Abstract
Although populations of amphibians are declining worldwide, there is no evidence that salamanders occupying small streams are experiencing enigmatic declines, and populations of these species seem stable. Theory predicts that dispersal through multiple pathways can stabilize populations, preventing extinction in habitat networks. However, empirical data to support this prediction are absent for most species, especially those at risk of decline. Our mark-recapture study of stream salamanders reveals both a strong upstream bias in dispersal and a surprisingly high rate of overland dispersal to adjacent headwater streams. This evidence of route-dependent variation in dispersal rates suggests a spatial mechanism for population stability in headwater-stream salamanders. Our results link the movement behavior of stream salamanders to network topology, and they underscore the importance of identifying and protecting critical dispersal pathways when addressing region-wide population declines.
Keywords: amphibian decline, dendritic ecological network, stream salamander, plethodontidae, movement
Despite the implications of spatial structure for the evolution of dispersal and resulting dynamics (1, 2), quantifying movement in real population networks has proven tremendously difficult (3). Of particular importance are specific, rarely observed dispersal pathways that are vital to network dynamics (2, 4), species distributions (5, 6), and population stability (7, 8). In theoretical network models, the propensity for small amounts of dispersal through multiple pathways is especially critical for population stability and metapopulation persistence (4, 9). However, empirical data for such dispersal probabilities in many organisms, particularly amphibians (10), are lacking.
Large-scale, enigmatic declines of amphibians have been documented worldwide (11), but existing data on headwater-stream salamanders in the Appalachian Mountains of eastern North America suggest stable populations. Estimated local extinction rates are near zero for species living in these habitats, and there are significantly smaller population fluctuations (in both change in population size and variance in population sizes) than for either pond- or river-breeding amphibians (12). Given ongoing modifications to the geometry and dynamics of riverine networks (13, 14) and the potential for large-scale changes in stream-network structure because of climate and landscape change (15), understanding the link between dispersal and network geometry may be crucial to addressing future impacts on demography, evolution, and community assembly in stream amphibians and other freshwater species.
The hierarchical geometry of stream systems distinguishes these ecological networks from other spatially structured habitats (16). In such dendritic networks, dispersal can follow two pathways: along network branches (within-network movement) or overland between branches (out-of-network movement). Variation in dispersal pathways may be related to species- or age-specific habitat associations, and may have strong effects on population persistence, evolutionary dynamics, and patterns of community composition.
In species that are restricted to network branches, dispersal is constrained by network structure (17), and consequently, population stability and local extinction risk are predicted to be highly sensitive to network position and direct connections among occupied branches (18). A capacity for overland dispersal between branches is predicted to increase population stability and decrease extinction risk significantly by providing periodic connectivity among spatially isolated reaches (4, 9). In the only reported decline that we could find in a stream-associated plethodontid (19), populations that were close in overland distance showed high genetic divergence, indicating that the overland movement pathway was not used in this population.
Because habitat conditions vary along the stream continuum (20), dispersal between branches at the same hierarchical level also reduces the probability of settlement in new and inhospitable habitat. Furthermore, dispersal distances between branches are generally shorter by overland routes than along stream corridors, especially in the uppermost reaches of river networks (i.e., the headwaters). This suggests that headwater specialists, including many amphibians and invertebrates, should evolve upstream-biased movement and overland dispersal strategies (21, 22).
Plethodontids (the family of lungless salamanders) have their greatest species diversity in upland, headwater areas of North and Central America, including the Appalachian Mountains (23). Stream-associated plethodontids have a complex life cycle (24) where aquatic larvae metamorphose into juveniles that, like adults, can be largely terrestrial. This life cycle allows the possibility of different movement behavior in each age class. In members of the genus Desmognathus, a dominant genus of plethodontids, the larval stage is brief and unlikely to allow significant dispersal within a stream network, but large postmetamorphic animals may disperse overland with less desiccation risk. Theoretical models and empirical data make clear that juveniles are more likely to disperse than adults in most species, reducing both kin competition and the potential for inbreeding (25). We would, therefore, expect postmetamorphic juvenile salamanders to have higher dispersal probabilities than adults.
