Abstract
Survival of differentiated cells is one of several processes regulated by Notch activity, although the general principles underlying this function remain to be characterized. Here, we probe the mechanism underlying Notch-mediated survival, building on emerging evidence that apoptotic responses coordinated by specialized intermediates converge on mitochondria, identifying a core event in death pathways. The Bcl-2 family protein Bax is one such intermediate, which in a unifying response to diverse apoptotic stimuli nucleates multiprotein assemblies on mitochondria, committing cells to irrevocable damage. Using Bax as the prototype stimulus, we analyze Notch signaling for potential interactions with mitochondria, probe intrinsic properties of the Notch receptor, and describe key intermediates in the Notch-activated signaling cascade. Ligand-dependent processing was necessary to generate the Notch intracellular domain (NIC) although signaling was independent of canonical interactions with nuclear factors. Notably, antiapoptotic activity was recapitulated by NIC recombinants, localized outside the nucleus, and compromised by enforced nuclear sequestration. NIC signaled via the kinase Akt to prevent the loss of mitochondrial function, contiguity, and consequent nuclear damage, outcomes critically depend on mitochondrial remodeling proteins Mitofusins-(Mfn)-1 and 2. Thus, the NIC-Akt-Mfn signaling cascade identifies a pathway regulating cell-survival, independent of canonical functions associated with NIC activity.
Keywords: apoptosis, Bax, mitofusins, signaling
Notch signaling, which regulates a variety of processes in different developmental contexts in metazoans (1), is initiated by interactions with ligands that trigger sequential, proteolytic cleavage of the transmembrane receptor. Typically, processing culminates in the release of the Notch intracellular domain (NIC), which localizes to the nucleus to initiate transcription (2). The pleitropic outcomes of Notch signaling arise from its interactions with other signaling networks, best understood in the context of nuclear functions of NIC (3). The more recently appreciated antiapoptotic functions of Notch are linked to interactions with molecules such as Akt and NF-κB (4–7), positioned as nodes across multiple signaling pathways, suggesting that Notch may function as a key molecular sensor regulating survival. Here, we probe the mechanism underlying Notch-mediated cell survival in an effort to reveal general principles underlying this function.
Bcl-2 family proteins determine mitochondrial involvement in death cascades, regulating commitment to apoptosis to diverse stimuli, which include developmental cues, nutrient-deprivation, and intrinsic cytotoxic stressors (8). Because Notch-mediated survival has been reported in several of these contexts, we build on previous characterizations to probe the mechanism underlying Notch-mediated antiapoptotic activity vis-à-vis intersections with mitochondrial events. Thus, we interrogated Notch-mediated survival using the proapoptotic protein Bax, which defines a core conserved event—mitochondria coordination—in mammalian death cascades (8, 9). Our results suggest a model for NIC function in the cytoplasm, which is distinct from its canonical roles. Further, we demonstrate functional interactions with proteins regulating mitochondrial fusion (10) revealing a mitochondrial link for NIC activity. These observations lay the foundations for detailed analysis of a noncanonical Notch pathway operating to regulate cell survival.
Results
Notch Activity Inhibited Bax-Induced Apoptosis.
We first investigated if Notch signaling inhibited Bax induced apoptosis. Bax tagged to enhanced GFP (Bax-GFP) was expressed in the HeLa cell line and apoptotic damage triggered within 60–80 min by the addition of 1 μM of the broad spectrum kinase inhibitor Staurosporine (STS) (Fig. 1 A and B). Coexpressing untagged or RFP-tagged NIC recombinants inhibited nuclear damage in HeLa (Fig. 1 C and D and Fig. S1A), COS-7 (Fig. S1B) and the HEK cell line, where Bax triggered apoptosis without the addition of STS (Fig. S1 C and D). NIC activity was independent of endogenous Notch1 (Fig. S1E) and inhibited apoptosis triggered by Bak another proapoptotic member of the Bcl-2 family (Fig. S1F).
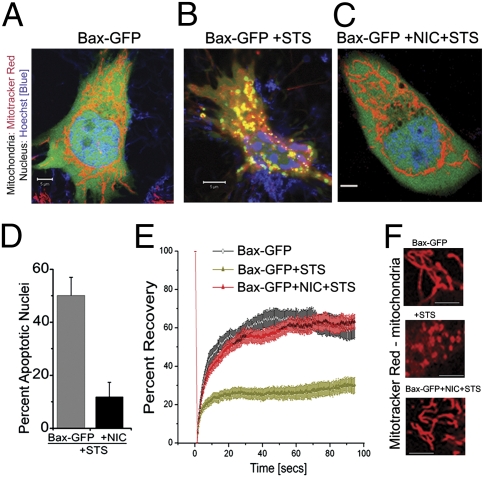
Fig. 1.
