Abstract
To address complications of pathogenic antibody or life-threatening anaphylactic reactions in protein replacement therapy for patients with hemophilia or other inherited protein deficiencies, we have developed a prophylactic protocol using a murine hemophilia B model. Oral delivery of coagulation factor IX fused with cholera toxin β-subunit (with or without a furin cleavage site; CTB-FFIX or CTB-FIX), expressed in chloroplasts (up to 3.8% soluble protein or 0.4 mg/g leaf tissue), bioencapsulated in plant cells, effectively blocked formation of inhibitory antibodies (undetectable or up to 100-fold less than controls). Moreover, this treatment eliminated fatal anaphylactic reactions that occurred after four to six exposures to intravenous F.IX. Whereas only 20–25% of control animals survived after six to eight F.IX doses, 90–93% of F.IX-fed mice survived 12 injections without signs of allergy or anaphylaxis. Immunostaining confirmed delivery of F.IX to Peyer's patches in the ileum. Within 2–5 h, feeding of CTB-FFIX additionally resulted in systemic delivery of F.IX antigen. This high-responder strain of hemophilia B mice represents a new animal model to study anaphylactic reactions. The protocol was effective over a range of oral antigen doses (equivalent to 5–80 μg recombinant F.IX/kg), and controlled inhibitor formation and anaphylaxis long-term, up to 7 months (∼40% life span of this mouse strain). Oral antigen administration caused a deviant immune response that suppressed formation of IgE and inhibitory antibodies. This cost-effective and efficient approach of antigen delivery to the gut should be applicable to several genetic diseases that are prone to pathogenic antibody responses during treatment.
Keywords: allergy, chloroplast, genetic disorders, oral tolerance, plant-made therapeutics
Current treatment of the X-linked bleeding disorder hemophilia is based on intravenous (i.v.) infusion of recombinant or plasma-derived coagulation factor VIII (hemophilia A) or factor IX (hemo-philia B) concentrate. In a subset of patients, humoral immune responses to the functional protein develop and pose a serious complication for factor replacement therapy. In hemophilia A, formation of inhibitory antibodies (inhibitors) directed against factor VIII (F.VIII), a helper-T-cell-dependent response, occurs in 20–30% of patients (1). There is much progress in the risk assessment of inhibitor formation in patients early in therapy, using a combination of genotyping (e.g., determination of the underlying F.VIII mutation and polymorphisms in the promoters of the cytokine genes IL-10 and TNFα), family history of inhibitor formation, ethnicity, and intensity of early treatment (2). Inhibitors increase the risk for bleeding-related morbidity and mortality. High-titer inhibitors (>5 Bethesda units, BU/mL) prevent treatment with clotting factor product. Bypass re-agents, such as activated factor VII, are expensive and pose throm-botic risks. Inhibitors can be eliminated by immune tolerance induction (ITI) protocols, which are based on frequent high-dose infusions of factor for months to several years, and often require amounts of products exceeding $1,000,000.
The overall incidence of inhibitors is considerably lower in hemophilia B (1.5–3%). However, 9–23% of patients with severe disease (<1% coagulation activity) form inhibitors, which are typically high-titer and are almost exclusively confined to subjects with gene deletions or early stop codons (3). ITI protocols are less effective for treatment of inhibitors to factor IX (F.IX) and sometimes have to be interrupted because of development of nephrotic syndrome. Importantly, studies found that up to 50% of patients with F.IX inhibitors may experience potentially life-threatening anaphylactic reactions to F.IX which also preclude the subject from home treatment and severely hinder ITI protocols (3). These acute severe and systemic type I hypersensitive allergic reactions, often attributed to IgE formation, have been reported for treatment of hemophilia A and B, lysosomal storage disorders, and others (4–9). No prophylactic protocols are currently available for prevention of these pathogenic antibody responses.
Oral delivery of coagulation factors has long been discussed as a potential approach to induce tolerance in hemophilia (10). However, realization has been elusive, likely because of inefficient delivery of the antigen to the gut-associated lymphoid tissue (GALT). There-fore, we sought to develop an alternative approach, using transplastomic technology as a means of efficient and potentially tolerogenic oral delivery of F.IX. This technology for robust expression and bioencapsulation of therapeutic proteins is based on transformation of plant cells by particle bombardment of plasmid DNA followed by homologous recombination with chloroplast genomes, selection, and regeneration of plants (11, 12). Transplastomic plants lack gene silencing, regardless of accumulating >100-fold higher transcripts than in nuclear transgenic plants and accumulation of foreign proteins at levels >70% of total leaf protein (13–16). Consequently, we and others have been able to produce high levels of several biopharmaceutical proteins and vaccine antigens via the chloroplast system (11, 17). Foreign proteins expressed in plant chloroplasts can be efficiently delivered to the immune or circulatory system upon oral delivery when they are fused with the transmucosal carrier cholera toxin B subunit (CTB) (14, 18, 19).
Although the prokaryotic nature of the chloroplast may not perform all of the posttranslational modifications required to produce biologically active F.IX, we reasoned that optimal delivery of the polypeptide to the GALT via bioencapsulation by the plant cell would result in tolerogenic antigen presentation that modulates immune response upon i.v. administration of functional F.IX protein. Results demonstrate that repeated oral delivery of chloroplast-derived F.IX suppressed inhibitor development and prevented life-threatening anaphylactic reactions to F.IX replacement therapy in a murine model of hemophilia B.
Results
Construction of Chloroplast Transformation Vector.