We combined theory on spatially structured networks, particularly dendritic networks, with empirical observations of marked animals to test the following predictions: (i) stream salamanders use multiple movement pathways, both within-stream dispersal (the primary pathway) and overland dispersal (the secondary pathway), (ii) juveniles have higher dispersal probabilities than other life stages, and (iii) dispersal through these multiple pathways stabilizes stream salamander populations. Specifically, with data from 491 D. fuscus and 2,043 D. monticola that were individually marked over a 2-year period in stream networks in Virginia, we directly quantified within-stream and overland dispersal probabilities. To investigate the consequences of the observed dispersal probabilities in dendritic metapopulations, we then conducted a simulation study in a fractal branching network of 15 habitat patches under different annual per-patch extinction probabilities.
Results
We first tested if D. fuscus and D. monticola differed in their relative movement behavior. For example, one species may prefer to move along the stream channel, whereas the other may make overland movements. A likelihood ratio test indicated little support for species-specific differences in movement rates (χ2 = 4.22; df = 2; P = 0.121), although a model specifying a different overall propensity for movement for each species provided a better fit to the data than one that estimated a single rate for each pathway (χ2 = 15.609; df = 3; P = 0.001). Investigation of the model-averaged point estimates for each dispersal probability indicated that D. fuscus has higher probabilities of moving via any given pathway than D. monticola (Fig. S1), although the estimate of the effect has a large SE. The results from these tests indicate that the movement ecology of the two desmognathine salamanders is similar and lends support to our hypothesis that the movement ecology of these species is related to their life-history characteristics. We, therefore, combined the data from both species for the remainder of our analysis. Two of five candidate models representing our hypotheses about stream salamander dispersal were supported by the data and had a combined model weight of 93% (Table 1). We calculated model-averaged estimates of the transition probabilities (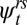 ) that were not conditional on a single model in the set (26).
) that were not conditional on a single model in the set (26).
Table 1.
Candidate model set
| Model | ΔAICc | w | Description |
| ψ (All movement pathways different) | 1.96 | 0.25 | Different overland movement for upper and lower reaches allowed different probabilities for movements to and from the adjacent reach. Within-stream movements were estimated separately. |
| ψ (All overland movements different; upstream = downstream) | 11.56 | 0.00 | Different overland movement probabilities for upper and lower reaches allowed different probabilities for movements to and from the adjacent reach. No bias was shown in within-stream movement. |
| ψ (Overland from upper ≠ lower; upstream ≠ downstream) | 0.00 | 0.67 | Different overland movements from upper and lower reaches are shown; overland movement to and from the adjacent reach was not estimated separately. Within-stream movements were estimated separately. |
| ψ (Overland from upper ≠ lower; upstream = downstream) | 14.57 | 0.00 | Different overland movements from the upper and lower reaches are shown; overland movement to and from the adjacent reach was not estimated separately. No bias was shown in within-stream movement. |
| ψ (Overland from upper = lower; upstream ≠ downstream) | 4.55 | 0.07 | Movement overland from the upper reach was equivalent to overland movement from the lower reach. Within-stream movements were estimated separately. |
Candidate model set showing constraints used on the transition probability parameter (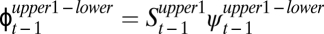 ), including the ΔAICc values and associated model weights (w) for each model. All models included the most parsimonious structure on p [stream reach, temporary removal pass (1–3), and sampling month] and
), including the ΔAICc values and associated model weights (w) for each model. All models included the most parsimonious structure on p [stream reach, temporary removal pass (1–3), and sampling month] and 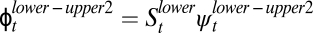 (age class and sampling month), and we estimated a separate movement probability for each type of age transition. We used model-averaging calculations to combine estimates from each model using their associated model weights (w) to provide an estimate of the predicted effect (Fig. 1).
(age class and sampling month), and we estimated a separate movement probability for each type of age transition. We used model-averaging calculations to combine estimates from each model using their associated model weights (w) to provide an estimate of the predicted effect (Fig. 1).
