Abstract
The Wnt/planar cell polarity (PCP) pathway regulates directed cell movement during development and was recently found to play a critical role in endothelial cell proliferation and angiogenesis [Zhang Y, et al. (2006) Chem Biol 13:1001–1009; Masckauchan TN, et al. (2006) Mol Biol Cell 17:5163–5172]. However, the mechanisms by which PCP signaling components regulate angiogenesis remain unknown. We report that expression of a constitutively active C-terminal domain of Dishevelled-associated activator of morphogenesis 1 (DAAM1) selectively inhibited endothelial cell proliferation. Moreover, this activated construct suppressed endothelial cell migration and the ability to form coordinated networks in vivo and in vitro. Although constitutively active DAAM1 (CDAAM1) induced both actin polymerization and microtubule (MT) stabilization, the stabilization of MTs alone was sufficient to inhibit endothelial cell growth selectively. Inhibition of actin polymerization alone by jasplakinolide treatment failed to reproduce the inhibitory effects of CDAAM1. These results indicate that DAAM1 regulates endothelial cell growth through MT stabilization in a cell type-selective manner and suggest that PCP signaling plays a pivotal role in angiogenesis by regulating MT stabilization.
Keywords: Wnt signaling, microtubule stabilization, zebrafish, dishevelled-associated activator of morphogenesis 1, HUVEC
Angiogenesis is the process by which blood vessels grow from existing vessels and is involved in many pathological conditions such as wound healing, inflammation, rheumatoid arthritis, endometriosis, diabetic retinopathy, macular degeneration, and tumor growth (1). TNP-470, a potent small-molecule angiogenesis inhibitor, has shown promising efficacy in vivo (2). Recently, our laboratory reported that TNP-470 disrupts planar cell polarity (PCP) signaling (3) and that inhibition of PCP signaling by either chemical or genetic means disrupts angiogenesis (4). This signaling pathway is one of several Wnt-driven signaling cascades. Wnt, as a ligand, interacts with a seven-transmembrane-spanning receptor Frizzled (Fz) to transduce signaling through a scaffolding protein Dishevelled (Dvl). Depending on the downstream effectors of Dvl, Wnt signaling can be classified as either β-catenin-dependent (canonical) or β-catenin-independent (noncanonical). This latter class encompasses a number of signaling cascades, including the Wnt/Ca2+ and PCP pathways (5). The PCP pathway was first studied in the cellular patterning of Drosophila wing epithelia (6) but has been shown to be involved in polarized cell movements such as convergent extension and gastrulation in vertebrates (7). Downstream of Dvl, effectors in the PCP pathway signal through the small GTP-binding protein Rho to coordinate actin polymerization and organization, giving rise to polarized cell movements (5).
Recently, binding of the formin Dishevelled-associated activator of morphogenesis 1 (DAAM1) to Dvl and the small GTPase Rho has been shown to coordinate Wnt signaling cues (8). DAAM1 is a member of a subgroup of formins, the diaphanous-related formins (DRFs), and contains multiple regulatory domains. These domains include an N-terminal GTPase-binding domain (GBD), followed by the diaphanous inhibitory domain (DID), two formin homology (FH1 and FH2) domains, and a diaphanous autoregulatory domain (DAD) at the C terminus (9) (Fig. 1A).
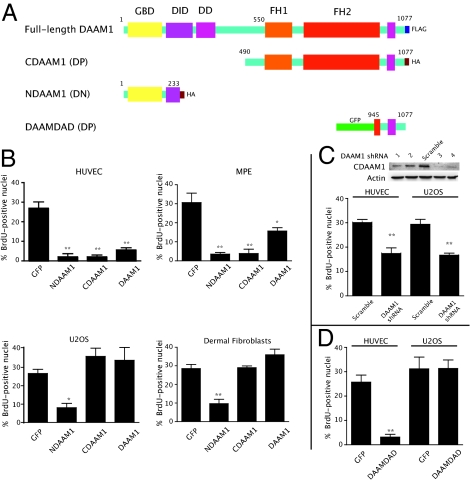
Fig. 1.
