Abstract
Caulobacter crescentus integrates phospho-signaling pathways and transcription factor regulatory cascades to drive the cell cycle. Despite the essential role of the CckA histidine kinase in the control of cell cycle events, the factors that signal its activation at a specific time in the cell cycle have remained elusive. A conditional genetic screen for CckA mislocalization mutants, using automated fluorescence microscopy and an image processing platform, revealed that the essential DivL protein kinase promotes CckA localization, autophosphorylation, and activity at the new cell pole. The transient accumulation of DivL at the new cell pole, but not its kinase activity, is required for the localization and activation of CckA. Because DivL and CckA accumulate at the same cell pole after the initiation of DNA replication and were found to interact in vivo, we propose that DivL recruits CckA to the pole, thereby promoting its autophosphorylation and activity.
Keywords: Caulobacter, CckA, DivL, polar localization
Cell division in Caulobacter crescentus is asymmetric, yielding daughters—a swarmer cell and a stalked cell—with different cell fates. Histidine kinases, the sensors of bacterial two-component signal transduction systems, govern many Caulobacter cell cycle transitions that culminate in this asymmetric cell division (1). Canonically, histidine kinases transduce environmental cues into specific behavioral responses via autophosphorylation and subsequent transphosphorylation of cognate response regulators, which in turn appropriately alter gene expression (2). Because two-component systems generally sense and respond to transitory environmental signals (3–7), it is unusual for histidine kinases and response regulators to be essential for viability. Caulobacter has several essential two-component systems, including CenK/CenR, which controls cell envelope biogenesis and integrity (8), and CckA/ChpT/CtrA, which regulates cell cycle progression (9–12) in a fashion analogous to eukaryotic cyclin-dependent kinases (13).
The CckA/ChpT/CtrA phospho-signaling cascade begins in stalked cells after the initiation of DNA replication with the autophosphorylation of CckA, a hybrid histidine kinase (10). The phosphoryl group of CckA-P is then transferred to an aspartate in the CckA C-terminal receiving domain, then to the ChpT histidine phosphotransferase, and finally to the response regulator CtrA (12). In its phosphorylated state, CtrA functions as a transcription factor that directly controls the expression of ∼95 cell cycle–regulated operons (14) and silences the initiation of DNA replication by binding to the chromosomal replication origin (15). CckA's critical role in CtrA phosphorylation, and thus its central role in cell cycle progression, led us to explore possible mechanisms for CckA localization and activation. CckA levels remain constant throughout the cell cycle (10), suggesting that controlled proteolysis and/or synthesis mechanisms are unlikely to be the reason for its cell cycle–dependent activity. The subcellular location of CckA changes during the cell cycle, however; polar localization of CckA after the initiation of DNA replication coincides with the peak of CckA autophosphorylation and phosphotransfer to CtrA (10, 11, 16). Because histidine kinases generally sense external environmental changes, the challenge was to find internal cellular signals or factors that temporally control the activation of CckA autophosphorylation and phosphotransfer reactions in stable and constant environment conditions.
In this study, we developed a chimeric reporter of CckA activity and demonstrated that CckA is active when localized to the new cell pole, opposite the stalk-presenting pole. Delocalized CckA and CckA localized to the old cell pole exhibited low CckA activity, connecting dynamic CckA localization to the new cell pole with the activation of CckA. A conditional genetic screen for CckA mislocalization mutants revealed that the essential DivL protein kinase is required for the localization, autophosphorylation, and activation of CckA at the new cell pole. DivL is an unusual member of the histidine kinase family of the two-component signal transduction system, in that the histidine phosphotransferase domain contains a tyrosine in place of a histidine (17). Although this tyrosine has been shown to be phosphorylated in vitro (17), we found that DivL tyrosine phosphorylation was not required for the localization or activity of CckA. Because DivL specifically localized to the new cell pole after initiation of replication (18) and interacted with CckA in vivo, we propose that DivL recruits CckA to the new pole, thereby promoting CckA autophosphorylation and activity.
Results
DivL Is Required to Localize CckA Specifically at the New Cell Pole.