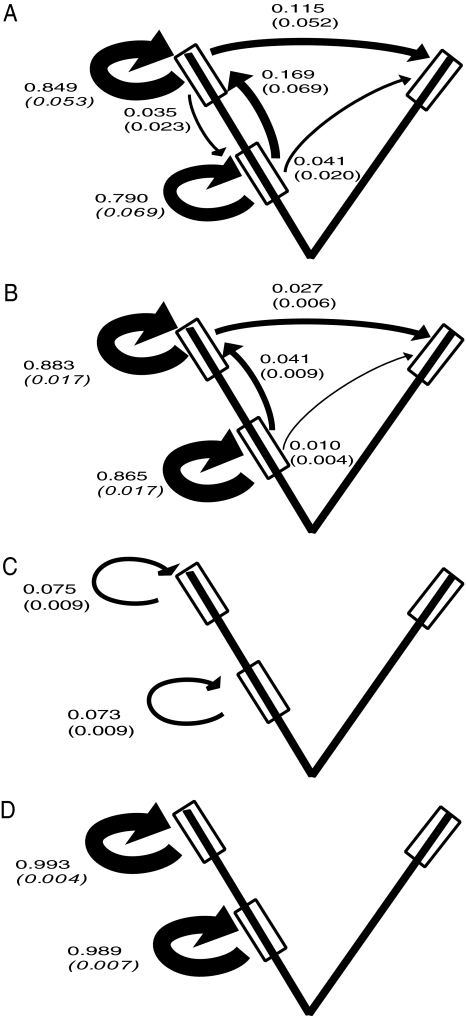
Newly metamorphosed juveniles (i.e., those individuals that were larvae in time t−1) had the highest probabilities of dispersing to other stream reaches; both a strong upstream bias in dispersal and a moderate probability of dispersing overland between reaches were apparent (Fig. 1A). Postmetamorphic juveniles (i.e., those individuals that were juveniles in time t−1) had higher site fidelity and lower probabilities of dispersing between reaches. However, like newly metamorphosed juveniles, they were more likely to move upstream than downstream and had a nonnegligible probability of dispersing overland to an adjacent reach (Fig. 1B). Adults exhibited the highest site fidelity with dispersal probabilities near 0 (Fig. 1 C and D).
Fig. 1.
Observed movement probabilities. Model-averaged monthly movement-age transition probabilities (and unconditional SEs) are shown. The letters refer to the type of age transition. (A) Larval to juvenile. (B) Remain juvenile. (C) Juvenile to adult. (D) Remain adult. Only movement probabilities ≥0.01 are shown for movements originating from the upper and lower reaches of the focal stream. The boxes represent the 40-m reaches, separated by 350–500 m along the stream or overland (figure not drawn to scale; distances along the stream channel were equal to the overland distance in each stream network sampled).
We were not able to explicitly separate overland from within-network movements between the uppermost reaches of our stream pairs. However, to travel between the two uppermost reaches along the stream corridors, an individual would have to survive and move first from the upper to the lower reach (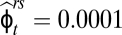 ) and then survive and move from the lower reach to the adjacent upper reach (
) and then survive and move from the lower reach to the adjacent upper reach (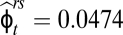 ). Across all age classes, this sequence of probabilities (
). Across all age classes, this sequence of probabilities (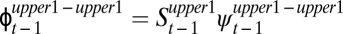 ) is much lower than the overland-movement probability calculated for the same two time periods (
) is much lower than the overland-movement probability calculated for the same two time periods (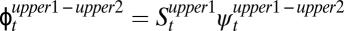 ): an individual could either survive and remain in the uppermost reach (
): an individual could either survive and remain in the uppermost reach (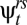 ) and then survive and move to the adjacent reach (
) and then survive and move to the adjacent reach (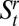 ) or vice versa. This suggests that movement between upper reaches along stream corridors is possible, but that such movements are of minor significance relative to overland movements.
) or vice versa. This suggests that movement between upper reaches along stream corridors is possible, but that such movements are of minor significance relative to overland movements.
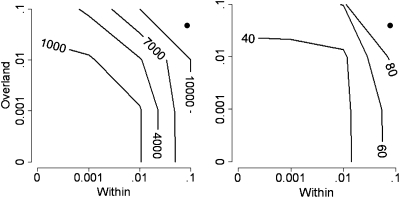
Comparing our observed movement rates with the results from the simulation model (Fig. 2), we found that our data support general theory predicting increased population stability in networks with even a small amount of overland dispersal (2, 9). This effect is most pronounced when extinction rates are low. Under markedly increased extinction rates (as might be expected given an epizootic) (27), populations are unlikely to persist, even with relatively high rates of dispersal.
Fig. 2.
Simulation results. Metapopulation persistence times in a 15-patch dendritic network with per-patch annual extinction risks of 0.01 (Left) and 0.1 (Right), given varying levels of within-network and overland movement probabilities, are shown. The contours indicate persistence time (years). The estimated annual movement probabilities from the mark-recapture study are plotted (•).