NIC inhibits Bax-induced cellular damage in HeLa cells. (A–C) Confocal images of cells stained with Mitotracker Red and H33342 in the following conditions: cells transfected with Bax-GFP and cultured for 14 h (A), followed by STS for 1 h (B), cells cultured for 14 h after transfection with NIC and Bax-GFP and then treated with STS for 1 h (C). Representative images of n = 50 in A and B and n = 55 in C. (Scale bar: 5 μm.) (D) Cells transfected with Bax-GFP or Bax-GFP+NIC were cultured overnight. For each condition, one set of dishes was untreated and another treated with STS for 1 h. Nuclear damage was estimated in GFP positive cells. The data are normalized to the group not treated with STS and the mean ± SD from three separate experiments plotted. (E) FRAP analysis of mitochondria in cells expressing Bax-GFP or Bax-GFP+NIC, stained with Mitotracker Red followed by STS for 1 h. Fluorescence intensities recovered after photobleaching were plotted over time (n = 10 cells for Bax-GFP, 18 cells for Bax-GFP+STS, and 13 cells for Bax-GFP+NIC+STS). Data plotted are mean ± SEM (F) Micrographs show details of mitochondrial organization in cells transfected with the indicated constructs, stained with Mitotracker Red, followed by STS for 1 h and then imaged.
Fluorescence recovery after photobleaching (FRAP) measures rate of refilling of a bleached area from connected neighboring fluorophore (such as Mitotracker Red) filled areas over a period and reflects organelle luminal contiguity. Mitochondrial fragmentation in STS-treated Bax-GFP cells resulted in dramatically reduced FRAP recoveries as compared to the untreated group (Fig. 1E), whereas recoveries were comparable to control untreated cultures in STS-treated Bax-GFP+NIC cells (Fig. 1E). These results were confirmed by visualizing mitochondrial organization in all three conditions (Fig. 1F). The mitochondrial network of live Bax-GFP expressing cells is rendered fragmented during apoptosis but is tubular and interconnected in cells coexpressing NIC (Fig. S1G). Additionally, partitioning of the fluorescent dye TMRM is dependent on mitochondrial transmembrane potential (MTP) and reduced uptake indicates a loss of outer mitochondrial membrane (OMM) integrity, which was inhibited in cells expressing NIC (Fig. S1H). At the onset of apoptosis, the OMM is rendered permissive to molecules, which irrevocably compromise organelle integrity. One signature is the change in Bax conformation and consequent multimerization at the MOM (11). Subsequent experiments assessed the consequences of NIC activity on these events.
NIC Inhibits Bax Multimerization.
Changes in Bax-GFP organization associated with onset of apoptosis were followed by live imaging and in fixed cells (Fig. S2). STS triggered the appearance of small puncta in the vicinity of mitochondria well before cells presented overt evidence of damage (Fig. 2A, Top and Movies S1 and S2). These puncta coalesced into aggregates, eventually culminating in the collapse of cellular structures by 1–1.5 h (Fig. 2A Middle, Fig. S3 A–G, and Movies S1 and S2), which was inhibited in Bax-GFP+NIC cells (Fig. 2A Bottom and Movie S3). Changes in Bax conformation at the mitochondrion reveals hitherto concealed epitopes as indicated by reactivity to specific antibodies (8, 12). Thus, reactivity to one such antibody clone 6A7, which recognizes an epitope in the Bax N terminus overlapped with Bax-GFP puncta (Fig. 2B), whereas Bax-GFP+NIC cells were 6A7 negative (Fig. 2C). Similar results were obtained in the HEK cell line (Fig. S2 B and C). A control antibody recognizing Bax independent of its conformation established the presence of Bax protein in all conditions (Fig. S2 A and D). In a related approach, chemical cross-linkers were employed to stabilize Bax multimers in cell lysates (13). In cell lysates treated with Dithiobis succinimidyl propionate (DSP), which is one such reagent, Bax-GFP appeared as a higher molecular weight species in immunoblots of cells undergoing apoptosis (Fig. 2D, lane 2), whereas only monomers were detected in cells coexpressing NIC (Fig. 2D, lane 6). The antiapoptotic protein Bcl-2, an antagonist of Bax also blocked the appearance of higher molecular weight species (Fig. 2D, lane 4). Thus, NIC signaling intersected with events regulating the structural reorganization of Bax-GFP, which is linked to events at the OMM. A rapidly growing literature documents the relationship between the dynamic behavior of molecules and their biological functions (14, 15), and we turned to a more sensitive measure of Bax dynamics to assess the effects of NIC activity on early events in apoptosis.
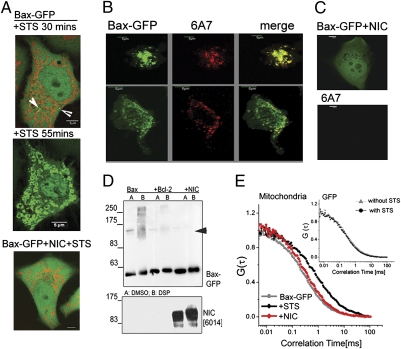
Fig. 2.