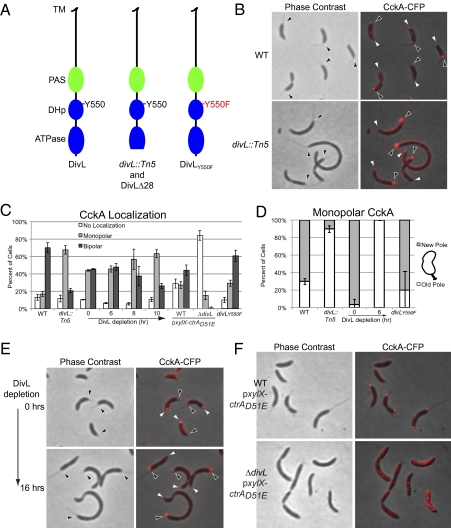
We carried out a genetic screen using conditional transposon-mediated mutagenesis and automated fluorescence microscopy (SI Experimental Procedures) to identify genes that control the localization of CckA. One of the mutants exhibiting an aberrant CckA localization pattern had a transposon insertion that mapped to divL, a gene encoding an essential cell cycle–dependent protein kinase (17). Sequence analysis revealed that the transposon was inserted in the ATPase domain, yielding a truncated protein lacking the final 28 C-terminal amino acids (Fig. 1A). Although this mutant strain retained viability, the cells exhibited marked growth and cell division defects. In divL::Tn5 cells, bipolar CckA was observed in 20% of the cells, compared with 70% in the WT strain (Fig. 1 B and C). Almost all of the divL::Tn5 cells with detectable stalks and monopolar CckA (Fig. 1B) had CckA at the old (stalked) cell pole (Fig. 1D). To confirm the phenotype of the divL::tn5 mutant, we generated two different divL mutant strains. First, we constructed a DivL depletion strain in which the chromosomal divL gene was deleted and the divL coding sequence was placed under the control of the Pvan promoter at the chromosomal vanA locus. Under depletion conditions, in which this strain yet still retained viability, we observed that the cells were elongated. After 6 h of DivL depletion, monopolar CckA-CFP was located exclusively at the stalked pole, whereas at time 0, monopolar CckA was found mostly at the new cell pole (Fig. 1 C and D). After 6 h of DivL depletion, 45% of the cells exhibited bipolar CckA-CFP, likely accounting for the persistent viability. After 8 h and 10 h of DivL depletion, the proportion of bipolar CckA was progressively reduced, yielding a significant reduction of CckA-CFP positioned at the new pole. After 16 h of growth in the absence of vanillate, viable cells exhibited CckA-CFP predominantly at the old cell pole (Fig. 1E), similar to the localization pattern observed in the divL::Tn5 strain (Fig. 1 B–D).
Fig. 1.
DivL promotes the new cell pole localization of CckA. (A) Diagram of the DivL protein, WT (Left), DivL lacking the last 28 amino acids or in divL::Tn5 strain (Center), and DivL with the Y550F mutation (Right), including the predicted transmembrane (TM), Per-ARNT-Sim (PAS), histidine phosphotransfer (DHp) containing the tyrosine Y550 at the conserved phosphorylation site, and the catalytic (ATPase) domains. (B) Phase contrast and fluorescence images of CckA-CFP in WT (NJH530) and divL::Tn5 (AA725) strains. (C) Histograms of CckA-CFP localization in WT (NJH530), divL::Tn5 (AA725), DivL depletion (NJH564, grown in presence of vanillate for divL expression), WT and ΔdivL strains in the presence of CtrAD51E (AA1001 and AA1003, respectively), and the divLY550F strain (AA972) supplemented with 0.5 mM vanillate for divLY550F expression (cells, n = 158–530). The time course for the DivL depletion strain was initiated by the removal of vanillate to deplete DivL. (D) Histograms of monopolar CckA-CFP partitioning between new and old cell poles in WT (NJH530), divL::Tn5 (AA725), DivL depletion (NJH564) cells, and divLY550F (AA972) (n = 10–57). (E) Phase contrast and fluorescence images of CckA-CFP in a DivL depletion strain (NJH564, grown in presence of vanillate for divL expression), before and 16 h after removal of vanillate. (F) Phase contrast and fluorescence images of CckA-CFP in WT and ΔdivL strains in the presence of CtrAD51E (AA1001 and AA1003, respectively). Black and white arrowheads represent the old pole (stalked) and the new pole, respectively.