Discussion
Using individually marked animals across multiple life stages, we quantified the specific dispersal pathways used by stream salamanders in headwater-stream networks, and we show that route-dependent variation in dispersal rates may be a spatial mechanism enhancing population stability in these species. High rates of overland dispersal in newly metamorphosed juveniles were particularly striking, especially considering that our surveys were conducted during two of the driest summers in recent history. Although desiccation risk may limit terrestrial activity of juvenile animals (28, 29), episodic afternoon thunderstorms (common in mountain systems) may provide windows of opportunity for overland movement. The existence of such temporally varying habitat conditions underscores the importance of using marked animals to identify specific dispersal pathways.
Dispersal is a key component of population dynamics (30). In stream networks, there are two dominant pathways for dispersal: within-network dispersal or overland dispersal between habitat branches. Within-network dispersal has been described for a number of stream-associated organisms [e.g., invertebrates (31), fish (32, 33), and salamanders (34, 35)]. In contrast, overland dispersal is poorly characterized for most species, although studies have documented this dispersal pathway in stream invertebrates (22, 36), observed short-distance overland movements in stream salamanders (35), and inferred overland dispersal in studies of genetic relatedness in stream salamanders (17) and invertebrates (37). The dendritic architecture of headwater-stream networks may influence the dispersal pathways used by organisms living in these networks, which may contribute to these striking differences in population trajectories among amphibian taxa (16). In spatially structured habitat networks, propensities for movement along specific pathways may represent evolutionary adaptations to network structure. We suggest that headwater specialists may have evolved overland dispersal as one strategy for persistence in dendritic habitat networks. This hypothesis is supported by our finding that the two Desmognathus species did not differ in their relative use of the overland versus within-stream movement pathways.
The observed rates of upstream and overland dispersal by juvenile salamanders support our prediction that the dendritic structure of stream systems affects the basic movement behavior of headwater specialists (16). Our data represent direct observations of salamander movement in headwater networks and identify a mechanism for increased occupancy and abundance in these systems (17, 38, 39). Within the restrictive spatial structure of dendritic networks, the use of multiple dispersal pathways is critical to population stability (2, 9). Understanding the population-level mechanisms for amphibian decline and persistence may require similarly detailed investigation into the movement ecology of these animals, with special attention to the pathways and life stages of dispersal. Our study was conducted in continuous forest habitat and designed to describe the movement ecology of headwater-stream salamanders in undisturbed habitat. Understanding how different types of habitat in the terrestrial matrix may influence movement rates would be a logical next step, especially because terrestrial habitat loss and alteration are a major threat to amphibian population persistence (40).
The magnitude and directional bias of movement has context-dependent effects on population dynamics (25). For example, low-to-moderate rates of dispersal can increase population stability and metapopulation persistence (8, 41), whereas higher rates of dispersal can synchronize populations (42–44), potentially increasing extinction risk (45, 46). Our results agree with theoretical models showing positive effects of low-to-moderate dispersal rates, but they also expand on these models by showing the importance of specific pathways of dispersal to population dynamics (2, 4, 44).
Overland dispersal may be a natural component of an organism's movement ecology (22, 31) or may result from anthropogenic actions. These actions can be intentional (e.g., translocation as part of a species’ recovery plan) (47) or accidental (e.g., introduction of an invasive species) (48). We find that the frequency of overland dispersal interacts with within-stream dispersal and local extinction probability to influence population persistence (Fig. 2). Specifically, our results suggest that freshwater species that cannot make overland movements, such as fish, sessile invertebrates, and mollusks, may benefit disproportionally from overland translocations (i.e., increasing the y-axis value in Fig. 2). Likewise, eliminating these overland connections may be especially crucial to slowing the spread of invasive species. More broadly, our results underscore the importance of identifying and protecting critical dispersal pathways when addressing region-wide population declines such as those observed in many amphibians.
Methods
Field Data Collection.
In each of two pairs of confluent streams in Shenandoah National Park, Virginia, we individually marked salamanders of all age classes in three 40-m reaches that were separated by 350–500 m either along the stream corridor or across terrestrial habitat (Fig. 1). All research was performed under appropriate Animal Care and Use Committee approval (University of Maryland #R-06-15). Each reach was surveyed using three temporary removal passes in May, June, July, and September of 2007 and 2008.
Age Classification.
Individuals were classified into three ages: larvae, juvenile, and adult. Animals were assigned to either the juvenile or adult age class based on the distribution of sizes: D. fuscus individuals were classified as adults when their snout-to-vent length exceeded 36 mm, and D. monticola individuals were classified as adults when their SVL exceeded 45 mm (49–51).
Multistate Modeling of Individual Capture Histories.
