Abstract
G-protein heterotrimers, composed of a guanine nucleotide-binding Gα subunit and an obligate Gβγ dimer, regulate signal transduction pathways by cycling between GDP- and GTP-bound states. Signal deactivation is achieved by Gα-mediated GTP hydrolysis (GTPase activity) which is enhanced by the GTPase-accelerating protein (GAP) activity of “regulator of G-protein signaling” (RGS) proteins. In a cellular context, RGS proteins have also been shown to speed up the onset of signaling, and to accelerate deactivation without changing amplitude or sensitivity of the signal. This latter paradoxical activity has been variably attributed to GAP/enzymatic or non-GAP/scaffolding functions of these proteins. Here, we validated and exploited a Gα switch-region point mutation, known to engender increased GTPase activity, to mimic in cis the GAP function of RGS proteins. While the transition-state, GDP·AlF4 −-bound conformation of the G202A mutant was found to be nearly identical to wild-type, Gαi1(G202A)·GDP assumed a divergent conformation more closely resembling the GDP·AlF4 −-bound state. When placed within Saccharomyces cerevisiae Gα subunit Gpa1, the fast-hydrolysis mutation restored appropriate dose–response behaviors to pheromone signaling in the absence of RGS-mediated GAP activity. A bioluminescence resonance energy transfer (BRET) readout of heterotrimer activation with high temporal resolution revealed that fast intrinsic GTPase activity could recapitulate in cis the kinetic sharpening (increased onset and deactivation rates) and blunting of sensitivity also engendered by RGS protein action in trans. Thus Gα-directed GAP activity, the first biochemical function ascribed to RGS proteins, is sufficient to explain the activation kinetics and agonist sensitivity observed from G-protein–coupled receptor (GPCR) signaling in a cellular context.
Keywords: bioluminescence resonance energy transfer, GTPase-accelerating protein activity, Regulator of G-protein Signaling proteins, signal onset and recovery, signal sensitivity
Heterotrimeric G proteins regulate numerous signaling pathways that elicit critical physiological responses in many organisms from Dictyostelium and fungi to plants and metazoans (1 –3). The G-protein heterotrimer consists of a guanine nucleotide-binding Gα subunit that, in its GDP-bound state, is tightly associated with an obligate Gβγ dimer (4, 5). Agonist binding to seven-transmembrane, G-protein–coupled receptors (GPCRs) activates this complex by catalyzing exchange of GDP for GTP in Gα that leads to a conformation change and Gβγ dissociation (6 –8). Proper responses rely on timely deactivation of heterotrimer signaling, achieved by intrinsic Gα-mediated hydrolysis of GTP to GDP (GTPase activity). This hydrolysis is enhanced by “regulator of G-protein signaling” (RGS) proteins that serve as GTPase-accelerating proteins (GAPs) (9, 10). The RGS domain common to these proteins elicits GAP activity by stabilizing Gα in its transition-state intermediate form, thus lowering the required reaction free energy for GTP hydrolysis and subsequent return to the Gα·GDP state (11, 12).
Early studies of the cellular effects of RGS proteins on GPCR signaling described accelerated signal onset as well as accelerated decay, particularly for Gβγ-gated ion channel responses measured by whole-cell electrophysiological recordings, as reviewed elsewhere (13). For example, the slow, nonphysiological rate of K+ current onset, normally seen with inward-rectifying potassium (GIRK/Kir3) channels expressed in heterologous systems (CHO-K1 cells, Xenopus oocytes), was dramatically sharpened upon coexpression of RGS proteins such as RGS4 and RGS8. Surprisingly, no demonstrable changes in signal amplitude nor agonist sensitivity were apparent in these early reports (14, 15). Both groups labeled these findings paradoxical (14, 15) in light of the negative regulatory role first ascribed to RGS proteins (i.e., Gα-directed GAP activity).
Models have been put forth to account for this “paradoxical” regulatory role of RGS proteins in GPCR signaling. Many RGS proteins have multiple, modular protein domains in addition to the signature RGS domain, as previously reviewed (9, 16). Thus, a model of “physical scaffolding” (17, 18) suggests that RGS proteins use these accessory protein domains (and/or non-GAP motifs within the RGS domain) to mediate interactions that modulate receptor/heterotrimer coupling or other aspects of receptor/G-protein/effector signaling output (19). Alternatively, the “kinetic scaffolding” or “spatial focusing” (20) model eschews evoking additional functions for RGS proteins and relies on their GAP activity alone. This model suggests that G-protein activation becomes saturated near spatially constrained “clusters” of agonist-activated GPCRs, causing GTP hydrolysis, rather than GDP release, to become rate limiting in the nucleotide cycle. RGS domain GAP activity is proposed to enhance steady-state pathway activation by preventing local depletion of Gα·GDP, thus providing receptors with additional Gαβγ heterotrimers to activate. Therefore, by promoting continuous cycling of G-protein activity, as opposed to prolonging the Gα·GTP state, rapid GTP hydrolysis alone may provide a mechanism of accelerating both signal onset and decay and shaping signal sensitivity (19 –21).