To confirm that DivL is required for the localization of CckA to the new pole, we generated a second mutant strain lacking any copy of divL. We constructed a divL deletion harboring a plasmid containing the phosphomimetic allele ctrAD51E under the control of the Pxyl promoter to support viability. It has been shown that the presence of CtrAD51E can rescue the lethality caused by the absence of divL (19). In the complete absence of DivL, CckA-CFP delocalized almost entirely, exhibiting few polar foci of CckA-CFP (Fig. 1 C and F), all of which were positioned at the old pole. Cumulatively, these results demonstrate that DivL promotes localization of CckA to the new cell pole.
DivL Activates the Autophosphorylation of CckA.
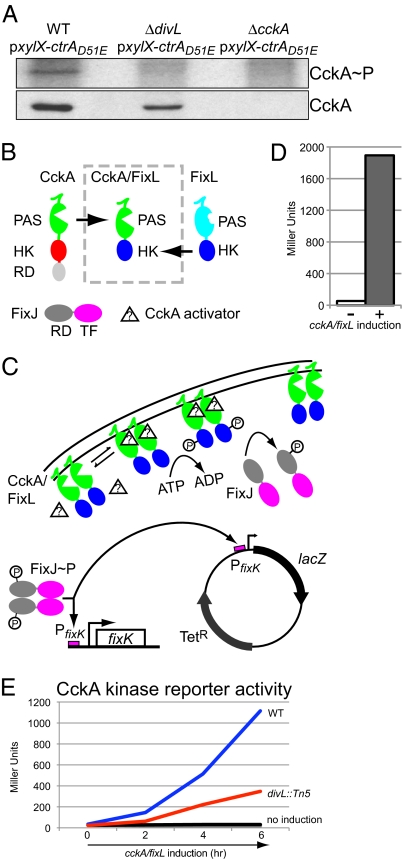
Conditional divL mutants exhibit reduced levels of phosphorylated CtrA when grown under restrictive conditions (19, 20), indicating that DivL plays a role in the pathway that controls the phosphorylation of CtrA. This observation, together with the fact that the lethality caused by the absence of either CckA or DivL can be rescued by the presence of the phosphomimetic protein CtrAD51E (11, 19), suggest that DivL and CckA act in the same signaling pathway. Because DivL is known to promote new pole CckA localization, we investigated whether DivL promotes the autophosphorylation of CckA. We performed in vivo phosphorylation experiments to examine whether the CckA phosphorylation state is affected by the absence of DivL, using WT and cckA deletion strains harboring CtrAD51E as positive and negative controls, respectively. As shown in Fig. 2A, no CckA phosphorylation was detected in a strain with a divL deletion, although Western blot analyses of the same samples demonstrated the presence of CckA protein. These findings indicate that DivL actively promotes both the autophosphorylation of CckA and its localization to the new cell pole.
Fig. 2.
DivL promotes the activity of CckA. (A) (Upper) PhosphorImager image obtained from an in vivo phosphorylation assay using anti-CckA antibodies performed with the WT, divL deletion, and cckA deletion strains (LS2715, AA980, and LS3360, respectively), all of which expressed the phosphomimetic allele ctrAD51E. (Lower) Corresponding immunoblots using anti-CckA antibodies. (B) Domain arrangements of CckA (sensor domain, PAS; histidine kinase domain, HK; receiver domain, RD), FixL, CckA/FixL (N-terminal transmembrane portion and sensor domain derived from CckA; C-terminal histidine kinase domain derived from FixL), and FixJ (transcription factor domain, TF). (C) In the CckA/FixL chimera strains, the presence of the hypothetical CckA activator results in CckA/FixL autophosphorylation, followed by phosphotransfer to the RD domain of FixJ. FixJ∼P then activates the transcription of chromosomal fixK, as well as the reporter plasmid pfixK-borne lacZ (3). (D) β-galactosidase activity of the CckA/FixL chimera strain (NJH227) was measured in the absence (−) and presence (+) of 0.3% xylose to induce cckA/fixL expression. (E) CckA kinase reporter activity for WT (NJH551, blue and black lines) and divL::Tn5 (NJH558, red and gray lines) strains. Cells were grown in exponential phase in M2G, and the expression of cckA/fixL was induced with xylose (blue and red lines) and then assayed for β-galactosidase activity. Control experiments without cckA/fixL induction are shown as indicated (black and gray lines).