Using multistate mark-recapture modeling (52), we estimated the probabilities of within-network versus overland, out-of-network movements. We used the Huggins formulation of the closed, robust-design, multistate model implemented in program MARK (53) to estimate survival (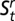 ) and capture (pt) probabilities at each site and transition probabilities (
) and capture (pt) probabilities at each site and transition probabilities (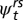 ) among age–reach states. The survival probability is the probability that an animal alive at time t survives to time t + 1 and remains in the study system (i.e., does not die or permanently emigrate), whereas the capture probability (pt) is the probability of capturing a marked individual in one of three temporary removal passes conditional on it being present in the sampled population at t.
) among age–reach states. The survival probability is the probability that an animal alive at time t survives to time t + 1 and remains in the study system (i.e., does not die or permanently emigrate), whereas the capture probability (pt) is the probability of capturing a marked individual in one of three temporary removal passes conditional on it being present in the sampled population at t.
We defined each state as a combination of age class and stream reach (52). The transition probability 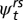 is, therefore, the probability that an individual in state r (age
is, therefore, the probability that an individual in state r (age 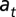 in reach
in reach 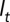 ) at time t is found in state s (age class
) at time t is found in state s (age class 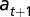 in reach
in reach 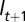 ) in time t + 1, conditional on survival from t to t + 1 and presence in the study system at t + 1. We investigated five models (Table 1) representing different constraints on the estimation of transition probabilities, and reflecting our hypotheses that stream salamander movement differed by pathway (overland vs. in-stream) and location in time t. Sources of variation in the age-reach transition probabilities (
) in time t + 1, conditional on survival from t to t + 1 and presence in the study system at t + 1. We investigated five models (Table 1) representing different constraints on the estimation of transition probabilities, and reflecting our hypotheses that stream salamander movement differed by pathway (overland vs. in-stream) and location in time t. Sources of variation in the age-reach transition probabilities (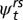 ) specify the type of movement (no movement, upstream, downstream, or overland) and the type of age transition (remain as larvae, remain juvenile, remain adult, transform from larvae to juvenile, or recruit from juvenile to adult).
) specify the type of movement (no movement, upstream, downstream, or overland) and the type of age transition (remain as larvae, remain juvenile, remain adult, transform from larvae to juvenile, or recruit from juvenile to adult).
Our global model included the additive effects of stream reach, age class, and sampling season as sources of variation in survival probabilities (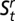 ); we included stream reach, temporary removal pass, and sampling season as sources of variation in capture probabilities (pt). Because of low capture probabilities of larval individuals, we borrowed information across age classes by assuming no age specificity within the closed-population removal model. Recapture probabilities for secondary sampling occasions within each primary occasion were constrained to zero to reflect our use of temporary removal passes. All transitions (
); we included stream reach, temporary removal pass, and sampling season as sources of variation in capture probabilities (pt). Because of low capture probabilities of larval individuals, we borrowed information across age classes by assuming no age specificity within the closed-population removal model. Recapture probabilities for secondary sampling occasions within each primary occasion were constrained to zero to reflect our use of temporary removal passes. All transitions (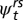 ) that were not structurally possible (e.g., from adult to larvae; overland movement of obligatorily aquatic larvae) were fixed at zero. Because all larvae were observed to metamorphose before July, larval transition probabilities for July to September were fixed at zero.
) that were not structurally possible (e.g., from adult to larvae; overland movement of obligatorily aquatic larvae) were fixed at zero. Because all larvae were observed to metamorphose before July, larval transition probabilities for July to September were fixed at zero.
We used program U-CARE (54) to perform the goodness-of-fit test on the global model. The global multisite test indicated some lack of fit in one of the stream pairs, and an investigation of Test3.Sr indicated that the problem was likely caused by transients. Transients were animals that were traveling through the study area (or experienced a handling effect), which led to extremely low probabilities of being present in future occasions. Within each age class, we, therefore, estimated different survival parameters for the time period immediately following initial capture versus subsequent periods.
We used a sequential modeling approach. We first found parsimonious models (using small-sample Akaike's Information Criterion (AICc) to rank models) for the closed-population capture probabilities and survival probabilities. Next, we used the resulting parameterizations to investigate hypotheses about stream salamander transition probabilities, which were conditional on age transition (Table 1).