Both chloroplast vectors pLD CTB-FIX and pLD CTB-FFIX (Fig. 1A) used in this study are based on the universal chloroplast vector pLD-Ctv that targets the expression cassette into the spacer region between the trnI and trnA genes of the chloroplast genome for integration via homologous recombination (20). In the CTB-FIX construct, a glycine-proline-glycine-proline (GPGP) hinge was created between the fusion elements to prevent steric hindrance, whereas in the CTB-FFIX construct, along with the GPGP hinge, a furin cleavage site was also introduced. The CTB-FIX and CTB-FFIX fusion genes were regulated by the psbA promoter and 5′ untranslated region (UTR) to achieve hyperexpression, as demonstrated previously for several other transgenes (21). The psbA 3′ UTR placed at the 3′ end of the fusion gene imparted transcript stability. The aadA gene driven by the tobacco plastid ribosomal operon promoter (Prrn) and the GGAG ribosome binding site conferring spectinomycin resistance were used for selection.
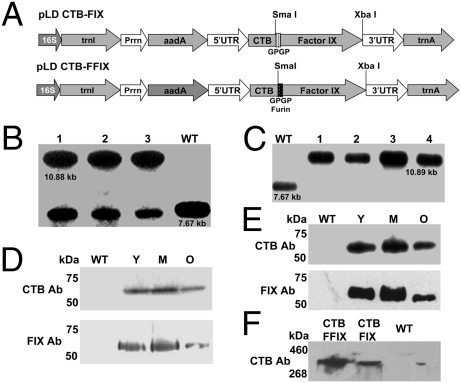
Fig. 1.
Characterization of transplastomic plants. (A) Schematic representation of chloroplast transformation vectors. Prrn, rRNA operon promoter; aadA, aminoglycoside 3′-adenylyltransferase gene; 5′ UTR, promoter and 5′ untranslated region of psbA gene; 3′ UTR, 3′ untranslated region of psbA gene. (B and C) Southern blot analysis of CTB-FIX and CTB-FFIX. WT, untransformed; 1–4, transplastomic lines. (D and E) Western blot analysis of transplastomic lines expressing CTB-FIX and CTB-FFIX probed with CTB antibody and FIX antibody. WT, untransformed plant extract; Y, M, and O, plant extract from young, mature, and old transplastomic leaves, respectively. (F) Native PAGE. Total soluble protein extracted from transplastomic leaves, resolved by native PAGE and probed with CTB antibody.
Regeneration of Transplastomic Plants and Evaluation of Transgene Integration by Southern Blot Analysis.
Transplastomic tobacco plants were produced as described earlier (12, 22). Chloroplast transgenic lines were examined by Southern blots to confirm site-specific transgene integration and to determine homoplasmy or heteroplasmy. Digestion of total plant DNA from untransformed plants (WT) with HindIII generated a 7.67-kbp fragment when hybridized with the 32P-labeled trnI-trnA flanking sequence probe, indicating no integration of foreign DNA (Fig. 1 B and C). All CTB-FIX transplastomic lines showed both 10.88-kb and 7.67-kb fragments, indicating heteroplasmy, whereas all CTB-FFIX transgenic lines showed only the 10.89-kb fragment, indicating homoplasmy. Southern blots confirmed site-specific integration of the transgenes into the spacer region between the trnI and trnA genes (Fig. 1 B and C). Homoplasmy in CTB-FIX transgenic lines was not achieved even after additional rounds of selection. This may be due to toxicity created by improperly folded F.IX due to steric hindrance in the absence of a furin cleavage site (GPGP alone was inadequate to prevent steric hindrance). Transplastomic lines with roots were transferred to jiffy pellets and kept under high humidity initially for 2 weeks in a growth chamber before plants were moved to the greenhouse.
CTB-FIX and CTB-FFIX Expression and Pentamer Assembly in Transgenic Tobacco.
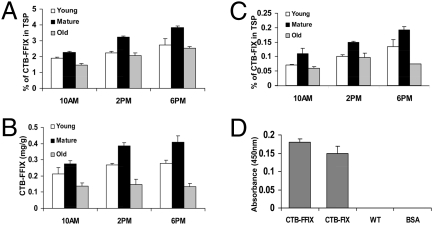
Immunoblots probed with CTB antibody showed the presence of an ∼58-kDa fusion protein in both CTB-FIX and CTB-FFIX transplastomic lines. A similar banding pattern was observed by immunodetection with FIX antibody (Fig. 1 D and E). Pentamer formation was observed in the native PAGE using CTB primary antibody (Fig. 1F). Quantitation of the fusion protein was carried out by densitometry on Western blots of plant samples using known amounts of purified CTB. The expression levels varied significantly depending on the developmental stages and time of leaf harvest (Fig. 2 A–C). Young (top five), mature (fully grown), and old (bottom three) leaves in transplastomic plants were investigated. Fig. 2 A–C show that transplastomic lines have higher F.IX expression in mature leaves. Younger leaf cells contain fewer chlo-roplasts and the psbA promoter is developmentally regulated; therefore, expression levels were less than in mature leaves. Older senescent leaves had lower accumulation of F.IX, probably due to higher proteolytic activity. Likewise, F.IX expression was higher at 6 PM than at 10 AM because the psbA promoter and 5′ untranslated regions located upstream of the FIX gene cassette are known to enhance translation in the light (15, 16, 23). Transplastomic lines of CTB-FIX and CTB-FFIX had expression levels of up to 0.19% and 3.8% (0.4 mg/g of leaf tissue) fusion protein, respectively, in the total soluble protein. Introduction of the furin cleavage site clearly stabilized FIX and enhanced its accumulation 20-fold.
Fig. 2.