A Chimeric Kinase Reporter System for CckA Kinase Activity.
A temporal correlation among CckA subcellular localization, autophosphorylation, and CckA phosphotransfer activity to CtrA has been reported previously (10, 11, 16). In the present study, we found that DivL promotes both the CckA's autophosphorylation and localization to the new pole. A direct way to approach the question of whether CckA's polar localization reflects its phosphotransfer activity was to assay the phosphorylation state of CtrA and CpdR, the targets of the CckA-ChpT pathway (12), in strains depleted of DivL. But the interpretation of these results would not be definitive, given the existence of multiple positive and negative feedback loops within the control network involving these regulatory proteins (1, 16, 19, 21). Consequently, we constructed a chimeric reporter of in vivo CckA activity that uses the LacZ reporter system previously developed for the FixL/FixJ two-component oxygen-sensing system (3). We grafted the N-terminal portion of CckA onto the C-terminal portion of FixL, resulting in a chimeric histidine kinase CckA/FixL that, when activated, phosphorylates the response regulator FixJ, which in turn activates the transcription of lacZ controlled by the PfixK promoter (Fig. 2 B and C). Before constructing the CckA/FixL chimera, we had to select grafting site residues for CckA and FixL (the last residue to be incorporated from the N terminus of CckA and the first residue to be incorporated from the C terminus of FixL). We generated multiple CckA/FixL chimeric constructs and, based on activity, selected grafting site K298 on CckA (including the transmembrane spanning region and the sensor domain with the PAS motif) and the graft site Q258 on FixL (including the histidine phosphotransfer domain and the catalysis-assisting ATP-binding domain). A strain containing a plasmid-borne xylose-inducible cckA/fixL chimera and a reporter plasmid pfixK-lacZ (3) was incubated in the absence or presence of the xylose inducer, and each strain was assayed for β-galactosidase activity (Fig. 2D).
Whereas background β-galactosidase activity was consistently low for the CckA reporter in the absence of cckA/fixL induction, xylose-induced expression of cckA/fixL resulted in a 38-fold increase in reporter activity. To validate and discriminate between constitutive (CckA activator–independent) and cell cycle–dependent CckA/FixL kinase activity, we assayed the de novo rates of LacZ synthesis as a function of the cell cycle (Fig. S1). The results suggest that CckA/FixL kinase activity is cell cycle–dependent, with activity increasing from a minimum level in swarmer cells to a maximum level in predivisional cells. This trend is largely in agreement with previously observed cell cycle– dependent rates of CckA autophosphorylation (11).
Having established the cell cycle–dependent kinase activity of the CckA/FixL chimera, we then compared the subcellular localization patterns of FixL-YFP and CckA/FixL-YFP with that of CckA-CFP (Fig. S2). In WT cells, FixL-YFP appeared to be fairly uniformly distributed throughout the cell and did not exclusively colocalize with CckA. In contrast, CckA/FixL colocalized with CckA at the cell poles. Thus, we have developed a chimeric reporter system that dynamically colocalizes with CckA, senses the cell cycle–dependent activation signals intended for CckA, and accurately transduces CckA's response to these signals into β-galactosidase activity units.
We used the CckA/FixL chimeric reporter system to study CckA activity in the divL:: Tn5 mutant strain, and found that the activity of the CckA/FixL chimera was 70% lower in the divL::Tn5 than in WT strain (Fig. 2E). Without induction of the CckA/FixL chimera, PfixK-lacZ reporter activity was at a very low basal level (Fig. 2E, black line). This reduced chimera activity in the divL::Tn5 mutant strain corresponded to the reduced bipolar CckA localization and reduced new pole CckA-CFP localization in cells presenting with monopolar CckA-CFP in the divL::Tn5 strain, resulting in a cumulative 65% decrease in new pole CckA-CFP localization (Fig. 1 C and D).