Activation of DAAM1 selectively inhibits endothelial cell growth. (A) Graphical representation of various DAAM1 constructs. (B) Growth effects of various DAAM1 domains in nonendothelial and endothelial cells. Cells were transfected with NDAAM1, CDAAM1, and full-length DAAM1 for 48 h; pulse-labeled with BrdU for 1 h; and then probed for the epitope (either FLAG- or HA-tag as appropriate) and for BrdU incorporation as described in Materials and Methods. Vector-expressing GFP served as a negative control for all experiments. Error bars indicate SEM. (C) shRNA-mediated knockdown of DAAM1 indiscriminately inhibits proliferation of both endothelial and U2OS cells. The immunoblot illustrates an approximately >70% knockdown of HA-CDAAM1 by shRNA3 and shRNA4. BrdU incorporation experiments were performed using shRNA3 against DAAM1. (D) Effect of DAAM1 DAD domain on HUVEC and U2OS cell proliferation. Each graph represents an average of three independent experiments (duplicate experiments for C with over 100 cells each). Statistical significance was determined by one-way ANOVA and denoted for *P < 0.05 and **P < 0.001. DD, dimerization domain; DP, dominant positive; DN, dominant negative.
FH1 and FH2 domains coordinate to induce actin nucleation and polymerization (10, 11). In the inactive state, the autoregulatory domain of a DRF is bound to the DID domain (12). Association of GTP-bound Rho or Cdc42 with the GBD results in the release of this inactive conformation, thus permitting the FH domains to catalyze actin nucleation and filament elongation (13, 14). In addition to actin polymerization, the most widely studied mammalian formin, mDia1, stabilizes microtubules (MTs) through its C-terminal FH2 domain (15).
Given that loss of PCP signaling suppressed endothelial cell proliferation and migration, thus inhibiting angiogenesis (4), we next examined the role of DAAM1 in endothelial cells and angiogenesis. We report here that constitutively active DAAM1 inhibited endothelial cell growth in a cell type-selective manner. Moreover, we found that CLASP1α, a plus end-tracking protein (+TIP) that induces MT stabilization independent of actin polymerization, also inhibited proliferation of endothelial cells selectively. Jasplakinolide, a chemical inducer of actin polymerization, did not suppress endothelial cell proliferation. In addition, activated DAAM1 blocked endothelial cell migration and network formation in vitro. These findings were further validated in vivo, because activated DAAM1 inhibited angiogenesis in zebrafish embryos. We therefore conclude that PCP signaling through DAAM1 coordinates cell growth and migration in endothelial cells predominantly through MT assembly and stabilization.
Results
CDAAM1 Selectively Inhibits Endothelial Cell Proliferation.
Our findings pertaining to the role of PCP signaling in angiogenesis (4) prompted us to investigate further downstream PCP signaling mediators to gain more detailed mechanistic insights into angiogenesis. In this study, we focused on DAAM1, which is activated in the PCP pathway independent of other Wnt signaling cascades (8). Because the N-terminal (NDAAM1) and C-terminal (CDAAM1) domains of DAAM1 (Fig. 1A) exert dominant-negative and dominant-positive (constitutively active) functions on PCP signaling, respectively (8), we compared the effects of PCP signaling on endothelial cell proliferation by expressing NDAAM1 and CDAAM1. BrdU incorporation into both human umbilical vein endothelial cells (HUVECs) and murine pulmonary endothelial (MPE) cells was diminished on overexpression of DAAM1 or its truncated mutants relative to GFP controls (Fig. 1B). To test if other cell types would respond in a similar manner, we introduced these constructs into human primary dermal fibroblasts and human U2OS osteosarcoma cells. Interestingly, neither CDAAM1 nor full-length DAAM1 significantly inhibited proliferation in these cells, although NDAAM1 blocked cell growth (Fig. 1B). Also, the loss of function of DAAM1 by shRNA-mediated knockdown indiscriminately inhibited both HUVEC and U2OS proliferation in a similar manner as the dominant negative NDAAM1 construct (Fig. 1C). This is reflected in vivo, where zebrafish embryos injected with an antisense morpholino to DAAM1 display notochord defects (16). Similar, although more pronounced, defects were observed with zebrafish NDAAM1 or in fish injected with morpholinos to both DAAM1 and DAAM2 (16). Therefore, whereas the antiproliferative effects of DAAM1 activation appear to be endothelial cell-specific, the loss-of-function data in vitro and in vivo support a broader role for DAAM1 in a variety of cell types, including endothelial cells.
However, because the proliferation inhibition resulting from up-regulation via expression of CDAAM1 appeared to be selective for endothelial cells (Fig. 1B), we tested whether this was true for other cell types that form continuous anisotropic tissues in vivo including smooth muscle cells (SMC) and Madin-Darby canine kidney (MDCK) cells. These cell types were not sensitive to the expression of CDAAM1 (Fig. S1). Therefore, the growth inhibition resulting from the expression of constitutively active CDAAM1 was indeed selective for endothelial cells.