Protein analysis in transplastomic tobacco plants. Accumulation of CTB-FFIX (A and B) and CTB-FIX (C) as a function of light and developmental stage. (D) GM1 binding assay of CTB-FIX and CTB-FFIX tobacco transformants. WT, untransformed; BSA, negative control. TSP, total soluble protein.
Vibrio cholerae secretes an 86-kDa toxin that is made up of two subunits, an α- and a β-subunit (CTB), that contains a binding site for the plasma membrane receptor of the intestinal epithelial cells (GM1) (24, 25). GM1-ganglioside has been shown to be the receptor for CTB protein in vivo (24), and a pentameric structure is required for binding to GM1 receptor (25). As illustrated in Fig. 2D, GM1 ELISA confirms that CTB-FIX and CTB-FFIX fusion proteins assembled properly to form pentameric structures within chloroplasts, which are essential for GM1-ganglioside receptor binding. This assay along with native PAGE confirmed the correct folding and disulfide-bond formation of CTB pentamers within transgenic chloroplasts.
Oral Delivery of F.IX Transplastomic Leaves Suppresses Inhibitor Formation and Fatal Anaphylaxis in Hemophilia B Mice.
Hemophilia B mice with targeted F9 gene deletion on C3H/HeJ genetic background form robust immune responses to human F.IX (hF.IX) upon protein or gene therapy (26). Therefore, three experimental groups received oral gavage of frozen powdered leaves (250 mg/dose) twice per week for 2 months, using leaves from untransformed (WT), CTB-FIX, or CTB-FFIX transplastomic plants (representing the equivalent of 0, 0.14, and 2 μg of recombinant hF.IX·dose−1·mouse−1, respectively). Dosage of CTB-FIX and CTB-FFIX for each batch of ground leaf powder was determined before each gavage and uniform doses were delivered. After 1 month, a therapeutic dose of recombinant hF.IX was given i.v. once per week (1 IU per mouse) for 2 months, that is, extending 1 month beyond the feeding protocol (Fig. 3A). Two more groups received i.v. injections of hF.IX only, without feeding.
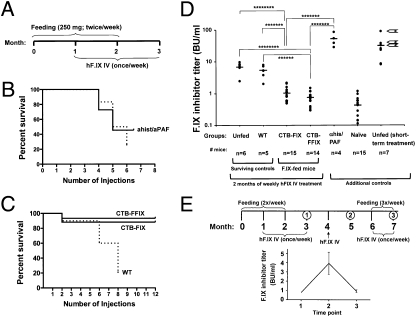
Fig. 3.
Prevention of inhibitor formation and of anaphylactic reactions against i.v. hF.IX by oral administration of hF.IX chloroplast transgenic plant material in hemophilia B mice. (A) Feeding and hF.IX administration schedule. (B) Survival of two control groups of unfed mice (starting with n = 11, solid line, and n = 12 mice, dotted line). Mice of one cohort (solid line) that survived five injections (n = 5) received antihistamine/anti-PAF before a sixth injection of hF.IX (ahist/aPAF), resulting in 100% survival. (C) Survival of mice fed with WT (n = 10 mice at the onset of protein therapy), CTB-FIX (n = 17), or CTB-FFIX (n = 15) plant material as a function of the number of i.v. injections of hF.IX protein. (D) Inhibitor titers (in BU/mL) at 3-month time-point mice (i.e., after 8 weekly i.v. injections of hF.IX) in unfed (pooled from several experiments), WT-fed, CTB-FIX-fed, and CTB-FFIX-fed. Additional controls: Titers in unfed mice are shown that received antihistamine/anti-PAF before a sixth injection of hF.IX, that were naïve, or that received four hF.IX administrations. For the last, arrows indicate animals that died after a subsequent fifth injection. Each dot represents an individual animal. Horizontal bars indicate average titers for each experimental group. (E) Long-term control of inhibitor formation with bioencapsulated hF.IX following the indicated schedule for oral administration of CTB-FFIX plant material and i.v. injection of hF.IX protein. Numbers indicate time points of blood collection. Below, average inhibitor titers (in BU/mL ± SEM) are graphed for the indicated time points.
In unfed animals (n = 11), severe allergic reactions were observed starting with the fourth i.v. injection of hF.IX, at which time fatal anaphylactic reactions started to occur, and continued subsequently with an incidence of 17–33% (Fig. 3B). Similarly, WT-fed mice showed severe allergic reactions immediately following the fourth (2 of 10 mice) and fifth injections (3 of 10 mice), including hunched position, piloerection, slowing of movement, tachypnea, and bronchospasms. During subsequent injections, ∼50% of the mice had such reactions. Moreover, starting from the sixth injection, 31–40% of animals died within 30 min to several hours after i.v. injection of hF.IX, apparently from respiratory arrest (Fig. 3C). We were able to collect blood samples from five remaining mice immediately after the eighth i.v. injection, within 2 h after which three additional mice died. In any case, 75–80% of mice treated with hF.IX were lost within six to eight weekly injections (Fig. 3 B and C), unless antianaphylactic drugs were given (see below).
In contrast, 90% of CTB-FIX- and 93% of CTB-FFIX-fed mice (n ≥ 14 per cohort) survived the initial 2-month period of eight weekly hF.IX injections and even subsequent injections (total of 12 exposures; Fig. 3C). In none of these mice did we observe signs of allergic or anaphylactic reactions. Bethesda assays showed nearly complete to complete suppression of inhibitor formation in mice fed with CTB-FIX (1 ± 0.5 BU/mL) or CTB-FFIX (0.7 ± 0.3 BU/mL) as compared with background inhibitor titers in naïve hemophilia B mice (no feeding, no treatment; 0.5 ± 0.3 BU/mL). Surviving WT-fed and unfed mice had significantly higher inhibitor titers, which were similar for these groups and ranged from 2 to 8 BU/mL and 2.5 to 10 BU/mL, respectively (Fig. 3D).