Taken together, our results indicate that in the complete absence of DivL, CckA localization to the new pole and CckA autophosphorylation is lost (Fig. 1 C and F and 2A). In addition, we observed a 70% reduction in CckA/FixL chimera activity in divL::Tn5 cells, commensurate with the loss of CckA-CFP at the new cell pole (Fig. 2E). Thus, CckA activity was enhanced when localized to the new pole, and this activation was dependent on the DivL protein kinase.
CckA Localization and Activity Are Independent of DivL Kinase Activity.
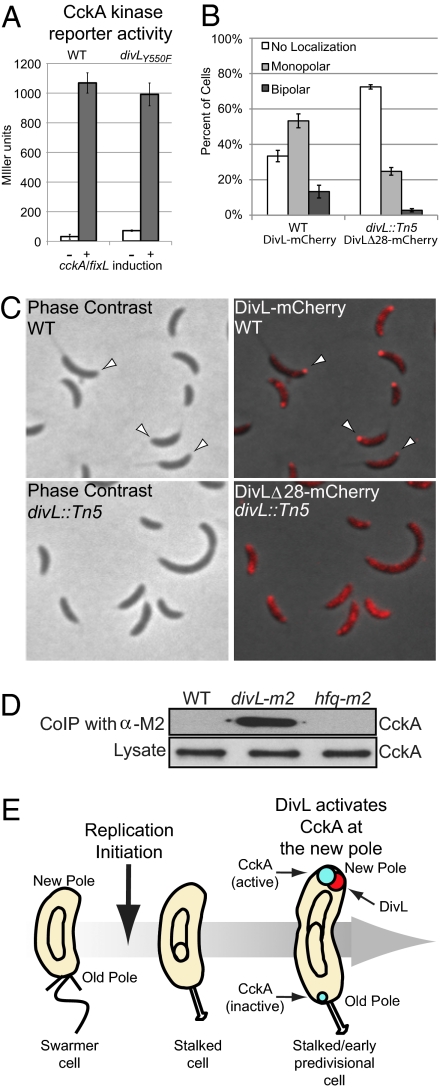
To determine whether CckA localization and phosphotransfer activity is dependent on the tyrosine kinase activity of DivL, we mutated the tyrosine residue shown to be phosphorylated in vitro to phenylalanine (divLY550F). This mutant strain was known to be not lethal (19). The divLY550F mutation did not significantly affect CckA-CFP subcellular localization (Fig. 1C) or the partitioning of monopolar CckA-CFP between new and old cell poles (Fig. 1D), and did not affect the activity of the CckA/FixL PfixK-lacZ reporter (Fig. 3A). This indicates that DivL kinase activity is not required for the appropriate polar localization or activation of CckA.
Fig. 3.
DivL promotes CckA activation by localization of CckA and DivL in the same polar protein complex. (A) CckA kinase reporter activity assays in WT (NJH551) and divLY550F (AA987) strains. Cells were grown to exponential phase in M2G with or without xylose overnight, to induce cckA/fixL expression, and then assayed for β-galactosidase activity. (B) Phase contrast and fluorescence images of DivL-mCherry in WT and DivLΔ28-mCherry localization in divL::Tn5 strains (AA889 and NJH576, respectively). Strains were grown in the presence of vanillate for 2 h before imaging, to induce the expression of divL-mCherry or divLΔ28-mCherry. (C) Histograms of DivL-mCherry localization in WT and DivLΔ28-mCherry localization in divL::Tn5 strains (AA889 and NJH576, respectively) (n = 505–608). (D) Western blots using anti-CckA antibodies showing the levels of CckA in the whole lysate (Lower), and in the elution sample after immunoprecipitation with anti-M2 epitope antibodies (Upper) from WT (CB15N), divL-m2 (AA1015), and hfq-m2 (LS4379) strains. (E) Representation of DivL activating CckA through the time. DivL and CckA are represented by red and blue circles, respectively. After initiation of DNA replication, DivL localizes at the new cell pole in stalked/early predivisional cell interacting and activating CckA. CckA at the old pole remains inactive. Circles inside the cells represent the chromosome/s.