We sought evidence from whole-cell GPCR signaling that could distinguish the contribution(s) of GAP and non-GAP functions of RGS proteins to the observations of accelerated kinetics, yet paradoxical lack of amplitude or sensitivity changes, attributed to RGS proteins. Our strategy was to impose the structural and functional changes in Gα brought about by RGS protein binding that enhance GTP hydrolysis, while avoiding other possible consequences of RGS protein binding (e.g., physical scaffolding). We used a Gα switch II point mutation (G202A in human Gαi1, G203A in human GαoA, G321A in Saccharomyces cerevisiae Gpa1) previously found to increase greatly the intrinsic rate of GTP hydrolysis (22). Our structural models obtained by x-ray crystallography show that the glycine-to-alanine substitution promotes a pretransition state mimicked by the Gα·GDP·AlF4 − complex (23), i.e., the same state most favored for RGS protein binding (11, 24). Placing this fast-GTPase mutation into the Gα integral to yeast pheromone sensing was sufficient to restore wild-type dose-responsive behavior in the absence of RGS domain GAP activity. Reconstituting neurotransmitter receptor signaling with fast-GTPase Gα accelerated both signal onset and decay and blunted sensitivity. This study thus provides experimental evidence that faster-than-wild-type GTP hydrolysis by Gα, whether accomplished via RGS protein GAP activity in trans or via accelerated intrinsic GTPase activity in cis, is sufficient to support rapid signal onset and altered sensitivity of agonist activation of GPCRs.
Results
Structures of Gαi1(G202A) Mutant Reveal a Pretransition State for GTP Hydrolysis.
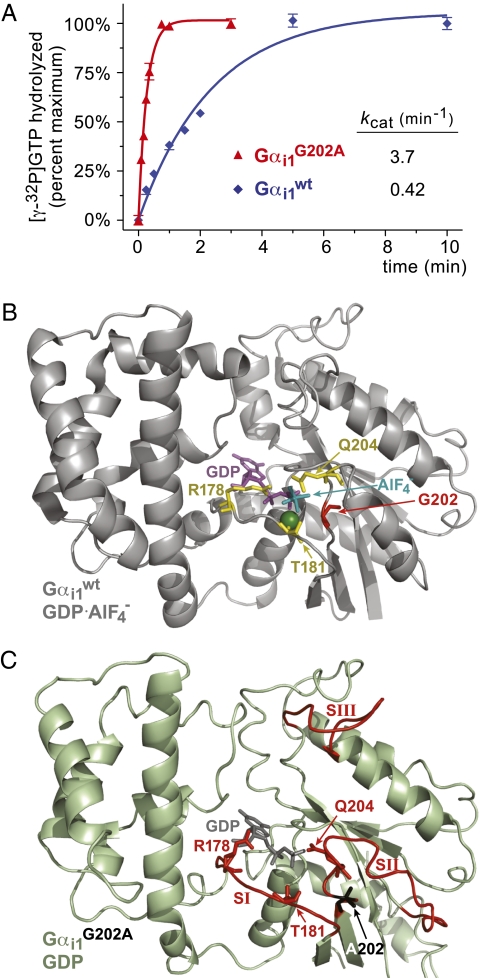
A glycine-to-alanine mutation at position 202 of the Gαi1 switch II region was originally identified as increasing the intrinsic GTPase activity at least 10-fold in vitro (22). We confirmed this enhanced GTPase activity in single-turnover [γ-32P]GTP hydrolysis assays (Fig. 1A ). RGS domains not only accelerate wild-type Gα GTPase activity, but also preferentially bind their transition-state mimetic form (Gα·GDP·AlF4 −) over the ground state (Gα·GDP) (24). Therefore, evidence of negligible RGS4-mediated enhancement of GTPase rate as well as increased RGS4 affinity for Gαi1(G202A)·GDP (KD 18 μM) vs. wild-type Gαi1·GDP (KD 430 μM) led Thomas et al. (22) to hypothesize that the G202A mutant, like RGS domain-bound Gα, is stabilized in a pretransition state for GTP hydrolysis, explaining its enhanced intrinsic GTPase activity. Here, to ascertain the structural determinants of enhanced GTP hydrolysis, we established structural models derived from x-ray crystallography for the GDP- and GDP·AlF4 −-bound forms of Gαi1(G202A) to 2.6 Å and 2.42 Å resolution, respectively; data collection and refinement statistics are listed in Table S1, and illustrations of model fit to experimental electron density are presented in Fig. S1.
Fig. 1.
G202A substitution in Gαi1 switch II leads to a pretransition state that accelerates intrinsic GTPase activity. (A) Single-turnover GTP hydrolysis assays were performed on ice using indicated recombinant wild-type and mutant Gα proteins, demonstrating the enhanced intrinsic GTPase rate of the Gαi1(G202A) mutant. The nearly order-of-magnitude GTPase rate enhancement observed with the G202A mutation is consistent with that reported by Thomas et al. (22); nevertheless, it must be acknowledged that some RGS proteins have been observed to accelerate Gα-mediated GTP hydrolysis by orders of magnitude under optimal conditions (24, 45). (B) Ribbon Cα tracing of the proposed transition state for GTP hydrolysis from the published structural model of wild-type Gαi1·GDP·AlF4 − (PDB id 1GFI; (23), highlighting the disposition of the three residues involved in GTP hydrolysis (Arg-178, Thr-181, Gln-204; yellow sticks), as well as the position of glycine-202 (red). GDP is colored magenta with the AlF4 − and magnesium ions colored teal and green, respectively. (C) Ribbon Cα tracing of our 2.6 Å structural model of GDP-bound Gαi1(G202A) derived from x-ray crystallography (PDB id 2PZ2). Switch regions (SI-SIII) are colored red, with the catalytic residues Arg-178, Thr-181, and Gln-204 depicted in red sticks and the mutant alanine-202 residue in black sticks.