We expected transition probabilities for D. monticola and D. fuscus to be similar, stemming from the similarity in life-history characteristics (55) (e.g., short larval period, close association with the stream channel, and overlapping body sizes within each life stage). To examine the evidence for such similarities in the recapture data, we first modeled the capture histories, including species–specific differences in movement probabilities for each movement pathway (overland, upstream, and downstream). Second, we compared this model against one specifying similar movement behaviors between the species but allowing one species to have higher movement probabilities overall. Third, we formally compared these hypotheses using a likelihood ratio test between a model with species–pathway interaction and a model with an additive effect of species. Finally, we tested whether or not the rates were identical between the two species by specifying a model with no species effect and testing this model against the additive model.
Simulation Model.
To investigate the consequences of the observed dispersal probabilities in dendritic metapopulations, we modified the model of ref. 56 to conduct a simulation study in a fractal branching network of 15 habitat patches. We investigated two different annual per-patch extinction probabilities (0.01 and 0.1) and varied the within-stream and overland dispersal probabilities. For our simulation of theoretical 15-patch dendritic networks, each patch was considered to be of equal quality. For each model run, we initialized full occupancy of all patches in the network and fixed the time-specific extinction probability in each patch. We investigated two extinction probabilities (0.1 and 0.01) and four movement probabilities (0, 0.1, 0.01, and 0.001). At each time step and after random extinctions of habitat patches throughout the network, we allowed colonization of extinct patches through one of three movement routes: (i) upstream, (ii) downstream (set equal to upstream movement), and (iii) overland colonization from one of the two closest neighboring patches within the same hierarchical level. Mortality of dispersers was not considered explicitly in our simulations. The model was run for a maximum of 10,000 time steps (or until full extinction of the network) for all parameter combinations, and each combination was replicated 100 times. To plot our empirical dispersal probabilities with the simulation results, we condensed our capture histories to two occasions (captured in 2007 or 2008). We fit a constant survival and capture probability model to these data, allowing different transition probabilities (equivalent among age–class transitions) for overland and within-stream dispersal.
Supplementary Material
Acknowledgments
We are indebted to our field assistants: C. Otto, K. Cecala, S. Mattfeldt, A. Dietrich, M. Stover, M. Weaver, E. Strauss, M. Michelson, and L. Bailey. Special thanks to P. Toschik for support during this study. This manuscript was substantially improved with comments from P. Toschik, L. Green, K. Lips, S. Converse, and H. Lynch. Funding for this research was provided by the Amphibian Research and Monitoring Initiative of the United States Geological Survey and the James S. McDonnell Foundation through a grant from the Studying Complex Systems program (220020138).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000266107/DCSupplemental.
References
- 1.Fortuna MA, Gomez-Ródriguez C, Bascompte J. Spatial network structure and amphibian persistence in stochastic environments. Proc R Soc Lond B Biol Sci. 2006;273:1429–1434. doi: 10.1098/rspb.2005.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland MD, Hastings A. Strong effect of dispersal network structure on ecological dynamics. Nature. 2008;456:792–794. doi: 10.1038/nature07395. [DOI] [PubMed] [Google Scholar]
- 3.Nathan R. The challenges of studying dispersal. Trends Ecol Evol. 2001;16:481–483. [Google Scholar]
- 4.Ranta E, Fowler MS, Kaitala V. Population synchrony in small-world networks. Proc R Soc Lond B Biol Sci. 2008;275:435–442. doi: 10.1098/rspb.2007.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey DJ, et al. Effects of landscape corridors on seed dispersal by birds. Science. 2005;309:146–148. doi: 10.1126/science.1111479. [DOI] [PubMed] [Google Scholar]