We suspected that these data underrepresented protein therapy-induced inhibitor titers in the control groups, because those mice with fatal hypersensitive responses likely had stronger immune responses. To address these points, we first analyzed plasma obtained from unfed hemophilia B mice after only four weekly hF.IX injections. These had substantially higher inhibitor titers of 8–90 BU/mL (Fig. 3D), and the three of seven mice with the highest titers died after the fifth hF.IX administration (arrows in Fig. 3D). Second, five mice that had survived a fifth injection received the sixth dose of hF.IX under coverage with antihistamine and anti-platelet activating factor (PAF). In this case, all five mice survived without allergic or anaphylactic response (Fig. 3B). Inhibitor titers were measured in four of these mice and were very high (29–87 BU/mL; Fig. 3D).
Oral Administration of Bioencapsulated hF.IX Can Control Inhibitor Formation Long Term.
One month after the last hF.IX injection, one additional i.v. administration of hF.IX was performed in nine CTB-FFIX-fed mice (i.e., 2 months after the last feeding; Fig. 3E). This resulted in an increase of inhibitors to an average of 4 BU/mL (Fig. 3E). Subsequently, treatment was resumed for 1 month (i.e., weekly i.v. infusions of hF.IX) while also continuing the feeding (three oral gavages per week). At the end of this regimen, inhibitor titers had declined to baseline (Fig. 3E), indicating that the protocol can be used to control inhibitor formation long-term. Again, no allergic or anaphylactic responses were observed.
Oral Administration of Bioencapsulated hF.IX Suppresses Formation of IgE and Inhibitors via an Immune Deviation Mechanism.
Analysis of hF.IX-specific Ig formation showed that CTB-FIX/CTB-FFIX-fed mice (and surviving WT-fed mice) had average circulating IgG1 titers which were significantly lower when compared with control mice that were kept alive with antihistamine/anti-PAF (Fig. 4A). Whereas IgG1 titers in control mice correlated well with inhibitor titers, there was no correlation in CTB-FIX/CTB-FFIX-fed mice, which therefore had formed noninhibitory antibodies (Fig. 4C). The noninhibitory antibodies in CTB-FFIX-fed mice that formed upon protein therapy, in contrast to the inhibitors that developed in WT-fed mice, did not cause enhanced clearance of i.v. infused hF.IX from the circulation (Fig. 4F). The noninhibitory antibodies also did not prevent correction of coagulation activity (as determined by activated partial thromboplastin time), which was raised from <1% at baseline to 16–25% of normal at 30 min after i.v. injection of 1 IU hF.IX (n = 5). Naïve mice treated in parallel showed comparable results (16–18% of normal at 30 min after treatment).
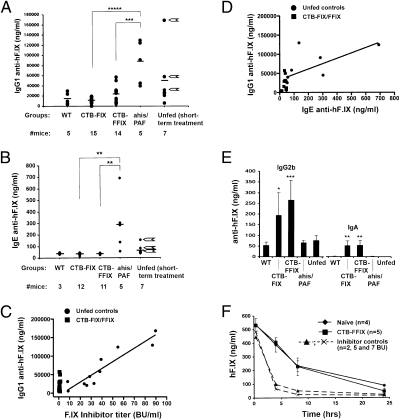
Fig. 4.
Suppression of high-titer IgG and of IgE Ig responses directed against hF.IX. (A) IgG1 titers at 3-month time-point mice (i.e., after 8 weekly i.v. injections of hF.IX) in WT-, CTB-, and CTB-FFIX-fed mice. Additional unfed control mice were as described in Fig. 3. (B) IgE titers for the same experimental groups. Arrows next to data points from unfed mice with four administrations of hF.IX protein indicate animals that died after a subsequent fifth injection. (C) Correlation between IgG1 titers and inhibitor titers in unfed control mice (solid circles) and mice fed with CTB-FIX or CTB-FFIX (solid squares) plant material. (D) Correlation between IgG1 titers and IgE titers in unfed control mice (solid circles) and mice fed with CTB-FIX or CTB-FFIX (solid squares) plant material. (E) Immune deviation induced by oral administration. IgG2b and IgA titers are elevated in CTB-FIX- and CTB-FFIX-fed mice compared with WT-fed and unfed control mice after repeated i.v. injections of hF.IX protein. (F) In comparison with naïve hemophilia B mice or CTB-FFIX-fed mice, WT plant material-fed mice with inhibitor development (shown are two individual mice with 5 and 7 BU) show enhanced clearance of i.v. injected hF.IX protein (1 IU/mouse). Shown are hF.IX antigen levels in plasma as a function of time after i.v. injection. Data points for naïve and CTB-FFIX-fed mice are average ± SEM.
Importantly, three of seven control mice that had received four injections of hF.IX had detectable IgE titers, and titers of >50 ng/mL were predictive of subsequent fatal responses (Fig. 4B, arrows). Higher-titer IgE was found in four of five analyzed mice that had received a sixth injection concomitant with antihistamine/anti-PAF (Fig. 4B). These control mice showed a correlation between high-titer IgG1 (>40 μg/mL) and IgE formation, indicating that repeated i.v. administrations boosted Th2-driven antibody formation, resulting in activation of additional B cells with class switching to IgE (Fig. 4D). This correlation was not seen in CTB-FFIX-fed mice, some of which developed IgG1 titers that would have been predicted to be associated with IgE (Fig. 4D). CTB-FIX/FFIX-fed mice had IgE titers of <40 ng/mL (the background of our assay) and no anaphylactic responses. Allergic reactions in rodents, although not previously identified in hemophilic mice, in general are caused by histamine and PAF release for IgE-dependent reactions, whereas an alternative, IgG-dependent pathway has also been described, which, however, does not involve histamine (27). To address this point, additional control mice received hF.IX administration under coverage with anti-PAF alone (i.e., without antihistamine), resulting in fatal anaphylaxis in all three animals tested, thereby further supporting an IgE-dependent mechanism in the hemophilia B mice.