DivL Promotes CckA Activation by Localization of CckA and DivL in the Same Polar Protein Complex.
As discussed earlier, the Tn5 transposon insertion in divL (which effectively truncates the last 28 C-terminal residues of DivL; Fig. 1A) showed a significant reduction in CckA new cell pole localization (Fig. 1 B–D). Because DivL itself localizes predominantly to the new cell pole, and because deletions of DivL C-terminal sequences significantly reduce the localization of DivL (18, 19), we reasoned that improper localization of truncated DivL could contribute to the reduction of CckA localization to the new pole and a concomitant reduction in CckA/FixL chimera activity. Accordingly, we constructed a mutant strain with divLΔ28-mCherry on the vanA locus in a divL::Tn5 background and examined the subcellular localization pattern of DivLΔ28-mCherry (Fig. 3C). We found an overall diminished DivLΔ28-mCherry polar localization of ∼60% compared with DivL-mCherry in a WT background (Fig. 3 B and C). We then investigated whether these two proteins interact in vivo. We generated a strain containing a copy of divL with its 3′ end fused in frame to a DNA sequence encoding the Flag epitope (M2) as the only copy of divL and at its native locus. We performed coimmunoprecipitation experiments using antibodies against the M2 epitope with the divL-m2 and WT strains, and found that CckA coimmunoprecipitated with DivL-M2 (Fig. 3D). As an additional control, we observed that CckA did not coimmunoprecipitate with Hfq-M2, an abundant RNA-binding protein (22), demonstrating that the coimmunoprecipitation of CckA with DivL-M2 is specific. Taken together, these results suggest that DivL promotes the autophosphorylation and activation of CckA, directly or indirectly, by localizing CckA and DivL at the new cell pole in the same protein complex.
Discussion
Activation of the essential CckA histidine kinase at a specific point in the cell cycle triggers a cascade of regulatory events that orchestrate the asymmetric cell division that is the hallmark of the Caulobacter cell cycle. The events that cue the temporally controlled autophosphorylation of CckA have remained elusive. We have presented evidence that activation of CckA occurs preferentially at the new pole, and that autophosphorylation of CckA is dependent on the DivL protein as it transiently localizes to the new cell pole where it interacts with CckA.
Polar Localization and Activation of the CckA Histidine Kinase.
One of the more curious aspects of the cell cycle–controlled activation of CckA is its transient localization at the cell poles. After the differentiation of the swarmer cell into the stalked cell and initiation of DNA replication, CckA accumulates at the new pole and also to some extent at the old cell pole, and it maintains this polar localization pattern up to the late predivisional cell stage (10, 16). Although CckA is present throughout the cell cycle, the timing of its autophosphorylation coincides with its transient localization to the cell poles (10, 11), suggesting a causal relationship. Using site-directed mutagenesis of the CckA hybrid kinase, it has been shown that replacing the phosphorylated amino acids, histidine H322 and/or aspartic acid D623, with alanine does not alter the localization pattern of CckA (16). These residues are essential for CckA function and viability, however, arguing that localization might precede and be required for autophosphorylation. Angelastro et al. (16) also determined that independent deletions of the PAS motif and the catalysis-assisting ATP-binding domain of CckA causes aberrant CckA localization accompanied by defects in CckA function, but not lethality (16). To gain insight into the relationship between CckA polar localization and its activation, we chose to study CckA activity by designing a chimeric CckA/FixL kinase with a reporter readout, and to use this assay to identify factors that control the temporal activation of CckA.
Polar Localization and Activation of the CckA Histidine Kinase by DivL.