Previous Gα structures, reviewed elsewhere (5, 7, 25), have detailed the conformational changes that three switch regions undergo during transition between GTP- and GDP-bound states. Key catalytic residues have also been identified, including a phosphate-neutralizing arginine and magnesium-coordinating threonine in switch-I (R178 and T181 in Gαi1), and a water-coordinating glutamine in switch II (Q204 in Gαi1). The side chains of these three residues are observed in their hydrolysis-coordinating roles within the structure of Gαi1 bound to GDP and to the planar ion AlF4 −, which induces a stable, transition-state mimetic form (PDB id 1GFI, ref (23); Fig. 1B ). The GDP·AlF4 −-bound conformation of the G202A mutant is nearly identical to wild-type Gαi1·GDP·AlF4 − (overall RMSD 0.93 Å; Fig. S2), with only slight alterations in the three switch regions.
Minor displacements in both the β2/α2 loop and switch I likely result from the G202A mutation itself to compensate for the additional methyl side chain of alanine. Despite these minor displacements, each of the catalytic residues (R178, T181, Q204) in Gαi1(G202A)·GDP·AlF4 − adopts an orientation similar to wild-type Gαi1·GDP·AlF4 − (i.e., an orientation fully competent for GTP hydrolysis).
Conversely, GDP-bound Gαi1(G202A) assumes a divergent conformation from that of wild-type Gαi1·GDP. In the GDP-bound state, switch II and switch III are normally unstructured and highly disordered when observed in crystal structures (26, 27); it is Gα activation, such as by GTP binding or addition of AlF4 −, that normally elicits structural order in the switch regions, including switch II adopting a rigid, α-helical conformation and switch III being suitably stabilized for visualization (23, 28). In contrast, GDP-bound Gαi1(G202A) displayed sufficient structural order and suitable electron density to model all three switch regions (e.g., Fig. 1C and Fig. S1A). Switch regions I and II of Gαi1(G202A)·GDP are strikingly similar to their conformation in the GDP·AlF4 −-bound state of both wild-type and G202A Gαi1, although minor alterations can be noted. Switch I does not approach the nucleotide-binding pocket as dramatically as in wild-type Gαi1·GDP·AlF4 − (Fig. S2); as previously stated, this may be in part due to the methyl side-chain of Ala-202 sterically hindering such a conformation. The catalytic R178 and T181 residues within switch I are, however, positioned similar to the wild-type Gαi1·GDP·AlF4 − structure (cf. Fig. 1 B vs. C; Fig. S2B). Whereas the switch II β3/α2 loop adopts a conformation somewhat distinct from wild-type Gαi1·GDP·AlF4 −, the catalytic Q204 residue is oriented toward nucleotide similar to the orientation seen in wild-type Gαi1·GDP·AlF4 − (Fig. 1B ). Together with the orientations of R178 and T181, this observation suggests that the G202A mutation causes allosteric conformational changes within Gα that orient key catalytic residues poised for GTP hydrolysis even when GDP-bound, confirming the hypothesis (22) that the G202A mutant is in a “pretransition” state resembling the changes brought about RGS domain binding (11, 29). Notwithstanding these structural changes to key catalytic residues, the G202A mutation did not appreciably affect the affinity of the GDP-loaded Gαi1 subunit for the Gβγ dimer (Fig. S3).
Glycine to Alanine Substitution in Switch II Accelerates Yeast Gpa1 GTPase Activity and Restores Wild-Type Pheromone Sensitivity to RGS-Insensitive Yeast.
Mating pheromone signaling in S. cerevisiae was one of the first signaling pathways shown to be regulated by an RGS protein (1, 30), with the yeast RGS protein Sst2 now well established as the principal trans-regulator of the pheromone response (31). Only one Gα subunit, Gpa1, is coupled to the yeast pheromone receptor, and it is highly similar to mammalian Gαi1, including a nearly identical switch II (Fig. S4A). A “RGS-insensitivity” point mutation within switch I of Gα was first identified in S. cerevisiae (namely, Gpa1G302S) (32); this mutation renders Gα insensitive to RGS domain GAP activity, but leaves unaffected its capacity for intrinsic GTP hydrolysis and coupling to Gβγ, receptors, and effectors (32, 33).