Because we were interested in the selectivity of endothelial cells to perturbations in PCP signaling and NDAAM1 appeared to inhibit cell proliferation in a cell-unselective manner, we focused on the cellular effects mediated by DAAM1 activation by CDAAM1 expression. To rule out the possibility that CDAAM1-mediated endothelial cell proliferation inhibition was an artifact of CDAAM1 overexpression, we subsequently tested whether endogenous DAAM1 activation resulted in similar endothelial cell growth inhibition as seen for CDAAM1 expression. Previously, expression of the C-terminally localized DAD domain of mDia1 has been shown to relieve autoinhibition of endogenous mDia (10). Taking advantage of the fact that, like mDia1, DAAM1 also possesses a DAD domain (17, 18), we expressed the DAAM1 DAD domain fused with GFP in HUVECs and control U2OS cells. DAAM1 DAD expression produced similar growth inhibition to that observed on CDAAM1 expression in HUVECs but not in U2OS cells (Fig. 1D). These results suggest that activation of endogenous DAAM1 by DAD can also inhibit the growth of endothelial cells in a similar manner as through overexpression of CDAAM1.
CDAAM1 Induces Actin Polymerization and MT Stabilization.
Because formin proteins are known to affect both actin polymerization and MT stabilization (8, 15, 19), we next determined which of these two activities (or both) could affect proliferation in an endothelial cell-selective manner. We first investigated whether CDAAM1-induced actin polymerization in endothelial cells was distinguishable from that in nonendothelial cells. CDAAM1 induced actin polymerization in both endothelial (HUVEC) cells (Fig. 2A) and nonendothelial (U2OS) cells (Fig. 2B) without any discernible differences.
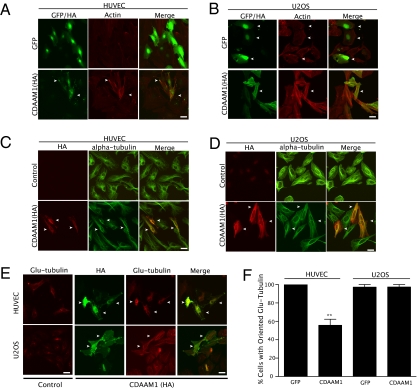
Fig. 2.
DAAM1 activation affects both actin and MT networks. (A) Expression of CDAAM1 on actin structure in HUVECs. (B) Expression of CDAAM1 on actin structure in U2OS cells. (C) Expression of CDAAM1 on overall MTs in HUVECs. (D) Expression of CDAAM1 on overall MTs in U2OS cells. (E) Expression of CDAAM1 on stable MTs in HUVECs and U2OS cells. Arrows indicate transfected cells. Note that in E, in HUVECs (Upper), the formation of stable MTs was proportional to expression of CDAAM1. In addition, both cell types displayed spontaneous formation of stable Glu-tubules. In each experiment, cells were transfected with CDAAM1 constructs for 24–48 h and then double-stained with anti-HA and with phalloidin, anti-α-tubulin, or anti-Glu-tubulin antibodies. (F) Quantification of HUVECs and U2OS cells displaying perinuclear-to-lamellipodia orientation of stabilized MTs. Commonly, spontaneous or (by adenoviral vector) CDAAM1-induced stabilized MTs were observed to orient from the perinuclear region outward. In HUVECs particularly, Glu-tubulin was predominantly perinuclear in most cells without projecting distally from the nucleus. The graph represents pooled data from two independent experiments (**P < 0.001). (Scale bar = 50 μm.)
Based on the high degree of homology between mDia1 and DAAM1 (20), we next speculated that CDAAM1 expression could induce MT stabilization in a manner similar to that observed on expression of the constitutively active mDia1 (15). To test this hypothesis, we first examined if CDAAM1 could differentially change overall MT structures in HUVECs and U2OS cells. No dramatic MT organizational changes were observed in CDAAM1-expressing HUVECs or in U2OS cells compared with their respective controls (Fig. 2 C and D). DAAM1 activation, therefore, did not affect overall MT organization, nor were there any outstanding cell type distinguishing features observed.
Although the overall MT organization was not affected, it was still possible that activated DAAM1 could stabilize MTs in a manner similar to mDia. In most cells, the subset of MTs possessing carboxyl-terminal detyrosinated tubulin (Glu tubulin) is more stable than other MTs (21). Because the FH1/FH2 domains of mDia1 are able to increase stable MTs (15), we examined whether there was an increase in MT stabilization in CDAAM1-expressing HUVECs and U2OS cells as measured by anti-Glu-tubulin immunofluorescence. As shown in Fig. 2 E and F, we found that CDAAM1 stabilized MTs in both HUVECs and U2OS cells proportional to the expression levels of CDAAM1 (Fig. 2E).