Notch activity inhibits changes in Bax conformation and dynamics. (A) Movie stills of Bax-GFP cells taken from Movie S2, at 30 min (Top) and 55 min (Middle) after STS addition. Arrow heads show nascent puncta. (Lower) A Bax-GFP+NIC cell 55 min post STS addition. (B and C) Confocal images (central plane) showing 6A7 staining in CHAPS permeabilized HeLa cells transfected with Bax-GFP (B) or Bax-GFP+ NIC (C) treated with STS for 1 h. Images are representative of 20 cells in each case. (Scale bar: 5 μm.) (D) Lysates made from HEK cells transfected 20 h before with indicated constructs, were treated with 1 mM crosslinker (lane B) or matched amounts of DMSO (lane A). Immunoblots were probed with antibodies to GFP and clone 6014 to detect Bax-GFP and NIC, respectively. The blot is representative of three separate experiments. (E) Cells expressing Bax-GFP+/− NIC were stained with Mitotracker Red and treated with vehicle or STS for 30 FCS analysis was performed as described in Methods. The associated autocorrelation curves of Bax-GFP at mitochondria under vehicle- or STS-treated condition are shown. (Inset) Autocorrelation curves of GFP at mitochondria in cells transfected with GFP (0.5 μg), stained with Mitotracker Red and treated with vehicle or STS for 30 min.
Fluorescence correlation spectroscopy (FCS) can report on diffusive properties of molecules, their dynamics and interactions in live, intact cells. This approach was used to measure the intracellular mobilities of Bax-GFP in the cytoplasm or in the vicinity of the mitochondria in different experimental conditions. Bax-GFP mobilities in the cytosol were consistent with free unhindered diffusion of molecules (Fig. 2E and Fig. S3H). In comparison with GFP (diffusion coefficient of approximately 19 ± 1.2 μm2/sec), the diffusion coefficient for Bax-GFP (approximately 10.85 ± 3 μm2/sec) is lower than expected for its molecular mass (approximately 48 kDa), suggesting a multimeric organization or interacting partner proteins of Bax-GFP in the cytoplasm (8, 16). Following the addition of STS (+30 min), a time point at which cells present no overt evidence of damage, we recorded the emergence of slower Bax-GFP diffusion time scales, at nascent puncta near mitochondria (Fig. 2E, filled black circles, and Fig. S3I). Thus, although there is no STS-induced change in Bax-GFP mobilities in the cytoplasm (Fig. S3H), slower diffusion time scales at mitochondria in these cells is consistent with the formation of higher order Bax complexes and were recorded in a substantial (60–65%) number of the total cells (n = 40) analyzed (Fig. S3J, +STS). We detect no STS-induced changes in mobilities in NIC+Bax-GFP cells (Fig. 2E). Overlapping autocorrelation curves of GFP in cells in the presence or absence of STS (Fig. 2E Inset) argued against changes in viscosity as the underlying cause. These approaches, suggest that NIC signaling intersects with early events in the apoptotic process, reflected in changes in Bax dynamics and conformational transitions in apoptotic cells.
Because NIC is a predominantly nuclear localized protein with emerging evidence of cytoplasmic functions (6, 17, 18), subsequent experiments dissected the spatial coordinates of NIC and the intrinsic properties of the receptor determining this function. Variously modified Notch/NIC recombinants were employed to assess the dependence on processing, canonical intermediates, and sustained nuclear localization of NIC for antiapoptotic activity.
Nuclear Sequestration Abrogates NIC-Mediated Antiapoptotic Activity.
A processing resistant Notch recombinant (19) disabled for interactions with ligand (NLNG), did not block apoptosis (Fig. 3A and Inset), whereas a recombinant (NLNG CC>>SS) corrected for this defect was active (Fig. 3A and Inset). The Notch target hes1 promoter activation by these constructs (Fig. S4A) was consistent with published reports (19). Antiapoptotic activity of full-length Notch (NFL) was abrogated in cells coexpressing an inhibitory form of its ligand—soluble Jagged-1 (sJag1) (Fig. 3B).
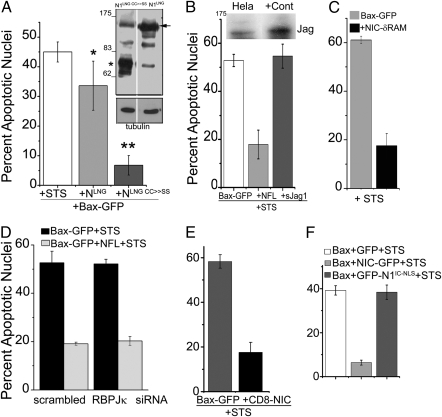
Fig. 3.