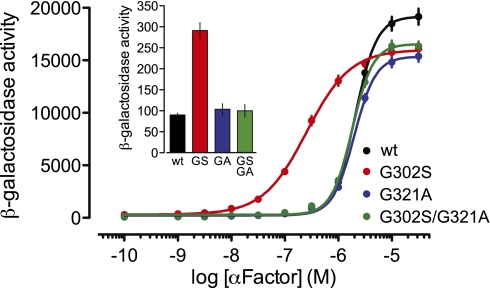
We first established that the fast-hydrolysis switch II mutation functions similarly in yeast Gpa1 (i.e., G321A). Single-turnover GTPase assays with recombinant Gpa1 demonstrated enhanced intrinsic GTPase activity for Gpa1(G321A) of at least 16-fold over wild-type (Fig. S4B); this rate enhancement was similar to that provided to wild-type Gpa1 by the RGS domain of Sst2. To assess the effect of enhanced intrinsic GTP hydrolysis on receptor-mediated signaling, pheromone response assays were carried out in a-haploid S. cerevisiae strains in which the wild-type GPA1 locus was replaced (via homologous recombination) with Gpa1 point mutants: either Gpa1G321A to provide faster-than-wild-type GTP hydrolysis, Gpa1G302S to render the protein RGS-insensitive, or Gpa1G302S/G321A to provide both activities. A pheromone pathway-specific lacZ reporter gene was used to assess dose-dependent changes in α-factor pheromone-induced transcriptional up-regulation (Fig. 2); pheromone signaling was independently measured using a “halo assay” (Fig. S5) indicative of pheromone-dependent growth inhibition (34, 35). Expression of RGS-insensitive Gpa1G302S led to a leftward-shifted pheromone dose–response curve, as well as larger halos and greater basal activity (Fig. 2 Inset; Fig. S5A), consistent with its original discovery as a “supersensitive allele” of Gpa1 (32) and demonstrating that endogenous Sst2 acts to decrease the sensitivity of pheromone receptor signaling through Gpa1. In contrast, expression of the fast-GTPase Gpa1G321A resulted in no change in basal activity or pheromone sensitivity, although a statistically significant, ∼20% decrease in maximal efficacy was noted (as also observed with the G302S allele) (Fig. S5C). Addition of faster-than-wild-type hydrolysis to the RGS-insensitive Gpa1 mutant (i.e., Gpa1G302S/G321A) was observed to supersede the effect of RGS-insensitivity in restoring wild-type basal activity and pheromone sensitivity (i.e., EC50), as well as the ultrasensitive nature of the response (i.e., Hill slope >1) (Fig. 2 and Fig. S5). Thus, the glycine-to-alanine fast hydrolysis mutation phenocopied the effects of the RGS protein Sst2 on agonist sensitivity.
Fig. 2.
The glycine-to-alanine switch II mutation Gpa1G321A restores wild-type pheromone sensitivity to RGS-insensitive yeast. Strain BY4741 of S. cerevisiae was transformed with integrating plasmids (pRS406) containing Gpa1WT, Gpa1G302S, Gpa1G321A, or Gpa1G302S/G321A as well as a plasmid (pRS423 FUS1-lacZ) containing the pheromone-inducible FUS1 promoter and lacZ reporter. Yeast grown to midlog growth phase were treated with the indicated concentrations of α-factor, and the resulting β-galactosidase activity was measured by fluorescence spectrophotometry. In the time frame of α-factor treatment and subsequent transcriptional readout (90 min), the yeast RGS protein Sst2 is induced (1). Data shown are the results of three independent experiments using multiple colonies (WT, n = 14; G302S, n = 14; G321A, n = 9; G302S/G321A, n = 13) performed in triplicate. Error bars, ±SE. (Inset) A similar transcriptional reporter experiment was conducted, but yeast were left untreated to measure basal β-galactosidase activity.
Glycine to Alanine Substitution in Switch II Restores Neurotransmitter Signaling Kinetics and Sensitivity to RGS-Insensitive GαoA.
To determine the effect of accelerating hydrolysis on GPCR signaling in mammalian cells, we used HEK 293 cells transiently expressing the D2 dopamine receptor (D2R) that couples to pertussis toxin (PTX)–sensitive Gαi/o proteins. Coupling to endogenous Gi/o heterotrimers was blocked by PTX pretreatment, and D2R signaling was reconstituted by expressing PTX-insensitive GαoA C351G. To measure GαoA activation, we used a recently developed bioluminescence resonance energy transfer (BRET) assay (36), wherein Gβγ dimers labeled with the YFP variant Venus (Gβ1γ2-V) are liberated by active Gα subunits and bind to a Renilla luciferase-labeled, membrane-associated C-terminal fragment of GRK3 (masGRKct-Rluc8). Energy transfer between masGRKct-Rluc8 and Gβ1γ2-V provides a biosensor of G-protein heterotrimer activation amenable to both steady-state measurements and time-resolved kinetic experiments (36).