However, there is an apparent difference in stable MT organization between these two cell types. Stabilized MTs in endothelial cells were predominantly localized in the perinuclear region, whereas stable MTs were dispersed throughout the cytoplasm within U2OS cells (Fig. 2E, Glu-tubulin panel). Quantification of cells displaying a typical perinuclear-to-lamellipodia orientation over total cells illustrated that CDAAM disrupted this orientation selectively and significantly in endothelial cells (Fig. 2F). Interestingly, the stable MTs were strongly colocalized with CDAAM1 in HUVECs and only moderately so in U2OS cells, suggesting a transport relation in endothelial cells. This relation is supported by data, whereby DAAM1 has been shown to mediate the endocytosis of EphB (16). Therefore, although DAAM1 can activate both actin polymerization and MT stabilization, the coordination of stabilized MTs appears to vary according to cell type, which is likely responsible for the varying effects of DAAM activation on proliferation.
MT Stabilization Rather Than Actin Polymerization Inhibits Endo-thelial Cell Proliferation.
Because CDAAM1 expression had antiproliferative effects in endothelial cells but not U2OS cells, although inducing actin polymerization and MT stabilization in both, we hypothesized that endothelial cells may respond to cytoskeletal changes (either actin polymerization, MT stabilization, or a combination of both) differently than other cell types. To test this hypothesis, we used agents that induced either actin polymerization or MT stabilization independent of each other. As with previous observations (22), treatment of HUVECs with the actin polymerization reagent jasplakinolide drastically induced actin polymerization at 10 nM (Fig. 3A). Nevertheless, the drug-induced actin polymerization, even at higher doses, failed to inhibit cell proliferation as measured by BrdU staining (Fig. 3B). Clearly, actin polymerization alone was insufficient to reproduce CDAAM1-mediated endothelial cell growth inhibition.
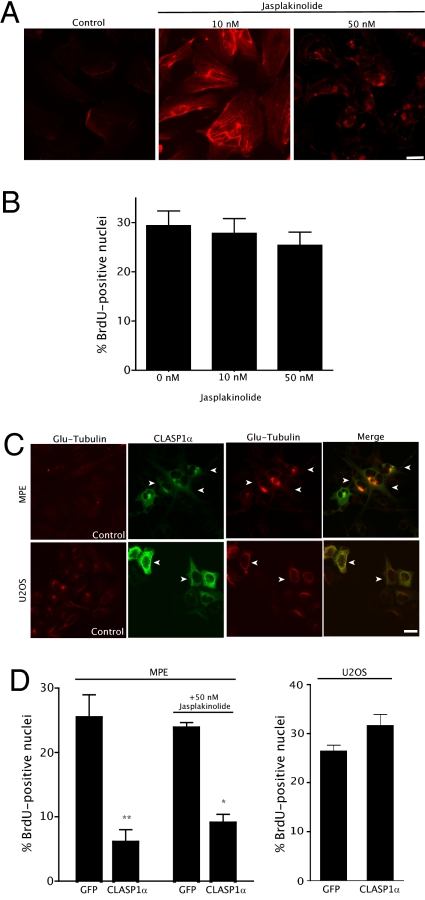
Fig. 3.
MTs rather than actin network changes are responsible for endothelial cell growth inhibition. (A) Jasplakinolide (10 or 50 nM, 16 h) induced actin polymerization. (B) Jasplakinolide did not affect endothelial cell growth. HUVECs were treated with either 10 or 50 nM of jasplakinolide for 16 h and then stained with either phalloidin or anti-BrdU antibody. S-phase cells were counted as in Fig. 1B. Error bars indicate SEM, representing an average of three independent experiments. (C) CLASP1α induces stable MTs in MPE cells. MPE cells were transfected with GFP-CLASP1α. After 24 h posttransfection, stable MTs were visualized with anti-Glu-tubulin antibody. Transfected cells were visualized with an anti-GFP antibody because GFP was quenched by methanol fixation. Arrows indicate transfected cells. (D) CLASP1α inhibited MPE but not U2OS cell growth. Cells were transfected with GFP-CLASP1α for 24 h, with or without 50 nM jasplakinolide for another 24 h, and cells were then fixed and visualized with anti-BrdU antibody. The percentage of S-phase cells among GFP-positive cells was counted as in Fig.1B. Error bars indicate SEM; averages of three independent experiments are displayed. Statistical analyses by one-way ANOVA were performed to yield respective P values (*P < 0.05; **P < 0.001). (Scale bar = 50 μm.)
