10.1038/ncb1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 12.Shimazu R, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, et al. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med. 2007;204:2963–2976. doi: 10.1084/jem.20071132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, et al. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanderling S, et al. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol Biol Cell. 2007;18:3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloor S, et al. Signal processing by its coil zipper domain activates IKK gamma. Proc Natl Acad Sci USA. 2008;105:1279–1284. doi: 10.1073/pnas.0706552105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krumbach R, Bloor S, Ryzhakov G, Randow F. Somatic cell genetics for the study of NF-{kappa}B signaling in innate immunity. Sci Signal. 2008;1:pt7. doi: 10.1126/scisignal.139pt7. [DOI] [PubMed] [Google Scholar]
- 20.Schlegel R, Tralka TS, Willingham MC, Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: Is phosphatidylserine a VSV-binding site? Cell. 1983;32:639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- 21.Coil DA, Miller AD. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J Virol. 2004;78:10920–10926. doi: 10.1128/JVI.78.20.10920-10926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eidelman O, Schlegel R, Tralka TS, Blumenthal R. pH-dependent fusion induced by vesicular stomatitis virus glycoprotein reconstituted into phospholipid vesicles. J Biol Chem. 1984;259:4622–4628. [PubMed] [Google Scholar]
- 23.Makarow M, Nevalainen LT, Kääriäinen L. Expression of the RNA genome of an animal virus in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1986;83:8117–8121. doi: 10.1073/pnas.83.21.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carneiro FA, et al. Probing the interaction between vesicular stomatitis virus and phosphatidylserine. Eur Biophys J. 2006;35:145–154. doi: 10.1007/s00249-005-0012-z. [DOI] [PubMed] [Google Scholar]
- 25.Coil DA, Miller AD. Enhancement of enveloped virus entry by phosphatidylserine. J Virol. 2005;79:11496–11500. doi: 10.1128/JVI.79.17.11496-11500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carneiro FA, Bianconi ML, Weissmüller G, Stauffer F, Da Poian AT. Membrane recognition by vesicular stomatitis virus involves enthalpy-driven protein-lipid interactions. J Virol. 2002;76:3756–3764. doi: 10.1128/JVI.76.8.3756-3764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelkmans L, et al. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 28.Randow F, Sale JE. Retroviral transduction of DT40. Subcell Biochem. 2006;40:383–386. doi: 10.1007/978-1-4020-4896-8_30. [DOI] [PubMed] [Google Scholar]
- 29.Ryzhakov G, Randow F. SINTBAD, a novel component of innate antiviral immunity, shares a TBK1-binding domain with NAP1 and TANK. EMBO J. 2007;26:3180–3190. doi: 10.1038/sj.emboj.7601743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaison C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]