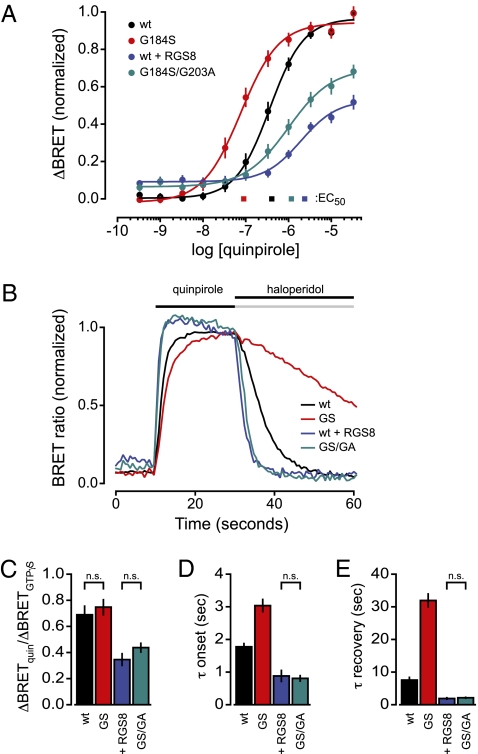
D2R activation with the agonist quinpirole (10−10–10−5 M) produced a graded increase in BRET between masGRKct-Rluc8 and Gβ1γ2-V (Fig. 3A ). In cells expressing PTX-insensitive GαoA, referred to here as wild-type (wt), the EC50 for this response was 405 ± 49 nM (n = 12). Agonist sensitivity was increased for responses mediated by RGS-insensitive GαoA G184S (EC50 = 90 ± 12 nM; n = 12; Fig. 3A ), suggesting that endogenous RGS proteins decrease agonist sensitivity in HEK 293 cells. Conversely, overexpression of RGS8 decreased agonist sensitivity even further (EC50 = 2.4 ± 0.4 μM; n = 8; Fig. 3A ), suggesting that wt Gα subunits are not saturated by endogenous RGS proteins. When the RGS insensitivity and fast hydrolysis mutations were combined (GαoA G184S/G203A), RGS insensitivity was preserved (Fig. S6), and yet the fast hydrolysis phenotype prevailed. Increased agonist sensitivity observed with the RGS insensitivity mutation alone was reversed by adding the fast hydrolysis mutation (EC50 = 1.2 ± 0.1 μM; Fig. 3A ). Responses to saturating concentrations of quinpirole were increased further by addition of hydrolysis-resistant GTPγS (Fig. 3C ), indicating that the reporter of heterotrimer activation (masGRKct-Rluc8) was not saturated and, thus, the maximum response should be sensitive to changes in Gα GTPase rate. Indeed, when compared with responses mediated by either wt GαoA or RGS-insensitive GαoA subunits, maximum responses after RGS8 overexpression and for RGS-insensitive/fast hydrolysis double mutants were significantly decreased (P < 0.01; repeated-measures ANOVA; Fig. 3C ). Importantly, maximal responses were equally blunted when GTP hydrolysis was accelerated by saturating RGS8 or by the fast hydrolysis mutation (P > 0.05; Fig. 3C ). These results are thus consistent with those observed for yeast pheromone signaling, and indicate that changes in Gα GTPase rate in both systems produce shifts in agonist sensitivity and response amplitude.
Fig. 3.
The glycine-to-alanine switch II mutation G203A restores rapid onset and recovery kinetics, as well as steady-state agonist sensitivity, to responses mediated by RGS-insensitive GαoA in HEK 293 cells. (A) Increases in BRET (ΔBRET) between masGRKct-Rluc8 and Gβ1γ2-V upon dopamine D2R activation are plotted against the concentration of the agonist quinpirole. Cells were pretreated with pertussis toxin (PTX) to inactivate native Gαi/o subunits, and responses were mediated by PTX-insensitive GαoA subunits: wild-type (wt), RGS-insensitive (G184S), or RGS-insensitive/fast hydrolysis double mutant (G184S/G203A; GS/GA). “+ RGS8” denotes ectopic overexpression of RGS8 in cells coexpressing PTX-insensitive, wt GαoA. Responses are normalized to the maximum response elicited by wt GαoA in the absence of RGS8 overexpression. (B) Normalized BRET between masGRKct-Rluc8 and Gβ1γ2-V is plotted against time during sequential addition of quinpirole (30 μM) and haloperidol (10 μM) for each of the GαoA variants. Traces represent the mean of 9–11 experiments, and are normalized to the response immediately before addition of haloperidol. (C) Maximal responses, elicited by saturating quinpirole (33 μM; “quin”) normalized to the responses elicited by permeabilization and addition of GTPγS, are plotted for the same four GαoA variants; n = 5, performed in quadruplicate; n.s., P > 0.05. (D) Average onset time constants (τ onset) plotted for each of the GαoA variants (± SEM, n = 5–12, performed in quadruplicate). (E) Average recovery time constants (τ recovery) plotted for each of the GαoA variants (±SEM, n = 3–10, performed in quadruplicate); n.s., P > 0.05; for C–E, all pairwise comparisons not marked “n.s.” were significantly different (P < 0.05, ANOVA).
We then took advantage of the ability of the BRET assay to indicate rapid changes in heterotrimeric G-protein activity to examine rates of response onset and recovery mediated by these GαoA mutants. BRET between Gβ1γ2-V and masGRKct-Rluc8 was monitored during sequential addition of saturating concentrations of quinpirole (30 μM) and the antagonist haloperidol (10 μM). Both the onset and recovery of responses mediated by RGS-insensitive GαoA G184S subunits were slowed when compared with wild-type (P < 0.01; n = 3–12 performed in quadruplicate; Fig. 3B ), as seen previously in numerous cell types (37, 38). In contrast, overexpression of RGS8 accelerated both onset and recovery of responses mediated by wt GαoA, again suggesting endogenous RGS proteins do not saturate these subunits (Fig. 3 B, D, and E ). Once again, when the RGS insensitivity and fast hydrolysis mutations were combined, fast hydrolysis compensated for RGS insensitivity. Both response onset and recovery mediated by G184S/G203A double mutant subunits were significantly faster than those observed with wild-type GαoA or RGS-insensitive GαoA (P < 0.01; Fig. 3 B, D, and E ), and were indistinguishable from those observed with RGS8 overexpression (P > 0.05; Fig. 3 D and E ). Similar effects were observed using analogous PTX-insensitive Gαi1 mutants in reconstituted signaling by the M4 muscarinic acetylcholine receptor, i.e., the fast hydrolysis mutation compensated for the loss of RGS sensitivity (Fig. S7 and Table S2). In this case, agonist (carbachol) sensitivity was less affected by changes in hydrolysis, similar to previous reports with native PTX-sensitive Gα subunits (14, 15). Taken together, these results indicate that accelerating GTP hydrolysis with a mutation that promotes a pretransition state for GTP hydrolysis reproduces the effects of RGS protein binding on agonist sensitivity and kinetics in mammalian cells.