To test if MT stabilization could inhibit endothelial cell proliferation independent of actin polymerization, we introduced GFP-fused CLASP1α into our cell lines. CLASP1α, a +TIP, has been shown to induce MT stabilization (23, 24) but has not been reported to inhibit cell proliferation. Expression of GFP-CLASP1α induced MT stabilization in MPE cells as indicated by the appearance of Glu-tubulin (Fig. 3C, Upper). Furthermore, BrdU incorporation assays revealed that expression of GFP-CLASP1α in MPE cells significantly decreased their proliferation compared with controls (Fig. 3D, Left). Adding jasplakinolide in addition to GFP-CLASP1α expression did not further inhibit growth (Fig. 3D, Left). Although GFP-CLASP1α also induced MT stabilization in U2OS cells (Fig. 3C, Lower), it did not inhibit cell growth (Fig. 3D, Right). Despite the recent report that CLASPs bind the actin network (25), our results indicate that CLASP1α did not induce actin polymerization in MPE cells (Fig. S2). Taken together, these results suggest that MT stabilization, rather than actin polymerization alone or a combination of the two, selectively inhibits endothelial cell growth. Therefore, endothelial cell growth arrest by CDAAM1 is most likely mediated through MT stabilization.
CDAAM1 Inhibits Endothelial Cell Migration and Tube Formation.
Because angiogenesis is a multicellular process that requires not only cell proliferation but migration and intercellular coordination (26), it was important to assess if DAAM1 activation could affect these complex processes. To test this, MPE cells expressing CDAAM1 were examined in migration and network formation assays. Expression of CDAAM1 drastically suppressed endothelial cell migration compared with the control (Fig. S3A). Not surprisingly, CDAAM1 also drastically reduced the number of cell extensions formed within cell networks on Matrigel (BD BioSciences) membranes (Fig. S3B). Because in vitro endothelial cell network formation correlates with angiogenic potential (27), these data suggest that proper coordination of DAAM1 activities is required for angiogenesis.
CDAAM1 Affects Angiogenesis in Vivo.
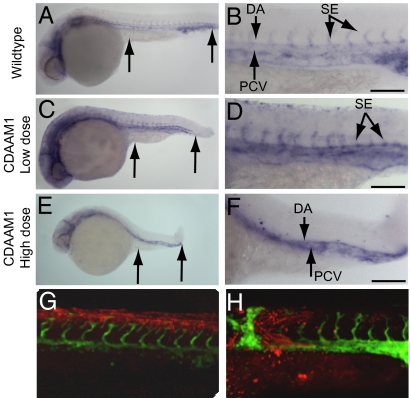
To address the role of DAAM1 in angiogenesis in vivo, we used fli:GFP transgenic zebrafish expressing GFP specifically within vascular endothelial cells (28). First, we investigated whether manipulation of DAAM1 activity by CDAAM1 RNA microinjection induced gastrulation defects as readout for reduced PCP signaling in zebrafish. Compared with WT (Fig. 4A), CDAAM1 RNA injection at a low dose (0.2 ng of RNA) generated moderate defects with obvious tail kinks and shorter anterior-posterior (A-P) length (Fig. 4C), although at a higher injection dose (0.4 ng), more severe convergence extension (C-E) defects were observed (Fig. 4E). These results indicated that overactivation of DAAM1 affects the vascularization of these embryos in a manner consistent with noncanonical Wnt signaling inhibition such as in pipetail (Wnt5 knockout) zebrafish (4). Analysis of an fli-1-driven vascular endothelial cell marker in the injected embryos revealed clear vascular defects to the trunk and tail in CDAAM1-injected zebrafish (Fig. 4 C and D). In a lateral view of a control 28-h postfertilization (hpf) embryo, normal vasculature in the trunk displayed a dorsal aorta (DA) and posterior cardinal vein (PCV) with intersegmental vessels (SEs) extending dorsally (Fig. 4B). In CDAAM1-injected embryos with moderate tail kink defects, the SEs were thinner and more diffuse, and some even disappeared (Fig. 4D). Moreover, CDAAM1-injected embryos with more severe defects lacked SE outgrowth and had gaps in the DA and PCV (Fig. 4 E and F).
Fig. 4.
CDAAM1 inhibits angiogenesis in zebrafish. (A and B) WT embryos demonstrating normal Fli-1 expression. (C and D) Embryos injected with 2 ng of CDAAM1 RNA. (E and F) Embryos injected with 4 ng of CDAAM1 RNA. A, C, and E (arrows) reveal the A-P length as readout for the PCP pathway. B, D, and F display vascular endothelial cell staining in the somite region. (G and H) Confocal images with Fli1-EGFP expression overlaid on lineage tracer identifying transplant location. Zebrafish embryo manipulation, RNA preparation, and imaging are described in Materials and Methods. (Scale bars = 100 μm.)