Nuclear localization is not essential for NIC activity. (A) HeLa cells transfected with Bax-GFP or Bax-GFP+ NLNG or NLNG CC>>SS were cultured overnight. Cultures were processed and scored for STS-induced apoptotic nuclear damage. * P < 0.01; **P < 0.001. (Inset) The recombinants detected by an antibody to myc in immunoblots. Arrow and the star indicate the unprocessed and processed form of the constructs, respectively. Representative of two experiments (B–E), HeLa cells transfected with different combinations of constructs shown in the panels were cultured overnight and then scored for STS-induced apoptotic damage as described in Methods. (Inset) The immunoblot shows Jag expression in HeLa cells. In (D) cells were pretreated with siRNA to RBP-Jκ or a scrambled control for 48 h before transfection. (F) HeLa cells transfected with Bax (2 μg) with and without NIC-GFP (1 μg) or GFP-N1IC-NLS (1 μg) were cultured overnight and scored for STS-induced apoptotic damage. (A–F) The data plotted are mean ± SD of three separate experiments.
Deletion of the RBP-Jκ/CBF1-associated module (RAM) domain, which mediates NIC-RBP-Jκ interactions (20), did not abrogate NIC function (Fig. 3C). siRNA-mediated ablation of RBP-Jκ or DN-CBF1 prevented NIC-mediated activation of the hes-1 promoter (Fig S4 B and D), but did not suppress NFL-mediated antiapoptotic activity (Fig. 3D and Fig. S4C) suggesting that canonical interactors were dispensable for Notch activity. A recombinant where the transmembrane domain of the CD8 receptor was incorporated to generate CD8-NIC-GFP/CD8-NIC-RFP is predominantly excluded from the nucleus (Fig. S4E), compromised for hes-1 transcription, (17) but inhibited apoptosis with an efficacy comparable to other active recombinants (Fig. 3E). Further, inclusion of an additional NLS (GFP-N1IC-NLS) compromised antiapoptotic activity (Fig. 3F). Collectively, these data suggest that canonical interactions in the nucleus or a sustained nuclear presence were dispensable for NIC functioning in its capacity as a regulator of cell survival. Building on previous observations (5, 17, 21, 22) and using a combination of siRNA and dominant negative constructs we established that the kinase Akt is an intermediate in the Notch-activated antiapoptotic pathway (Fig. 4 A and B and Fig. S5 A–C). In subsequent experiments we tried to elucidate the mechanism by which NIC activity impinged on mitochondrial integrity.
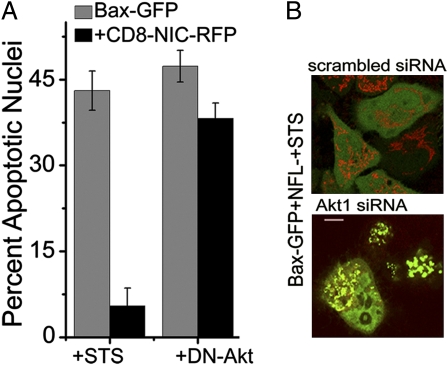
Fig. 4.
Akt is required for NIC activity (A) HeLa cells transfected with Bax-GFP or Bax-GFP+CD8-NIC-RFP+/− DN-Akt were cultured overnight and scored for STS-induced apoptotic damage. Data plotted are the mean ± SD of three separate experiments. (B) Representative image of cells (n = 30) pretreated with siRNA to Akt1 or a scrambled control for 48 h, cotransfected with Bax-GFP+NFL. Cells counterstained with Mitotracker were imaged 60 min after the addition of STS. (Scale bar: 5 μm.)
Distinctively, unlike Bcl-xL, a well-characterized Bax antagonist, NIC-mediated antiapoptotic activity restored mitochondrial connectivity, suggesting a mechanism that may integrate with the machinery regulating organelle morphology for NIC function. Mitochondrial morphology and dynamics are the outcome of a balance between the opposing processes of organelle fusion and fission (10). The cellular roles of mitochondria including energy conversion, maintenance of transmembrane potential, and calcium homeostasis are thought to be intimately linked to molecules that regulate mitochondrial remodeling (23, 24), although the mechanisms remain to be elucidated. Mitofusins are core conserved components of the fusion machinery, and we first asked if NIC-Akt signaling converged on these molecules.
Notch-Akt–Mediated Antiapoptotic Activity Requires Mitofusins.