Discussion
The discovery of RGS proteins resolved a longstanding discrepancy between the slow intrinsic GTPase rate of Gα subunits seen in vitro and rapid termination of heterotrimeric G-protein signals seen in vivo (39, 40). However, early studies demonstrating this important role of RGS proteins for timely response termination (14, 15) described new puzzling observations. By increasing the rate of GTP hydrolysis, RGS proteins were expected to speed response recovery, and this expectation was fulfilled. It was also expected that accelerating GTP hydrolysis would decrease steady-state agonist sensitivity, but this was not observed (14, 15). It appeared that the reason for the maintained agonist sensitivity in these studies was an unexpectedly large increase in the rate of response onset. The authors of these studies concluded that RGS proteins increased not only the rate of G-protein deactivation, but also the rate of G-protein activation, the latter so much so that agonist sensitivity and response amplitude were left unchanged (14, 15).
Two primary models have been proposed to account for RGS-protein–mediated acceleration of response onset. The first of these stems from the observation that RGS proteins can interact directly or indirectly with GPCRs in addition to G proteins, as reviewed elsewhere (16, 18, 41). Thus RGS proteins might facilitate GPCR coupling to G proteins by directly promoting their physical interaction, i.e., by serving as physical scaffolds. The second model is similar to that originally proposed for activation of the Gαq-effector and -GAP protein PLCβ (42), and suggests that rapid hydrolysis allows for rapid reactivation of Gα by active receptor (9, 19, 20, 43). Such a mechanism could promote coupling by reducing the need for GPCRs and G proteins to associate by slow diffusion and collision events. The positive effect of RGS proteins because of this kinetic scaffolding mechanism would depend entirely on acceleration of GTP hydrolysis. A third potential mechanism, GAP-mediated enhancement of receptor-stimulated GDP release, was proposed recently based on modeling studies incorporating steady-state GTPase activity (19). Experimental data consistent with these models have been reported (17, 20, 44, 45). However, little experimental evidence was previously available to directly support or exclude any mechanism.
In the present study, we show that a mutation that imposes a pretransition state and accelerates GTP hydrolysis mimics the influence of RGS proteins on response kinetics and agonist sensitivity. Therefore, the GAP function of RGS proteins is sufficient to explain both the increase in the rate of deactivation and (independently) the rate of response onset. Our study leaves the underlying mechanism for this independent acceleration of response onset unresolved and thus our understanding of the GAP function of RGS proteins remains incomplete. Our findings are generally consistent with, but do not definitively establish, a kinetic scaffolding model and/or other positive regulatory mechanisms that depend only on structural changes in Gα. Furthermore, our results do not exclude the possibility that RGS proteins may also serve as physical scaffolds, perhaps as a means of achieving receptor-selective regulation (18, 46). However, our results do imply that physical scaffolding or other non-GAP functions of RGS proteins are not necessary to explain the effects of RGS proteins on response kinetics or agonist sensitivity.
Materials and Methods
Protein Purification, Single Turnover GTPase Assays, and Structure Determinations.
Recombinant Gpa1 (aa 39–472) and Sst2 RGS domain (aa 408–698) were each subcloned with N-terminal His6- and TEV-cleavage sites in a modified pET expression vector (Novagen). His6/TEV-tagged human Gαi1, both wild-type and G202A mutant, and the two yeast proteins were separately purified from clarified Escherichia coli lysate using sequential Ni-NTA affinity, TEV cleavage, anion exchange, and size-exclusion chromatographies essentially as previously described (47). Single-turnover GTPase assays using recombinant Gα subunits were conducted essentially as described previously (48, 49). For crystallization experiments, purified Gαi1(G202A) protein was concentrated to 10 mg/mL and stored in crystallization buffer (50 mM Hepes pH 8, 5 mM DTT, 1 mM EDTA, 1 mM MgCl2, and 100 μM GDP) with or without added AlCl3 (30 μM) and NaF (10 mM). Details of crystal growth and structure determinations are presented in SI Materials and Methods.
Pheromone Transcriptional Reporter Assay and Halo Assay of Growth Inhibition.
Standard methods for manipulating S. cerevisiae expression plasmid DNA and the growth, maintenance, and transformation of yeast were used throughout, as previously described (34, 35). Yeast strains bearing integrated gpa1 mutation(s), created by homologous recombination and validated by PCR as described in SI Materials and Methods, were transformed with the transcriptional reporter plasmid pRS423 FUS1-lacZ, and β-galactosidase activity was determined as described elsewhere (34, 35). The pheromone-induced growth inhibition assay has also been described previously (50).
BRET Measurements.
Agonist-dependent cellular measurements of bioluminescence resonance energy transfer between masGRKct-Rluc8 and Gβ1γ2-V were performed as previously described (36). Details, including curve-fitting examples (Fig. S8), are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Henrik G. Dohlman (UNC) for advising on the yeast work by S.D.C., Ryan Shanks for initial input into the project, and Dr. Jason Snyder of the UNC Protein Core for the ΔN38-Gpa1 expression vector and assistance with generating biotinylated Gβγ. This work was funded by National Institutes of Health Grants R01 GM078319 (to N.A.L.) and R01 GM082892 (to D.P.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: Atomic coordinates are present in the Protein Data Bank, www.pdb.org [PDB id 2PZ2 for Gαi1(G202A)·GDP and 2PZ3 for Gαi1(G202A)·GDP·AlF4 −].