To rule out the possibility that the C-E defects contributed to the vascular disruptions, we performed transplantation of CDAAM-1-expressing cells into WT hosts. A majority of the host embryos (12 of 13) had transplanted cells outside of the somite/intersegmental region and showed normal vascularization (Fig. 4G). Of the host embryos with CDAAM-1 expression in the somite/intersegmental region, all (5 of 5) showed dramatic alterations to vascularization (Fig. 4H). Because the vascular disruptions observed in the transplant model were limited to the region of transplanted cells, the predominant effect of CDAAM on angiogenesis is within endothelial cells in an autonomous manner. CDAAM1-induced phenotypes are also highly penetrant compared with control injections (Table 1). Taken together, the data indicate that overactivation of DAAM1 signaling disrupts A-P extension of the embryo and angiogenesis in vivo.
Table 1.
Statistical representation of defects caused by CDAAM1
| RNA | Normal | Mild-moderate defects | Severe defects | % Defects |
| GFP, 0.5 ng | 32 | — | — | 0 |
| CDAAM1, 0.2 ng | 8 | 32 | 5 | 82 |
| CDAAM1, 0.4 ng | 9 | 17 | 6 | 72 |
Discussion
In this study, we found that CDAAM1 expression selectively inhibited cell growth in endothelial cells. In addition, we show that, like the constitutively active mDia1, constitutively active CDAAM1 induces MT stabilization, indicating that MT stabilization may be a central function for formin proteins. More importantly, aberrant MT stabilization, rather than actin polymerization mediated by expression of CDAAM1, is responsible for the growth inhibition of endothelial cells. This report shows that up-regulation of PCP signaling mediated by activation of DAAM1 can lead to growth inhibition in a cell-selective manner. This study provides a mechanism by which precise Wnt/PCP signaling regulates endothelial cell proliferation, because both overactivation (i.e., CDAAM1 expression) and underactivation (i.e., NDAAM1 expression) resulted in proliferation suppression. This may explain the conflicting results from two groups studying the roles of Wnt signaling in endothelial cells. Goodwin et al. (29) found that Wnt5a decreased cell growth and capillary length of bovine aortic endothelial cells, whereas others (30) reported that Wnt5a signaling promoted endothelial cell proliferation and survival. Under our experimental conditions, we failed to detect endothelial cell growth inhibition or stimulation with Wnt5a-conditioned medium (4). One possibility is that endothelial cells may have developed certain mechanisms to restrain Wnt5a signaling. For instance, we observed that almost all Fz4, the presumed Wnt receptor for the PCP pathway, when overexpressed in endothelial cells, is localized within punctuate aggregates or in the perinuclear region rather than on the plasma membrane (Fig. S4). Conversely, Fz4 was found to localize on the plasma membrane when expressed in HEK293 cells (31). This may suggest that endothelial cells maintain PCP signaling at a proper level by regulating the availability of Wnt receptors.
Several clinical studies from MT-targeted agents (MTAs) have indicated that both MT stabilization and depolymerization increase MT dynamics specifically in endothelial cells at low doses and inhibit endothelial cell proliferation, migration, and network formation on Matrigel (32–34). These results suggest that endothelial cells may likely differ from other cell types with respect to MT composition, such as by tubulin isotypes or proteins that regulate MT dynamics (33). In this study, we demonstrated that MT stabilization mediated by CDAAM1 or CLASPα can selectively inhibit similar functions as MTAs, further supporting the hypothesis that endothelial cells may regulate MT networks differently than other cell types.
Our present study shows that overactivation of DAAM1 inhibits angiogenesis. This inhibition occurs at multiple levels: Not only is endothelial cell proliferation inhibited but migration and network formation are suppressed. Moreover, zebrafish data show that injection of constitutively active CDAAM1 results in diminished and miscoordinated vasculature, a phenotype similar to the Wnt5 loss-of-function mutant (pipetail, ppt). Clearly in vivo, overactive PCP signaling can be as detrimental as diminished Wnt signaling, indicating that the PCP signaling pathway must be properly coordinated in cellular and developmental processes. Studies investigating other PCP mediators such as vang/stbm support this hypothesis (35, 36), because either overexpression or inhibition of vang/stbm in Xenopus led to a foreshortened embryo with disrupted convergent extension.