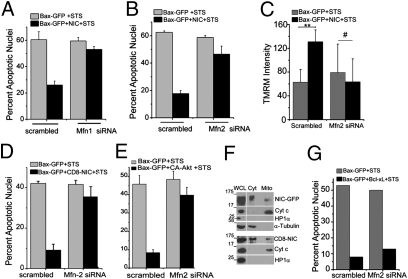
Mitofusins (Mfn)-1 and Mfn-2, are mitochondrial outer membrane GTPases regulating the coordinated and sequential fusion of mitochondrial membranes (10). Depletion of Mfn1 or Mfn2 (using siRNA) resulted in punctate mitochondria (Fig. S6 A and B) and abrogated NIC-mediated inhibition of nuclear damage and restoration of MTP (Fig. 5 A–C). CD8-NIC and CA-Akt demonstrated a similar dependence on Mfns for activity (Fig. 5 D and E and Fig. S6C) positioning Mfns as intermediates in the NIC-Akt signaling module. Both NIC-GFP and CD8-NIC-GFP were detected in cellular fractions enriched for the mitochondrial resident cytochrome c (Fig. 5F), indicating a possible (temporally regulated) association with mitochondria. Distinctively, Bcl-xL-mediated antiapoptotic activity was independent of Mfns (Fig. 5G and Fig. S6 E and D).
Fig. 5.
Notch-mediated antiapoptotic activity requires Mitofusins. (A and B) Cells pretreated with Mfn1(A), Mfn2 (B) or scrambled siRNA for 48 h were transfected with Bax-GFP or Bax-GFP+NIC, cultured overnight, and scored for STS-induced apoptotic damage. (C) Cells pretreated with M or scrambled siRNA for 48 h, transfected with Bax-GFP or Bax-GFP+NIC, cultured overnight, treated with STS, and scored for TMRM dye intensity by confocal microscopy. Differences in TMRM intensities in Bax transfected scrambled and M siRNA-treated cells are not significant (P = 0.91). ** P < 0.001; # P = 0.54. (D) Cells pretreated with Mfn-2 or scrambled siRNA for 48 h were transfected with Bax-GFP or Bax-GFP+CD8-NIC-RFP. Cultures were processed as described in Methods. (E) Cells pretreated with Mfn2 or scrambled siRNA for 48 h were transfected with Bax-GFP or Bax-GFP+CA-Akt and processed as in A. (A–E) Data plotted are the mean ± SD from three individual experiments. (F) Representative immunoblot analysis of NIC-GFP or CD8-NIC-GFP transfected cells fractionated and probed for GFP, Cytochrome c, tubulin, and HP1α. (G) Cells pretreated with Mfn2 or scrambled siRNA for 48 h were transfected with Bax-GFP or Bax-GFP+Bcl-xL. Cultures were processed for STS-induced apoptotic damage. The data in the graph shows one experiment representative of two separate trials.
To identify a possible physiological correlate of the interaction between NIC and Bax function described by these experiments we turned to the analysis of activated T cell apoptosis in response to cytokine deprivation.
Notch Regulates Bax Activation in Primary T Cells.
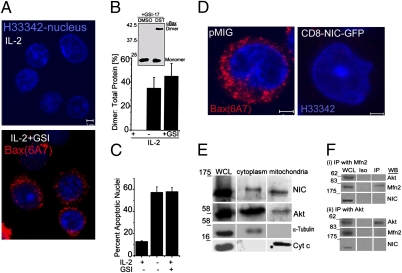
Bax is one of three proapoptotic molecules of the Bcl-2 family implicated in activated T-cell apoptosis for the down-regulation of the immune response (25). Key aspects of this deletion can be recapitulated in vitro in T cells activated using surrogate antigens. Because Notch signaling inhibited activated T cell apoptosis following cytokine deprivation (26), we explored interactions between NIC and Bax in activated T cells. 6A7 reactivity, a signature of Bax activation, was not detected in live cells cultured in cytokine (Fig. 6A Upper) but, as seen in T cells deprived of cytokine (Fig. S6F), was induced following treatment with a gamma secretase inhibitor (GSI), which abrogates Notch processing (Fig. 6A Lower), triggering T cell apoptosis (Fig. 6C). Similarly, Bax dimers stabilized using cross-linkers were detected in cells following cytokine deprivation or treatment with GSI (Fig. 6B). Thus, inhibition of NIC activity recapitulated apoptotic features triggered by cytokine deprivation in T cells. Further, recombinant CD8-NIC, which blocks activated T cell apoptosis (17), also inhibited Bax activation (Fig. 6D). As seen in the cell lines, NIC and Akt were detected in the tubulin negative, mitochondrial fraction of T cells enriched for cytochrome c (Fig. 6E). However, immunoprecipitation analysis did not indicate stable associations between NIC, Mfn2, and Akt in T cells (Fig. 6F).
Fig. 6.