This article contains supporting information online at www.pnas.org/cgi/content/full/0912934107/DCSupplemental.
References
- 1.Dohlman HG, Thorner JW. Regulation of G protein-initiated signal transduction in yeast: Paradigms and principles. Annu Rev Biochem. 2001;70:703–754. doi: 10.1146/annurev.biochem.70.1.703. [DOI] [PubMed] [Google Scholar]
- 2.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 3.Johnston CA, Willard MD, Kimple AJ, Siderovski DP, Willard FS. A sweet cycle for Arabidopsis G-proteins: Recent discoveries and controversies in plant G-protein signal transduction. Plant Signal Behav. 2008;3:1067–1076. doi: 10.4161/psb.3.12.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilman AG. G proteins: Transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 5.Siderovski DP, Kimple AJ, Willard FS. In: Wiley Encyclopedia of Chemical Biology. Begley T, editor. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 6.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat Struct Mol Biol. 2006;13:772–777. doi: 10.1038/nsmb1129. [DOI] [PubMed] [Google Scholar]
- 7.Johnston CA, Siderovski DP. Receptor-mediated activation of heterotrimeric G-proteins: Current structural insights. Mol Pharmacol. 2007;72:219–230. doi: 10.1124/mol.107.034348. [DOI] [PubMed] [Google Scholar]
- 8.Digby GJ, Lober RM, Sethi PR, Lambert NA. Some G protein heterotrimers physically dissociate in living cells. Proc Natl Acad Sci USA. 2006;103:17789–17794. doi: 10.1073/pnas.0607116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 10.Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov. 2002;1:187–197. doi: 10.1038/nrd747. [DOI] [PubMed] [Google Scholar]
- 11.Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4—activated G(i alpha1): Stabilization of the transition state for GTP hydrolysis. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 12.Berman DM, Kozasa T, Gilman AG. The GTPase-activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J Biol Chem. 1996;271:27209–27212. doi: 10.1074/jbc.271.44.27209. [DOI] [PubMed] [Google Scholar]
- 13.Zerangue N, Jan LY. G-protein signaling: Fine-tuning signaling kinetics. Curr Biol. 1998;8:R313–R316. doi: 10.1016/s0960-9822(98)70196-4. [DOI] [PubMed] [Google Scholar]
- 14.Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of gbetagamma-activated inwardly rectifying K+ channels. Proc Natl Acad Sci USA. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 1997;390:525–529. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- 16.Willard MD, Willard FS, Siderovski DP. In: Handbook of Cell Signaling. Bradshaw R, Dennis E, editors. San Diego: Elsevier; 2008. [Google Scholar]
- 17.Benians A, Nobles M, Hosny S, Tinker A. Regulators of G-protein signaling form a quaternary complex with the agonist, receptor, and G-protein. A novel explanation for the acceleration of signaling activation kinetics. J Biol Chem. 2005;280:13383–13394. doi: 10.1074/jbc.M410163200. [DOI] [PubMed] [Google Scholar]
- 18.Neitzel KL, Hepler JR. Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling. Semin Cell Dev Biol. 2006;17:383–389. doi: 10.1016/j.semcdb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Turcotte M, Tang W, Ross EM. Coordinate regulation of G protein signaling via dynamic interactions of receptor and GAP. PLOS Comput Biol. 2008;4:e1000148. doi: 10.1371/journal.pcbi.1000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong H, et al. A spatial focusing model for G protein signals. Regulator of G protein signaling (RGS) protien-mediated kinetic scaffolding. J Biol Chem. 2003;278:7278–7284. doi: 10.1074/jbc.M208819200. [DOI] [PubMed] [Google Scholar]
- 21.Mukhopadhyay S, Ross EM. Quench-flow kinetic measurement of individual reactions of G-protein-catalyzed GTPase cycle. Methods Enzymol. 2002;344:350–369. doi: 10.1016/s0076-6879(02)44727-1. [DOI] [PubMed] [Google Scholar]
- 22.Thomas CJ, et al. Uncoupling conformational change from GTP hydrolysis in a heterotrimeric G protein alpha-subunit. Proc Natl Acad Sci USA. 2004;101:7560–7565. doi: 10.1073/pnas.0304091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman DE, et al. Structures of active conformations of Gi alpha 1 and the mechanism of GTP hydrolysis. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- 24.Popov S, Yu K, Kozasa T, Wilkie TM. The regulators of G protein signaling (RGS) domains of RGS4, RGS10, and GAIP retain GTPase activating protein activity in vitro. Proc Natl Acad Sci USA. 1997;94:7216–7220. doi: 10.1073/pnas.94.14.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprang SR, Chen Z, Du X. Structural basis of effector regulation and signal termination in heterotrimeric Galpha proteins. Adv Protein Chem. 2007;74:1–65. doi: 10.1016/S0065-3233(07)74001-9. [DOI] [PubMed] [Google Scholar]