Overall, we show that perturbations in PCP signaling via DAAM1 overactivation inhibit angiogenesis through MT disorganization. These results provide a possible mechanism by which PCP signaling governs angiogenesis and offer unique opportunities to inhibit pathological angiogenesis without negative collateral effects. Nevertheless, further questions remain. For example, the mechanistic steps leading from ectopic MT stabilization to endothelial cell cycle arrest have yet to be determined. Moreover, it remains uncertain how DAAM1 can affect the polarity of cells and, in turn, influence their migration and ability to coordinate with other cells. Also, the selective inhibitory effects on endothelial cells by DAAM1 activation suggest a unique role for DAAM1 in endothelial cells not shared with other similar cell types. Finally, given the fact that DAAM1 is strongly expressed in the differentiating nervous system (37) and the PCP/Wnt cascade is implicated in the growth and guidance of axons and dendrites (38), it will be interesting to examine whether MT stabilization or actin polymerization plays an essential role in neuronal guidance. Answers to these questions will determine whether the role of DAAM1 in MT stabilization is confined only to endothelial cells and angiogenesis.
Materials and Methods
Cell Culture and Transfection.
HUVECs and human dermal fibroblasts were obtained from the Yale Cell Culture Core facility. MPE cells were isolated as described previously (39). HUVECs were cultured in EGM-2 (Lonza). MPE cells, human dermal fibroblasts, SMCs, MDCKs, and U2OS cells were cultured in DMEM with 10% (vol/vol) FBS. Cells were transfected with Lipofectamine 2000 (Invitrogen) or Fugene HD (BD BioScience) according to the manufacturer's instructions. MISSION shRNA lentiviral vectors (Sigma) were used to deliver scrambled or anti-DAAM1 shRNA according to the manufacturer's instructions. Finally, adenoviral vectors (pAd/MV/V5-DEST; Invitrogen) were used in certain experiments (according to the manufacturer's protocol) in which chemical transfections were insufficiently effective (as described in the text). PCR and plasmid construction are further detailed in SI Text and Table S1.
Immunofluorescence and BrdU Double-Staining.
The reader is referred to SI Text for details.
Migration Assays and Tube/Network Formation Assay on Matrigel.
Migration assays were performed in a 24-well plate (BD Biocoat Control Inserts, catalog no. 354578; BD Biosciences) according to the manufacturer's instructions. Matrigel assays were performed as described in the product literature (27). Details are provided in SI Text.
Microinjection of Zebrafish.
Zebrafish (Danio rerio) embryos collected from natural matings were processed using standard techniques (40). RNA was introduced into one to eight cell-stage embryos with a pressure injector. CDAAM RNA was synthesized from linearized templates using the mMessage mMachine kit (Ambion). Injected embryos were analyzed for morphological defects and fixed (4% formaldehyde) at 28 hpf. Whole-mount in situ hybridization was performed using antisense Fli1 riboprobe. The Fli1 riboprobe was generated using primers Fli-1F1 (5′-CTGCTGCTCCTTTACCCAAG-3′) and Fli-1R1 (5′-AGCTCCAGTATGGGGTTGTG-3′), which were ampli-fied by PCR from embryonic cDNA and cloned into pCRII TOPO vector. Antisense Fli1 riboprobe was then made through a digestion with BamH1 and in vitro transcription with T7 RNA polymerase. Embryos were photographed using a Zeiss Axiocam.
For the transplantation experiment, donor Fli1:EGFP embryos were microinjected with cDAAM RNA and 0.5 pmol lissamine-conjugated standard control morpholino (MO) as a lineage tracer (Gene Tools, LLC). Donor and host Fli1:EGFP embryos were manually dechorionated before transplantation. At the sphere stage, 20–30 cells were removed from donor embryos and transplanted into the blastodermal margin of each host embryo. At 30 hpf, host embryos were anesthetized with tricaine (4 mg/mL) and imaged on a Leica SP2 confocal microscope. Datasets were collected from the trunk region focusing on the SEs at a magnification of ×20.
Statistical Analysis.
GraphPad Prism v4.0b software was used to perform all statistical analyses. Student's t tests and one- or two-way ANOVA analyses were followed by Tukey's multiple comparison tests or Bonferroni's posttests as appropriate (*P < 0.05; **P < 0.01).