- 6.Muneepeerakul R, et al. Neutral metacommunity models predict fish diversity patterns in Mississippi-Missouri basin. Nature. 2008;453:220–222. doi: 10.1038/nature06813. [DOI] [PubMed] [Google Scholar]
- 7.Ruxton GD, Gonzalez-Andujar JL, Perry JN. Mortality during dispersal stabilizes local population fluctuations. J Anim Ecol. 1997;66:289–292. [Google Scholar]
- 8.Dey S, Joshi A. Stability via asynchrony in Drosophila metapopulations with low migration rates. Science. 2006;312:434–436. doi: 10.1126/science.1125317. [DOI] [PubMed] [Google Scholar]
- 9.Hill MF, Hastings A, Botsford LW. The effects of small dispersal rates on extinction times in structured metapopulation models. Am Nat. 2002;160:389–402. doi: 10.1086/341526. [DOI] [PubMed] [Google Scholar]
- 10.Lips KR, Reeve JD, Witters L. Ecological traits predicting amphibian population declines in Central America. Conserv Biol. 2003;17:1078–1088. [Google Scholar]
- 11.Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 12.Green DM. The ecology of extinction: Population fluctuation and decline in amphibians. Biol Conserv. 2003;111:331–343. [Google Scholar]
- 13.Montgomery DR, et al. Distribution of bedrock and alluvial channels in forested mountain drainage basins. Nature. 1996;381:587–589. [Google Scholar]
- 14.Walter RC, Merritts D. Natural streams and the legacy of water-powered mills. Science. 2008;319:299–304. doi: 10.1126/science.1151716. [DOI] [PubMed] [Google Scholar]
- 15.Hayhoe K, et al. Past and future changes in climate and hydrological indicators in the US Northeast. Clim Dyn. 2007;28:381–407. [Google Scholar]
- 16.Campbell Grant EH, Lowe WH, Fagan WF. Living in the branches: Population dynamics and ecological processes in dendritic networks. Ecol Lett. 2007;10:165–175. doi: 10.1111/j.1461-0248.2006.01007.x. [DOI] [PubMed] [Google Scholar]
- 17.Rissler LJ, Wilbur HM, Taylor DR. The influence of ecology and genetics on behavioral variation in salamander populations across the Eastern Continental Divide. Am Nat. 2004;164:201–213. doi: 10.1086/422200. [DOI] [PubMed] [Google Scholar]
- 18.Labonne J, Virginie R, Parisi B, Gaucherel C. Linking dendritic network structures to population demogenetics: The downside of connectivity. Oikos. 2008;117:1479–1490. [Google Scholar]
- 19.Means DB, Travis J. Declines in ravine-inhabiting dusky salamanders of the Southeastern US coastal plain. Southeast Nat. 2007;6:83–96. [Google Scholar]
- 20.Vannote RL, et al. The river continuum concept. Can J Fish Aquat Sci. 1980;37:130–137. [Google Scholar]
- 21.Bruce RC. Upstream and downstream movements of Eurycea bislineata and other salamanders in a southern Appalachian stream. Herpetologica. 1986;42:149–155. [Google Scholar]
- 22.Macneale KH, Pecharsky BL, Likens GE. Stable isotopes identify dispersal patterns of stonefly populations living along stream corridors. Freshw Biol. 2005;50:1117–1130. [Google Scholar]
- 23.Vieites DR, Min MS, Wake DB. Rapid diversification and dispersal during periods of global warming by plethodontid salamanders. Proc Natl Acad Sci USA. 2007;104:19903–19907. doi: 10.1073/pnas.0705056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilbur HM. Complex life cycles. Annu Rev Ecol Syst. 1980;11:67–93. [Google Scholar]
- 25.Bowler DE, Benton TG. Causes and consequences of animal dispersal strategies: Relating individual behaviour to spatial dynamics. Biol Rev Camb Philos Soc. 2005;80:205–225. doi: 10.1017/s1464793104006645. [DOI] [PubMed] [Google Scholar]
- 26.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information–Theoretic Approach. New York: Springer; 2002. [Google Scholar]
- 27.Lips KR, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keen WH. Influence of moisture on the activity of a plethodontid salamander. Copeia. 1984;1984:684–688. [Google Scholar]
- 29.Feder ME, Londos PL. Hydric constraints upon foraging in a terrestrial salamander, Desmognathus ochrophaeus (Amphibia: Plethodontidae) Oecologia. 1984;64:413–418. doi: 10.1007/BF00379141. [DOI] [PubMed] [Google Scholar]
- 30.Nathan R, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci USA. 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilton DT, Freeland JR, Okamura B. Dispersal in freshwater invertebrates. Annu Rev Ecol Syst. 2001;32:159–181. [Google Scholar]