NIC regulates Bax activation in T cells. (A) Activated T cells were cultured with IL-2 or IL-2 + 10 μM gamma secretase inhibitor (GSI-17) for 12 h, stained with 6A7 and H33342, and imaged, (Scale bar: 2 μm.) (B) The cross-linking assay was performed in cell lysates in the different conditions and assessed for Bax multimers by immunoblot analysis (Inset). The graph shows band intensities of dimer Bax relative to total Bax protein by densitometric analysis (mean ± SD from three independent assays). (C) Cells treated as in A were scored for nuclear damage using H33342. Data are the mean ± SD from three separate experiments. (D) Activated T cells expressing GFP or CD8NIC-GFP were cultured without IL-2 for 14 h and stained for 6A7 (red) and Hoechst 33342. (n = 40 cells in two experiments). (E) Representative immunoblot analysis of activated T cells fractionated and probed for NIC, Akt, cyt c, and tubulin in cytoplasmic and mitochondrial fractions and whole cell lysates (WCL). (F) Immunoblot of immunoprecipitation analysis of Akt, Mfn-2, and NIC in activated T cells. Iso: isotype control Ig. White spacers indicate empty lanes in the original membranes.
Discussion
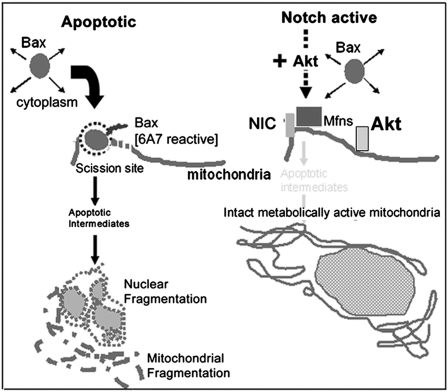
Emerging evidence indicates roles for mitochondria in the regulation of cellular signaling cascades, extending their functions beyond the primary, well-characterized role in the control of cell metabolism (24). This study positions the mitochondrion as an integral part of a cytoplasmic Notch-activated signaling cascade that regulates cell survival (Fig. 7).
Fig. 7.
Schematic summarizing key features of the NIC-activated antiapoptotic cascade. Cytoplasmic NIC activity, inhibited apoptosis coordinated by mitochondria in cell lines (triggered using Bax), and primary T cells. The mitochondrial remodeling proteins—Mitofusins—are identified in this work as key intermediates in the antiapoptotic cascade, regulating mitochondrial function and connectivity downstream of the NIC-Akt signaling pathway. Despite evidence of mitochondrial pools of NIC and Akt, we do not detect stable, direct associations between NIC-MFN-AKT suggesting the possibility of dynamic interactions among these molecules regulating signaling outputs.
NIC signaling was investigated using the Bcl-2 family protein Bax as a probe, which converges on the OMM for apoptotic output. Antiapoptotic activity was demonstrated using multiple approaches (in live and fixed cells) monitoring changes in Bax dynamics, conformation, and oligomerization in established cell lines and apoptosis induced by cytokine-deprivation (mediated by the mitochondrion), in ex vivo activated primary T cells, supporting the link between NIC signaling and mitochondrial function. Experiments designed to elucidate spatial coordinates positioned cytoplasmic NIC, as opposed to the nuclear localized form, as the active signaling intermediate. This was based on evidence that membrane-targeting or NES addressing tags did not compromise antiapoptotic activity and supported by the previous demonstration of cytoplasmic pools of the transgene (ascertained by FCS) in NIC-GFP expressing cells (17).
The mitochondrion is central to several apoptotic cascades, and the OMM is a primary target of antiapoptotic proteins. A possible link between NIC activity and the mitochondrion was suggested by the unexpected observation that the NIC-Akt signaling cascade, unlike Bcl-2 family antiapoptotic proteins (27), restored both organelle connectivity and integrity in cells where it blocked death, suggesting interactions with molecules that maintain organelle structure. The Mfns are required for maintenance of organelle morphology and architecture and linked to the regulation of the myriad metabolic activities of mitochondria (23, 24, 28). Depleting Mfns did not perturb Bcl-xL activity but abrogated NIC and Akt function, identifying an essential role for these molecules in the cascade. Although NIC and Akt are detected in the cellular fractions enriched for mitochondria, we do not detect stable associations between NIC-Akt and Mfns, although the possibility of dynamic interactions underlying functional outputs remains to be addressed. The mitochondrial link suggested in this work is supported by the recent report that phosphorylated Akt-S473—an output of cytoplasmic NIC activity as demonstrated by our previous work (17)—promotes mitochondrial retention of the kinase (29).
Altered bioenergetic capabilities associated with loss of Notch activity have been previously reported in Drosophila melanogaster (30). However, the mechanism(s) translating changing mitochondrial dynamics into altered functional outputs is not understood, given instances where respiratory competency and maintenance of MTP are decoupled from organelle shape regulating activities (31). Because Mfns are essential intermediates in the NIC-Akt signaling module, this cellular system provides both a molecular handle to explore NIC interactions with mitochondria and a means to explore mechanistic connects between mitochondrial function, morphology, and the coupling to extracellular cues. Apart from their well defined role in cellular bioenergetics, mitochondria are increasingly thought to function as hubs that coordinate signaling cascades in response to extracellular cues (24, 28). It is tempting to speculate that a signaling module comprising evolutionarily conserved intermediates—NIC, Akt, and Mfn—may constitute a generalized mechanism modulating mitochondrial function to meet changing cellular demands encountered during development and differentiation (21, 32) in diverse biological contexts.