None of the WT-fed or unfed mice formed IgG2a against hF.IX, thereby ruling out an IgG2a/complement-dependent mechanism of anaphylactoid reactions. Whereas IgG2a formation was highly variable and inconsistent in CTB-FIX/CTB-FFIX-fed mice, tolerized mice consistently had circulating low titers of TGF-β-dependent antibodies IgG2b and IgA, the hallmark subclass of a mucosal antibody response (Fig. 4E).
CTB-FFIX Material Delivers hF.IX Antigen to the GALT and into the Circulation.
Feeding by CTB-FIX or CTB-FFIX material by itself did not cause a detectable systemic antibody response against hF.IX. Immunostaining demonstrated that feeding resulted in delivery of hF.IX antigen to the ileum, predominantly to the interphase between microfold (M) cells and CD11c+ dendritic cells in Peyer's patches (Fig. 5 A and B). Within 2–5 h, feeding of CTB-FFIX material also resulted in systemic delivery of hF.IX antigen to the liver and into plasma (Fig. 5 D and F). In the case of unfed mice, no signal was detected in ileum (Fig. 5C) or liver (Fig. 5E).
Fig. 5.
Delivery of hF.IX antigen to the GALT and into circulation. (A–E) Immunostains (original magnification: 200×). (A) Peyer's patch and villi of ileum of CTB-FFIX-fed mouse stained for hF.IX (red), M cells (UEA-1, green), and CD11c (blue). Inset shows zoom-in on part of Peyer's patch with positive hF.IX staining. (B) Peyer's patch and villi of ileum of CTB-FFIX-fed mouse stained for hF.IX (red), F4/80+ macrophages (green), and nuclei (DAPI, blue). (C) Negative control: stain for hF.IX (red) and nuclei (DAPI; blue) in ileum of unfed mouse. (D and E) Stain for hF.IX (green) and nuclei (DAPI; blue) of liver of CTB-FFIX-fed (D) and unfed (E) mouse. (F) In two independent experiments, hF.IX antigen was detectable in plasma in two of three mice 2 or 5 h after oral gavage with CTB-FFIX material.
Discussion
Oral tolerance for hemophilia has been discussed for the past decade but has not been realized, in part because of challenges in effective delivery of coagulation factor antigen to the gut. A recent study concluded that the amount of coagulation factor required for feeding would be cost-prohibitive even for mouse experiments and therefore was not pursued (28). Here we used an alternative technology that allows for efficient production, bioencapsulation, and delivery of antigens to the GALT (14, 18, 29, 30).
Human F.IX was produced at a high level in plant chloroplasts as a fusion protein. The CTB fusion ensures stability of the transgenic protein in the chloroplast, and the nontoxic B subunit exhibits specific and high-affinity binding to the oligosaccharide domain of ganglioside GM1 (a lipid-based membrane receptor), thereby tethering the protein to the plasma membrane of host cells of the intestinal epithelium upon release from the plant cells in the ileum (18, 31).
Priming with CTB-FIX via an oral route reduced Th2-dependent antibody responses against i.v. hF.IX, and in particular protected from IgE formation, thereby eliminating allergic and anaphylactic reactions. Moreover, reintroduction of the antigen via the oral route suc-cessfully controlled inhibitor formation long-term without a need for immune-suppressive drugs. Interestingly, plant materials expressing 20-fold different antigen levels were effective, which should facilitate clinical translation. The furin cleavage site, which directs release and exocytosis of the fused protein from gut epithelia, was not required for treatment (but improved expression and accumulation of FIX in plant cells). These results imply that introduction of F.IX to the GALT was key to successful immune-modulatory therapy. It is generally thought that persistence of the antigen is required to maintain tolerance. Thus, it is likely that oral delivery has to be repeated beyond the induction phase, perhaps every 2 months, to maintain hyporesponsiveness long-term. It should be possible to optimize the protocol so that less intensive repeated oral dosing is effective for maintenance.
Repeated injections of hF.IX caused fatal reactions in most control animals, so that only mice survived that were either low responders or, possibly, spontaneously controlled the pathogenic antibody response. The magnitude of the antibody response was unmasked by pharmaceutical blockage of histamine release, thereby allowing animals to survive despite high production of IgE against hF.IX. Tests to detect IgE responses in patients with hemophilia have been developed, such as RAST (radioallergosorbent test) (9). However, there are no protocols for preventing or suppressing IgE formation, nor has there been an animal model that mimics this aspect of hemophilia pathophysiology. Similar to observations in humans, the strain of mice we identified as prone to anaphylactic reactions has an F9 gene deletion (8). A lack of prior reports on anaphylactic reactions to coagulation factors may be because protein replacement therapy has been largely studied using F.VIII infusions to hemophilia A mice (which, similar to human treatment, is less likely to cause anaphylaxis). Hemophilia B mice were primarily used for gene therapy studies and/or bred on C57BL/6 background (which shows weaker immune responses to F.IX) (32).