- 26.Lambright DG, Noel JP, Hamm HE, Sigler PB. Structural determinants for activation of the alpha-subunit of a heterotrimeric G protein. Nature. 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- 27.Mixon MB, et al. Tertiary and quaternary structural changes in Gi alpha 1 induced by GTP hydrolysis. Science. 1995;270:954–960. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 28.Noel JP, Hamm HE, Sigler PB. The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature. 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- 29.Soundararajan M, et al. Structural diversity in the RGS domain and its interaction with heterotrimeric G protein alpha-subunits. Proc Natl Acad Sci USA. 2008;105:6457–6462. doi: 10.1073/pnas.0801508105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan RK, Otte CA. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982;2:21–29. doi: 10.1128/mcb.2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chasse SA, et al. Genome-scale analysis reveals Sst2 as the principal regulator of mating pheromone signaling in the yeast Saccharomyces cerevisiae. Eukaryot Cell. 2006;5:330–346. doi: 10.1128/EC.5.2.330-346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiBello PR, et al. Selective uncoupling of RGS action by a single point mutation in the G protein alpha-subunit. J Biol Chem. 1998;273:5780–5784. doi: 10.1074/jbc.273.10.5780. [DOI] [PubMed] [Google Scholar]
- 33.Lan KL, et al. A point mutation in Galphao and Galphai1 blocks interaction with regulator of G protein signaling proteins. J Biol Chem. 1998;273:12794–12797. doi: 10.1074/jbc.273.21.12794. [DOI] [PubMed] [Google Scholar]
- 34.Chasse SA, Dohlman HG. Identification of yeast pheromone pathway modulators by high-throughput agonist response profiling of a yeast gene knockout strain collection. Methods Enzymol. 2004;389:399–409. doi: 10.1016/S0076-6879(04)89024-4. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman GA, Garrison TR, Dohlman HG. Analysis of RGS proteins in Saccharo-myces cerevisiae. Methods Enzymol. 2002;344:617–631. doi: 10.1016/s0076-6879(02)44744-1. [DOI] [PubMed] [Google Scholar]
- 36.Hollins B, Kuravi S, Digby GJ, Lambert NA. The c-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell Signal. 2009;21:1015–1021. doi: 10.1016/j.cellsig.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Lambert NA. Endogenous regulators of G protein signaling proteins regulate presynaptic inhibition at rat hippocampal synapses. Proc Natl Acad Sci USA. 2000;97:12810–12815. doi: 10.1073/pnas.230260397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong SW, Ikeda SR. Endogenous regulator of G-protein signaling proteins modify N-type calcium channel modulation in rat sympathetic neurons. J Neurosci. 2000;20:4489–4496. doi: 10.1523/JNEUROSCI.20-12-04489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arshavsky VY, Pugh EN., Jr Lifetime regulation of G protein-effector complex: Emerging importance of RGS proteins. Neuron. 1998;20:11–14. doi: 10.1016/s0896-6273(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 40.Chen CK, et al. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 2000;403:557–560. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- 41.Sierra DA, Popov S, Wilkie TM. Regulators of G-protein signaling in receptor complexes. Trends Cardiovasc Med. 2000;10:263–268. doi: 10.1016/s1050-1738(00)00072-4. [DOI] [PubMed] [Google Scholar]
- 42.Biddlecome GH, Berstein G, Ross EM. Regulation of phospholipase C-beta1 by Gq and m1 muscarinic cholinergic receptor. Steady-state balance of receptor-mediated activation and GTPase-activating protein-promoted deactivation. J Biol Chem. 1996;271:7999–8007. doi: 10.1074/jbc.271.14.7999. [DOI] [PubMed] [Google Scholar]
- 43.Smith B, et al. Dual positive and negative regulation of GPCR signaling by GTP hydrolysis. Cell Signal. 2009;21:1151–1160. doi: 10.1016/j.cellsig.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Jaén C, Doupnik CA. RGS3 and RGS4 differentially associate with G protein-coupled receptor-Kir3 channel signaling complexes revealing two modes of RGS modulation. Precoupling and collision coupling. J Biol Chem. 2006;281:34549–34560. doi: 10.1074/jbc.M603177200. [DOI] [PubMed] [Google Scholar]
- 45.Mukhopadhyay S, Ross EM. Rapid GTP binding and hydrolysis by G(q) promoted by receptor and GTPase-activating proteins. Proc Natl Acad Sci USA. 1999;96:9539–9544. doi: 10.1073/pnas.96.17.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willard MD, et al. Selective role for RGS12 as a Ras/Raf/MEK scaffold in nerve growth factor-mediated differentiation. EMBO J. 2007;26:2029–2040. doi: 10.1038/sj.emboj.7601659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimple RJ, et al. RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor activity. J Biol Chem. 2001;276:29275–29281. doi: 10.1074/jbc.M103208200. [DOI] [PubMed] [Google Scholar]
- 48.Johnston CA, et al. Minimal determinants for binding activated G alpha from the structure of a G alpha(i1)-peptide dimer. Biochemistry. 2006;45:11390–11400. doi: 10.1021/bi0613832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross EM. Quantitative assays for GTPase-activating proteins. Methods Enzymol. 2002;344:601–617. doi: 10.1016/s0076-6879(02)44743-x. [DOI] [PubMed] [Google Scholar]
- 50.Sprague GF., Jr Assay of yeast mating reaction. Methods Enzymol. 1991;194:77–93. doi: 10.1016/0076-6879(91)94008-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.