Methods
Antibodies.
Antibodies to mNIA (NIC), GFP and cytochrome c were from BD Biosciences; Bax-Clone 6A7 and α-Tubulin from Neomarker; Bax (clone P-19), Notch1, Akt, Mfn-2, and Jagged from Santa Cruz, c-myc and HP1α from Cell Signaling Technology or Millipore-Upstate.
Plasmids.
The Bax-GFP, untagged Bax constructs NIC-GFP and NIC-RFP have been described (17, 33). CD8-NIC was cloned into the plasmid RFP-N3 between the BglII and EcoRI sites to make the CD8-NIC-RFP recombinant and subcloned into pMIG as described elsewhere (17). The following plasmids were obtained as gifts: AcN1 (NIC) and DN-CBF1 from J. Aster (Harvard Medical School, Boston); NotchLNG and NLNGCC>>SS from R. Kopan (Washington University, St. Louis); GFP-N1IC-NLS from B. A. Osborne (Massachusetts University Amherst, Amherst, MA), and Notch full-length (NFL) from A. Rangarajan (Indian Institute of Science, Bangalore, India). All other constructs were from Millipore-Upstate. Plasmids were used at the following concentrations unless specified otherwise: Bax-GFP, 0.5 μg; NFL, NICδRAM, and CD8-NIC-RFP, 2 μg; NIC, NLNG, NLNG CC>>SS, sJag1, DN-Akt, CA-Akt, Bcl-2, and Bcl-xl, 3 μg. Empty vector pcDNA was used to equalize DNA concentrations across experimental groups.
Assays of Cellular Damage.
HeLa, HEK 293, and the COS-7 cells, maintained as described (17), were plated at a density of 3 × 105 cells per 35-mm dish 14–16 h before transfection with Lipofectamine 2000 (Invitrogen). All siRNA were from Dharmacon, Inc. and were used at 100 nM concentration as per the manufacture's instructions. For HeLa and COS-7 cells 14 h posttransfection, cells were cultured for an additional hour with or without 1 μM STS (Sigma) to induce apoptotic damage. The data are normalized to apoptotic damage in the group not treated with STS. HEK cells were analyzed for apoptotic nuclear damage 18–24 h posttransfection. Generation of activated T cells, treatment with GSI, transductions using retroviruses and isolation of transfected cells by flow-sorting were performed as described (26). To analyze nuclear morphology, cells were stained (for 5–7 min at room temperature) with the DNA binding dye Hoechst (H) 33342 (1 μg/mL) and nuclear morphology in GFP positive cells scored by microscopy. In cells expressing RFP- and GFP-tagged recombinants, only cells positive for both tags were scored. For the analysis of mitochondrial morphology, adherent cells were incubated with 100 ng/mL of MitoTracker Red 580 (Invitrogen) for 30–45 min at 37 °C. Cells were imaged by confocal microscopy after two PBS washes. Bax oligomerization was assessed in cell lysates as described (12). Cell fractionations used the mitochondria isolation kit for mammalian cells (Pierce) as per the manufacture's instructions.
Quantitative Fluorescence Assays.
Cells were maintained at 37 °C with a temperature and CO2 stage controller and examined using Olympus Confocal FV1000, 60×, NA1.4 Objective Lens. FCS and FRAP analysis were performed as described (14). The analysis of Bax-GFP puncta number and kinetics was performed using a custom-made Labview program, which analyzed Bax-GFP puncta in each frame. Puncta were set as described (Fig. S3), the number of Bax-GFP puncta in each frame was plotted as a function of time and averaged for 16 to 20 cells.
Statistical Analysis.
Data are presented as mean ± SD derived minimally from three to five independent experiments unless stated otherwise. FRAP and Bax-GFP puncta analyses are presented as mean ± SEM. Statistical significance was calculated using the two population Student's t test.
Supplementary Material
Acknowledgments
Jon Aster (Harvard Medical School, Boston), R. Kopan (Washington University, St. Louis), B. A Osborne (Amherst, MA), and A. Rangarajan (Indian Institute of Science, Bangalore, India) made generously gifts of several constructs. We thank G. V. Shivashankar [National Centre for Biological Sciences (NCBS), Bangalore, India] and members of his laboratory, for their assistance and inputs on the design and analysis of experiments with live cells. We acknowledge G. V. Shivashankar (NCBS) and Veronica Rodrigues (Department of Biological Sciences, Tata Institute of Fundamental Research Mumbai) for critical comments and the NCBS Central Imaging and Flowcytometry Facility [supported by grants from the Department of Science and Technology, India (Centre of Nanotechnology SR/S5/NM-36/2005; Grant 43/2003-SF and the Wellcome Trust, United Kingdom]. The study is funded by an International SRF in Biomedical Sciences in India, Wellcome Trust, United Kingdom, and a grant from the Department of Science & Technology, India, to A.S. M.N. is supported by a postdoctoral fellowship from the Department of Biotechnology, India, and L.P. is supported by a fellowship from the Council of Scientific and Industrial Research, India.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910060107/DCSupplemental.