Symptoms in the hemophilia B mice were similar to IgE-mediated severe anaphylactic reactions observed in the treatment of other ge-netic diseases, including hemophilia A and the lysosomal storage disorders Pompe disease (acid α-glucosidase deficiency) and Fabry disease (α-galactosidase A deficiency) (5–7, 33). In treatment of hemophilia B, strong evidence has been provided for IgE-mediated anaphylactic reactions (9, 34). However, IgG/complement-mediated mechanisms have also been proposed (4), and IgE formation may therefore not be the only source of anaphylactoid reactions in humans with hemophilia.
Protection of hemophilia B mice from inhibitor and IgE responses involved a deviant immune response characterized by suppression of IgE and of high-titer IgG1, formation of nonneutralizing antibodies instead of inhibitors, and production of additional Ig subclasses (albeit at very lower titers). Interestingly, the presence of nonneutralizing antibodies has also been described in clinical ITI protocols (4). IL-4 production by CD4+ T cells, that is, by Th2 cells, promotes both IgG1 and IgE class switching. However, it has been proposed that gut-associated regulatory T cells expressing IL-10 may suppress IgE formation (35). Our findings on induction of IgG2b and IgA suggest a role for TGF-β, possibly derived from Th3 cells, in modulation of F.IX-specific B-cell activation.
Notably, the response to the allo- or autoantigen introduced to the GALT by oral administration of chloroplast transgenic plant material modulates responses to the antigen in other tissues, which we exploited to develop oral tolerance protocols (14). The oral delivery protocol described here offers solutions for complications encountered with alternative strategies such as gene transfer or transient immune-suppressive regimens (36). Genetic manipulation poses risks of immunotoxicities and insertional mutagenesis; immune-suppressive drugs have side effects and increase the risk for opportunistic infections, and long-term consequences of use in pediatric patients are unclear. Administration of peptides to mucosal surfaces has also been described, which, however, is complicated in an outbred population because it requires knowledge of CD4+ T-cell epitopes (37).
The chloroplast system overcomes major limitations of protein production by elimination of highly expensive fermentation and puri-fication systems, cold storage, transportation, and sterile injections. Genetically modified chloroplast genomes of most crops are maternally inherited, and the absence of any reproductive structures offers efficient foreign gene containment (38, 39) and therefore facilitates their safe production in the field (23). Plant cells provide bioencapsulation and protect therapeutic proteins from degradation in the stomach from acids and enzymes (30). Plants also reduce concerns about pathogen contamination as may be the case for production of proteins in transgenic animals. Whereas these initial studies were carried out in nonfood tobacco plants, the let-tuce chloroplast transformation system has been well-developed for expression of human therapeutic proteins (14, 16).
With the recent development of novel protein replacement therapies for genetic diseases, more reports are coming forward on immunotoxicities caused by protein infusion. Oral administration of transplastomic plant tissue provides an avenue for prevention and control of pathogenic antibody responses and anaphylactic reactions to systemically delivered therapeutic proteins.
Materials and Methods
Vector Construction.
Two CTB fusion constructs were made with human factor IX (FIX). To prevent steric hindrance, a glycine-proline-glycine-proline (GPGP) hinge was introduced between the CTB and FIX in one of the constructs. A furin cleavage site was also introduced along with the GPGP hinge in the second construct. The cDNA sequence encoding F.IX was obtained from the American Type Culture Collection and amplified using sequence-specific restriction-site flanking primers and a cDNA clone of human factor IX as template. The PCR product was then cloned into the pCR BluntII Topo vector (Invitrogen) and the sequence was verified. Following SmaI/XbaI digestion, the FIX gene was ligated into the pLD Ctv 5CP chloroplast transformation vector (14) to create pLD-CTB FIX (only a GPGP hinge between CTB and FIX) and pLD-CTB FFIX (with a GPGP hinge and furin cleavage site between CTB and FIX) vectors.
Regeneration of Transplastomic Plants and Confirmation of Transgene Integration by Southern Blot.
Transplastomic plants using chloroplast expression vectors pLD CTB-FIX and pLD CTB-FFIX were regenerated as described elsewhere (12, 22). Total plant DNA (1–2 μg) isolated from leaves using a Qiagen DNeasy Plant Mini Kit was digested with HindIII, and Southern blot analysis was carried out to confirm integration and determine homoplasmy as described previously (22). Southern blots were hybridized with a [32P]α[dCTP]-labeled chloroplast flanking sequence probe (0.81 kb) containing the trnI-trnA genes. The transplastomic plants with roots were transferred to a greenhouse as described previously (12).
Immunoblot Analysis.
Young, mature, and old leaves from transformed plants grown in a greenhouse were collected at 10 AM, 2 PM, and 6 PM. Chloroplast-derived CTB-FIX and CTB-FFIX proteins were extracted from 100 mg of powdered leaf material as described earlier (12, 22). Equal amounts of total protein along with known amounts of purified bacterial CTB (Sigma) were separated by SDS/PAGE and transferred to a nitrocellulose membrane by electroblotting. Immunoblotting with rabbit anti-CTB primary polyclonal antibody (1:3,000; Sigma) and horseradish peroxidase (HRP) -conjugated donkey anti-rabbit secondary antibody (1:5,000; Southernbiotech) was used for densitometric analysis (12, 16, 19). A SuperSignal West Pico HRP Substrate Kit (Pierce) was employed for detection of chemiluminescence signal by exposure to film. Fusion protein was also detected using goat anti-human factor IX (1:3,000; Enzyme Research) antibody followed by donkey anti-goat IgG-HRP-conjugated secondary antibody (1:5,000; Santa Cruz Biotechnology). Native PAGE was also carried out and fusion protein was detected as described above. GM1 receptor binding assays were performed as described previously (40).
Animal Experiments.