- 32.Skalski GT, Gilliam JF. Modeling diffusive spread in a heterogeneous population: A movement study with stream fish. Ecology. 2000;81:1685–1700. [Google Scholar]
- 33.Gowan C, Young MK, Fausch KD, Riley SC. Restricted movement in resident stream salmonids: A paradigm lost? Can J Fish Aquat Sci. 1994;51:2626–2637. [Google Scholar]
- 34.Lowe WH. Linking dispersal to local population dynamics: A case study using a headwater salamander system. Ecology. 2003;84:2145–2154. [Google Scholar]
- 35.Grover MC, Wilbur HM. Ecology of ecotones: Interactions between salamanders on a complex environmental gradient. Ecology. 2002;83:2112–2123. [Google Scholar]
- 36.Petersen I, Masters Z, Hildrew AG, Ormerod SJ. Dispersal of adult aquatic insects in catchments of differing land use. J Appl Ecol. 2004;41:934–950. [Google Scholar]
- 37.Hurwood DA, Hughes JM. Nested clade analysis of the freshwater shrimp, Caridina zebra (Decapoda: Atyidae), from north-eastern Australia. Mol Ecol. 2001;10:113–125. doi: 10.1046/j.1365-294x.2001.01175.x. [DOI] [PubMed] [Google Scholar]
- 38.Campbell Grant EH, Green LE, Lowe WH. Salamander occupancy in headwater stream networks. Freshw Biol. 2009;54:1370–1378. [Google Scholar]
- 39.Lowe WH, Bolger DT. Local and landscape-scale predictors of salamander abundance in New Hampshire headwater streams. Conserv Biol. 2002;16:183–193. doi: 10.1046/j.1523-1739.2002.00360.x. [DOI] [PubMed] [Google Scholar]
- 40.Cushman SA. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol Conserv. 2006;128:231–240. [Google Scholar]
- 41.Roff DA. The analysis of a population model demonstrating the importance of dispersal in a heterogeneous environment. Oecologia. 1974;V15:259–275. doi: 10.1007/BF00345182. [DOI] [PubMed] [Google Scholar]
- 42.Paradis E, Baillie SR, Sutherland WJ, Gregory RD. Dispersal and spatial scale affect synchrony in spatial population dynamics. Ecol Lett. 1999;2:114–120. [Google Scholar]
- 43.Kendall BE, Bjornstad ON, Bascompte J, Keitt TH, Fagan WF. Dispersal, environmental correlation, and spatial synchrony in population dynamics. Am Nat. 2000;155:628–636. doi: 10.1086/303350. [DOI] [PubMed] [Google Scholar]
- 44.Earn DJD, Levin SA, Rohani P. Coherence and conservation. Science. 2000;290:1360–1364. doi: 10.1126/science.290.5495.1360. [DOI] [PubMed] [Google Scholar]
- 45.Harrison S, Quinn JF. Correlated environments and the persistence of metapopulations. Oikos. 1989;56:293–298. [Google Scholar]
- 46.Liebhold A, Koenig WD, Bjornstad ON. Spatial synchrony in population dynamics. Annu Rev Ecol Syst. 2004;35:467–490. [Google Scholar]
- 47.Griffith B, Scott JM, Carpenter JW, Reed C. Translocation as a species conservation tool: Status and strategy. Science. 1989;245:477–480. doi: 10.1126/science.245.4917.477. [DOI] [PubMed] [Google Scholar]
- 48.Johnson LE, Ricciardi A, Carlton JT. Overland dispersal of aquatic invasive species: A risk assessment of transient recreational boating. Ecol Appl. 2001;11:1788–1799. [Google Scholar]
- 49.Bruce RC, Hairston NG. Life-history correlates of body-size differences between two populations of the salamander, Desmognathus monticola. J Herpetol. 1990;24:126–134. [Google Scholar]
- 50.Bruce RC. Life history of the salamander Desmognathus monticola, with a comparison of the larval periods of D. monticola and D. ochrophaeus. Herpetologica. 1989;45:144–155. [Google Scholar]
- 51.Orser PN, Shure DJ. Population cycles and activity patterns of the dusky salamander, Desmognathus fuscus fuscus. Am Midl Nat. 1975;93:403–410. [Google Scholar]
- 52.Lebreton J-D, et al. Modeling individual animal histories with multistate capture-recapture models. Adv Ecol Res. 2009;41:87–173. [Google Scholar]
- 53.White GC, Burnham KP. Program MARK: Survival estimation from populations of marked animals. Bird Study. 1999;46:120–138. [Google Scholar]
- 54.Choquet R, et al. User's Manual for U-Care, Utilities—CApture-REcapture, V2. 02. Montpellier, France: CEFE/CNRS; 2003. [Google Scholar]
- 55.Organ JA. Studies of the local distribution, life history, and population dynamics of the salamander genus Desmognathus in Virginia. Ecol Monogr. 1961;31:189–220. [Google Scholar]
- 56.Fagan WF, Campbell Grant EH, Lynch HJ, Unmack PJ. Riverine landscapes: ecology for an alternative geometry. In: Cantrell RS, Cosner C, Ruan S, editors. Spatial Ecology. Boca Raton: CRC/Chapman Hall Press; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.