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Mumm JS, Kopan R. Notch signaling: From the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 3.Hurlbut GD, Kankel MW, Lake RJ, Artavanis-Tsakonas S. Crossing paths with Notch in the hyper-network. Curr Opin Cell Biol. 2007;19:166–175. doi: 10.1016/j.ceb.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 5.Rangarajan A, et al. Activated Notch1 signaling cooperates with papillomavirus oncogenes in transformation and generates resistance to apoptosis on matrix withdrawal through PKB/Akt. Virology. 2001;286:23–30. doi: 10.1006/viro.2001.0867. [DOI] [PubMed] [Google Scholar]
- 6.Androutsellis-Theotokis A, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 7.Osipo C, Golde TE, Osborne BA, Miele LA. Off the beaten pathway: The complex cross talk between Notch and NF-kappaB. Lab Invest. 2008;88:11–17. doi: 10.1038/labinvest.3700700. [DOI] [PubMed] [Google Scholar]
- 8.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–113. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17:563–569. doi: 10.1016/j.tcb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Lalier L, et al. Bax activation and mitochondrial insertion during apoptosis. Apoptosis. 2007;12:887–896. doi: 10.1007/s10495-007-0749-1. [DOI] [PubMed] [Google Scholar]
- 12.Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 13.Mikhailov V, et al. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem. 2001;276:18361–18374. doi: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]
- 14.Lippincott-Schwartz J, Snapp E, Kenworthy A. Studying protein dynamics in living cells. Nat Rev Mol Cell Biol. 2001;2:444–456. doi: 10.1038/35073068. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya D, Mazumder A, Miriam SA, Shivashankar GV. EGFP-tagged core and linker histones diffuse via distinct mechanisms within living cells. Biophys J. 2006;91:2326–2336. doi: 10.1529/biophysj.105.079343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel T, et al. The G2/M regulator 14-3-3sigma prevents apoptosis through sequestration of Bax. J Biol Chem. 2001;276:45201–45206. doi: 10.1074/jbc.M106427200. [DOI] [PubMed] [Google Scholar]
- 17.Perumalsamy LR, Nagala M, Banerjee P, Sarin A. A hierarchical cascade activated by non-canonical Notch signaling and the mTOR-Rictor complex regulates neglect-induced death in mammalian cells. Cell Death Differ. 2009;16:879–889. doi: 10.1038/cdd.2009.20. [DOI] [PubMed] [Google Scholar]
- 18.Le Gall M, De Mattei C, Giniger E. Molecular separation of two signaling pathways for the receptor, Notch. Dev Biol. 2008;313:556–567. doi: 10.1016/j.ydbio.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mumm JS, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 20.Aster JC, et al. Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jkappa or nuclear localization sequences retain the ability to associate with RBP-Jkappa and activate transcription. J Biol Chem. 1997;272:11336–11343. doi: 10.1074/jbc.272.17.11336. [DOI] [PubMed] [Google Scholar]
- 21.Ciofani M, Zúñiga-Pflücker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 22.Sade H, Krishna S, Sarin A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem. 2004;279:2937–2944. doi: 10.1074/jbc.M309924200. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 24.McBride HM, Neuspiel M, Wasiak S. Mitochondria: More than just a powerhouse. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 26.Bheeshmachar G, et al. Evidence for a role for notch signaling in the cytokine-dependent survival of activated T cells. J Immunol. 2006;177:5041–5050. doi: 10.4049/jimmunol.177.8.5041. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan C, Delivani P, Cullen SP, Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell. 2008;31:570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Chan DC. Mitochondria: Dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Antico Arciuch VG, et al. Akt1 intramitochondrial cycling is a crucial step in the redox modulation of cell cycle progression. PLoS One. 2009;4:e7523. doi: 10.1371/journal.pone.0007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thörig GE, Heinstra PW, Scharloo W. The action of the notchlocus in Drosophila melanogaster. II. Biochemical effects of recessive lethals on mitochondrial enzymes. Genetics. 1981;99:65–74. doi: 10.1093/genetics/99.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park KS, et al. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem. 2008;283:33347–33356. doi: 10.1074/jbc.M806251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, et al. Mitofusins Mfn1. and Mfn2. coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parikh N, Sade H, Kurian L, Sarin A. The Bax N terminus is required for negative regulation by the mitogen-activated protein kinase kinase and Akt signaling pathways in T cells. J Immunol. 2004;173:6220–6227. doi: 10.4049/jimmunol.173.10.6220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.