Supplementary Material
Acknowledgments
We thank Drs. Taavi Neklesa, Tim Corson, and Stephanie Leuenroth-Quinn for critical comments and discussion. We also thank Drs. Greg Gundersen and Niels Galjart for the kind gifts of the Glu-tubulin antibody and the GFP-CLASP1α plasmid, respectively. This work was supported by National Institutes of Health Grants CA083049 (to C.M.C.) and CA112369 (to D.C.S.). P.C. is the recipient of a Leukemia and Lymphoma Society Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/1001075107/DCSupplemental.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Satchi-Fainaro R, et al. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat Med. 2004;10:255–261. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, et al. A chemical and genetic approach to the mode of action of fumagillin. Chem Biol. 2006;13:1001–1009. doi: 10.1016/j.chembiol.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirone P, et al. A role for planar cell polarity signaling in angiogenesis. Angiogenesis. 2008;11:347–360. doi: 10.1007/s10456-008-9116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006;7:41–49. doi: 10.1007/s11154-006-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- 7.Klein TJ, Mlodzik M. Planar cell polarization: An emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 8.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 9.Faix J, Grosse R. Staying in shape with formins. Dev Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276:2824–2830. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- 11.Sagot I, Klee SK, Pellman D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat Cell Biol. 2002;4:42–50. doi: 10.1038/ncb719. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Higgs HN. Dissecting requirements for auto-inhibition of actin nucleation by the formin, mDia1. J Biol Chem. 2005;280:6986–6992. doi: 10.1074/jbc.M411605200. [DOI] [PubMed] [Google Scholar]
- 13.Evangelista M, et al. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe N, et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palazzo AF, Cook TA, Alberts AS, Gundersen GG. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol. 2001;3:723–729. doi: 10.1038/35087035. [DOI] [PubMed] [Google Scholar]
- 16.Kida YS, Sato T, Miyasaka KY, Suto A, Ogura T. Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord. Proc Natl Acad Sci USA. 2007;104:6708–6713. doi: 10.1073/pnas.0608946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aspenström P, Richnau N, Johansson AS. The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Exp Cell Res. 2006;312:2180–2194. doi: 10.1016/j.yexcr.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, et al. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci USA. 2008;105:210–215. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizaki T, et al. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat Cell Biol. 2001;3:8–14. doi: 10.1038/35050598. [DOI] [PubMed] [Google Scholar]
- 20.Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell. 2005;16:1–13. doi: 10.1091/mbc.E04-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: Tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- 22.Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- 23.Akhmanova A, et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104:923–935. doi: 10.1016/s0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 24.Drabek K, et al. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr Biol. 2006;16:2259–2264. doi: 10.1016/j.cub.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 25.Tsvetkov AS, Samsonov A, Akhmanova A, Galjart N, Popov SV. Microtubule-binding proteins CLASP1 and CLASP2 interact with actin filaments. Cell Motil Cytoskeleton. 2007;64:519–530. doi: 10.1002/cm.20201. [DOI] [PubMed] [Google Scholar]
- 26.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 27.Nicosia RF, Ottinetti A. Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: A comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol. 1990;26:119–128. doi: 10.1007/BF02624102. [DOI] [PubMed] [Google Scholar]
- 28.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin AM, Kitajewski J, D'Amore PA. Wnt1 and Wnt5a affect endothelial proliferation and capillary length; Wnt2 does not. Growth Factors. 2007;25:25–32. doi: 10.1080/08977190701272933. [DOI] [PubMed] [Google Scholar]
- 30.Masckauchán TN, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006;17:5163–5172. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 32.Pasquier E, et al. Antiangiogenic concentrations of paclitaxel induce an increase in microtubule dynamics in endothelial cells but not in cancer cells. Cancer Res. 2005;65:2433–2440. doi: 10.1158/0008-5472.CAN-04-2624. [DOI] [PubMed] [Google Scholar]
- 33.Pasquier E, André N, Braguer D. Targeting microtubules to inhibit angiogenesis and disrupt tumour vasculature: Implications for cancer treatment. Curr Cancer Drug Targets. 2007;7:566–581. doi: 10.2174/156800907781662266. [DOI] [PubMed] [Google Scholar]
- 34.Pourroy B, et al. Antiangiogenic concentrations of vinflunine increase the interphase microtubule dynamics and decrease the motility of endothelial cells. Cancer Res. 2006;66:3256–3263. doi: 10.1158/0008-5472.CAN-05-3885. [DOI] [PubMed] [Google Scholar]
- 35.Darken RS, et al. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J. 2002;21:976–985. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- 37.Nakaya MA, et al. Identification and comparative expression analyses of Daam genes in mouse and Xenopus. Gene Expr Patterns. 2004;5:97–105. doi: 10.1016/j.modgep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: New insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 39.Yeh JR, Mohan R, Crews CM. The antiangiogenic agent TNP-470 requires p53 and p21CIP/WAF for endothelial cell growth arrest. Proc Natl Acad Sci USA. 2000;97:12782–12787. doi: 10.1073/pnas.97.23.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene, OR: Univ of Oregon Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.