Male C3H/HeJ mice with targeted deletion of the F9 gene (hemophilia B mice, 8–10 weeks old at the onset of experiments) were used as published (26, 41). Animals were housed under special pathogen-free conditions at the University of Florida and treated under Institutional Animal Care and Use Committee-approved protocols. The leaf material for oral delivery was ground in liquid nitrogen with mortar and pestle and stored at −80 °C until the day of administration. Oral doses (250 mg each) of either untransformed or transgenic leaf material were resuspended in 200 μL of sterile PBS and homogenized on ice for 5 min with an OMNI International (GLH-2596) probe. The plant cell suspension was stored on ice until oral delivery via oral gavage using a tuberculin syringe and a 20-G bulb-tipped gastric gavage needle (Popper and Sons) while maintaining the animals under light anesthesia. Recombinant hF.IX (Benefix) was injected into the tail vein at 1 IU (4–5 μg hF.IX) per mouse. Plasma samples were collected by tail bleed into citrate buffer as described (26). For clearance studies, mice were bled from the retro-orbital plexus using heparinized microcapillary tubes. To block anaphylactic reactions, 150 μg antihistamine (triprolidine; Sigma) and 50 μg antiplatelet-activating factor (PAF) CV-3988 were coinjected i.v. (in 100 μL PBS) 5 min before administration of hF.IX.
Antigen and Antibody Measurements.
Plasma levels of hF.IX antigen were measured by ELISA and inhibitory antibody titers were determined by Bethesda assay as published (26). One Bethesda unit (BU) represents the degree of inhibition that results in 50% residual coagulation activity. Immunocapture assays to determine titers of hF.IX-specific Ig subclasses were as described using IgA, IgE, IgG1, IgG2a, and IgG2b standards from Sigma. To measure IgE titers, IgG was removed from plasma samples using protein G agarose (Pierce; Thermo Scientific) before addition to microtiter plates coated with hF.IX protein. Statistical comparisons between experimental groups were assessed with unpaired Student's t test. Differences were considered significant and reported with *P < 0.05, **P < 0.01, ***P < 0.001, and so on.
Immunohistochemistry.
Mice were fed with CTB-FFIX material (250 mg) twice per day for 2 days and killed 5 h after the last gavage, and tissue was collected as described (26). Cryosections (10-μm thick) were fixed in acetone for 5 min, air-dried, and then rehydrated in PBS. Sections were blocked with 2% donkey serum in PBS for 30 min. Goat α-hF.IX (1:400; Affinity Biologicals), rat α-F4/80 (clone: C1:A3-1; 1:200; AbD Serotec), and biotinylated-α-CD11c (1:200; BD Biosciences) were applied in 2% donkey serum for 30 min. After a washing, tissue sections were incubated with secondary antibody Alex Fluor-488 donkey α-rat IgG, Alex Fluor-568 donkey (or FITC) α-goat IgG, and streptavidin-Alexa Fluor-350 (1:100 dilution; Invitrogen). Some sections were incubated with FITC-labeled Ulex europaeus agglutinin (UEA-1; Vector Labs; 10 μg/mL) for 10 min before being washed and mounted with or without DAPI. Images were captured using a Nikon Eclipse 80i fluorescence microscope and Retiga 2000R digital camera (QImaging) and analyzed with Nikon Elements software.
Acknowledgments
We thank Clive Wasserfall and David Markusic for their help. This work was supported by NIH Grant R21 HL089813 to R.W.H. and H.D., R01 AI/HL51390 to R.W.H., and R01 GM 63879 to H.D.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.DiMichele DM. Inhibitor treatment in haemophilias A and B: Inhibitor diagnosis. Haemophilia. 2006;12(Suppl 6):37–41. doi: 10.1111/j.1365-2516.2006.01364.x. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh K, Shetty S. Immune response to FVIII in hemophilia A: An overview of risk factors. Clin Rev Allergy Immunol. 2009;37:58–66. doi: 10.1007/s12016-009-8118-1. [DOI] [PubMed] [Google Scholar]
- 3.DiMichele D. Inhibitor development in haemophilia B: An orphan disease in need of attention. Br J Haematol. 2007;138:305–315. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 4.Franchini M, et al. Anaphylaxis in patients with congenital bleeding disorders and inhibitors. Blood Coagul Fibrinolysis. 2009;20:225–229. doi: 10.1097/MBC.0b013e328329f265. [DOI] [PubMed] [Google Scholar]
- 5.Kadar JG, Schuster J, Hunzelmann N. IgE-mediated anaphylactic reaction to purified and recombinant factor VIII in a patient with severe haemophilia A. Haemophilia. 2007;13:104–105. doi: 10.1111/j.1365-2516.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- 6.Mohrenschlager M, Ollert M, Ring J. A study on serum IgE and clinical symptomatology of atopy in patients suffering from the lysosomal storage disorder Fabry disease. J Eur Acad Dermatol Venereol. 2008;22:692–695. doi: 10.1111/j.1468-3083.2008.02638.x. [DOI] [PubMed] [Google Scholar]
- 7.Nicolino M, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med. 2009;11:210–219. doi: 10.1097/GIM.0b013e31819d0996. [DOI] [PubMed] [Google Scholar]
- 8.Thorland EC, et al. Anaphylactic response to factor IX replacement therapy in haemophilia B patients: Complete gene deletions confer the highest risk. Haemophilia. 1999;5:101–105. [PubMed] [Google Scholar]
- 9.Warrier I, et al. Factor IX inhibitors and anaphylaxis in hemophilia B. J Pediatr Hematol Oncol. 1997;19:23–27. doi: 10.1097/00043426-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Terada K, Yagi Y, Niizuma T, Kataoka N. Is oral tolerance therapy possible for haemophilia with inhibitors? Vox Sang. 2001;80:61–62. doi: 10.1046/j.1423-0410.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 11.Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14:669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma D, Samson NP, Koya V, Daniell H. A protocol for expression of foreign genes in chloroplasts. Nat Protoc. 2008;3:739–758. doi: 10.1038/nprot.2007.522. [DOI] [PubMed] [Google Scholar]
- 13.De Cosa B, Moar W, Lee SB, Miller M, Daniell H. Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat Biotechnol. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts—Oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniell H, Ruiz G, Denes B, Sandberg L, Langridge L. Optimization of codon composi-tion and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol. 2009;9:33. doi: 10.1186/1472-6750-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruhlman T, Verma D, Samson N, Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010 doi: 10.1104/pp.109.152017. 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davoodi-Semiromi A, Samson N, Daniell H. The green vaccine: A global strategy to combat infectious and autoimmune diseases. Hum Vaccin. 2009;5:488–493. doi: 10.4161/hv.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limaye A, Koya V, Samsam M, Daniell H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 2006;20:959–961. doi: 10.1096/fj.05-5134fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davoodi-Semiromi A, et al. Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol J. 2010;8:223–242. doi: 10.1111/j.1467-7652.2009.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniell H, Datta R, Varma S, Gray S, Lee SB. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat Biotechnol. 1998;16:345–348. doi: 10.1038/nbt0498-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma D, Daniell H. Chloroplast vector systems for biotechnology applications. Plant Physiol. 2007;145:1129–1143. doi: 10.1104/pp.107.106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh ND, Ding Y, Daniell H. Chloroplast-derived vaccine antigens and bio-pharmaceuticals: Protocols for expression, purification, or oral delivery and functional evaluation. Methods Mol Biol. 2009;483:163–192. doi: 10.1007/978-1-59745-407-0_10. [DOI] [PubMed] [Google Scholar]
- 23.Arlen PA, et al. Field production and functional evaluation of chloroplast-derived interferon-α2b. Plant Biotechnol J. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Haan L, Verweij W, Agsteribbe E, Wilschut J. The role of ADP-ribosylation and G(M1)-binding activity in the mucosal immunogenicity and adjuvanticity of the Escherichia coli heat-labile enterotoxin and Vibrio cholerae cholera toxin. Immunol Cell Biol. 1998;76:270–279. doi: 10.1046/j.1440-1711.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji T, Watanabe K, Miyama A. Monomer of the B subunit of heat-labile enterotoxin from enterotoxigenic Escherichia coli has little ability to bind to GM1 ganglioside compared to its coligenoid. Microbiol Immunol. 1995;39:817–819. doi: 10.1111/j.1348-0421.1995.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 26.Cao O, et al. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17:1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelman FD. Anaphylaxis: Lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516–517. [DOI] [PubMed] [Google Scholar]
- 28.Rawle FE, et al. Induction of partial immune tolerance to factor VIII through prior mucosal exposure to the factor VIII C2 domain. J Thromb Haemost. 2006;4:2172–2179. doi: 10.1111/j.1538-7836.2006.02118.x. [DOI] [PubMed] [Google Scholar]
- 29.Arlen P, et al. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect Immun. 2008;76:3640–3650. doi: 10.1128/IAI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason HS, Warzecha H, Mor T, Arntzen CJ. Edible plant vaccines: Applications for prophylactic and therapeutic molecular medicine. Trends Mol Med. 2002;8:324–329. doi: 10.1016/s1471-4914(02)02360-2. [DOI] [PubMed] [Google Scholar]
- 31.Haq TA, Mason HS, Clements JD, Arntzen CJ. Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science. 1995;268:714–716. doi: 10.1126/science.7732379. [DOI] [PubMed] [Google Scholar]
- 32.Monahan PE. Factor IX: Insights from knock-out and genetically engineered mice. Thromb Haemost. 2008;100:563–575. [PubMed] [Google Scholar]
- 33.Sun B, et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine Pompe disease. Mol Ther. 2010;18:353–360. doi: 10.1038/mt.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dioun AF, Ewenstein BM, Geha RS, Schneider LC. IgE-mediated allergy and desensitization to factor IX in hemophilia B. J Allergy Clin Immunol. 1998;102:113–117. doi: 10.1016/s0091-6749(98)70061-2. [DOI] [PubMed] [Google Scholar]
- 35.von der Weid T, Bulliard C, Fritsche R. Suppression of specific and bystander IgE responses in a mouse model of oral sensitization to β-lactoglobulin. Int Arch Allergy Immunol. 2001;125:307–315. doi: 10.1159/000053831. [DOI] [PubMed] [Google Scholar]
- 36.Arruda VR, Favaro P, Finn JD. Strategies to modulate immune responses: A new frontier for gene therapy. Mol Ther. 2009;17:1492–1503. doi: 10.1038/mt.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao O, et al. Immune deviation by mucosal antigen administration suppresses gene-transfer-induced inhibitor formation to factor IX. Blood. 2006;108:480–486. doi: 10.1182/blood-2005-11-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daniell H. Molecular strategies for gene containment in transgenic crops. Nat Biotechnol. 2002;20:581–586. doi: 10.1038/nbt0602-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniell H. Transgene containment by maternal inheritance: Effective or elusive? Proc Natl Acad Sci USA. 2007;104:6879–6880. doi: 10.1073/pnas.0702219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniell H, Lee SB, Panchal T, Wiebe PO. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J Mol Biol. 2001;311:1001–1009. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin HF, Maeda N, Smithies O, Straight DL, Stafford DW. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood. 1997;90:3962–3966. [PubMed] [Google Scholar]