A conditional transposon insertion screen for CckA mislocalization mutants revealed that the DivL protein kinase is required for CckA polar localization and autophosphorylation, as well as for efficient CckA/FixL chimera activity. The divL mutant identified in this screen had a transposon insertion in the sequence encoding its ATPase domain, yielding a DivL protein lacking the 28 C-terminal amino acids, corresponding to the ATPase domain (Fig. 1A). Although DivL is essential for viability, a strain lacking the last 28 amino acids of DivL was not lethal, but did exhibit morphological and growth defects. In agreement with this, Reisinger et al. (19), analyzing multiple transposon insertions in divL, concluded that the ATPase domain of DivL is dispensable for viability. Importantly, the DivL mutant protein with a 28-aa truncation exhibited aberrant subellular localization (Fig. 3 B and C).
We found that DivL is required for the localization of CckA to the new cell pole. In a strain with divL deleted and expressing the phosphomimetic ctrAD51E, to support viability, CckA was never seen at the new cell pole, although a very small percentage of cells had CckA localized at the old pole (Fig. 1 C and F). Because autophosphorylation of CckA did not occur in the complete absence of DivL (Fig. 2A), the localization of CckA to the new cell pole correlates with its phosphorylation state in a DivL-dependent manner. The phosphorylation state of histidine kinases is usually considered a valid measure of their activity. It has been reported that phosphorylated CtrA, a CckA phosphorylation–dependent target, is reduced in a divL510 mutant (a recessive loss-of-function allele) (19). Furthermore, the phosphomimetic protein CtrAD51E can rescue the lethality of the ΔdivL mutant (Figs. 1 C and F and 2A) (19), as well the ΔcckA mutant (11). Taken together, these observations argue that DivL influences the phosphorylation state of CtrA in a CckA-dependent manner; we propose that it does so by regulating the phosphorylation state of CckA.
The existence of multiple positive and negative feedback loops within the CckA/CtrA/CpdR regulatory network, which includes differential transcription, phosphorylation, dephosphorylation, and proteolysis of the CckA phosphorylation–dependent targets CtrA and CpdR, makes the study and interpretation of CckA activity based on the phosphorylation state of these targets difficult to untangle (1, 16, 19, 21). For this reason, we assessed the phospho-transfer activity of CckA by constructing a validated chimeric kinase reporter system (Fig. 2 B–D). The sensor domain of CckA was fused to the histidine kinase domain of FixL. Autophosphorylation of the chimeric kinase in response to a cell cycle signal resulted in the cell cycle–regulated phosphorylation of the FixJ response regulator. FixJ∼P in turn activated the fixK promoter, which when fused to lacZ provided a quantitative readout of chimeric CckA/FixL kinase activity. Using this reporter system, we found significantly reduced CckA activity in a divL::Tn5 strain (Fig. 2E). Considering these results along with those obtained from CckA polar localization and autophosphorylation experiments, we conclude that DivL promotes the autophosphorylation of CckA at the new cell pole, and that the CckA observed at the stalked pole is not active (Fig. 3E). A recent quantitative analysis of the spatial distribution of CckA during the cell cycle argued that localization of CckA at the new pole is likely important for CckA function and regulation, and that the localization at the old pole might not play an important role, serving only as a “depot” for excess CckA (16).
Although DivL is a protein kinase (17), its kinase activity was not required for the localization or activation of CckA (Figs. 1 C and D and 3A). Just how DivL, which is devoid of kinase activity, regulates the phosphorylation of CckA remains a matter of conjecture. Given our findings that the localization of CckA-CFP at the new cell pole was significantly reduced in the divL::Tn5 strain (Fig. 1 A–D), and that the polar localization of DivLΔ28-mCherry was reduced to the same extent in the divL::Tn5 strain (Fig. 3 B and C), we propose that the localization of DivL at the new cell pole functions to promote CckA localization at that pole, where CckA finds its niche for autophosphorylation and activation. In support of these observations, we found that CckA interacts in vivo, directly or indirectly, with DivL. CckA localization occurs at a specific time in the cell cycle after the initiation of replication (10, 11). In preliminary experiments, we found that inhibiting the initiation of DNA replication prevents the localization of both DivL and CckA to the new cell pole, providing a link to cell cycle progression.
In summary, we conclude that on the initiation of DNA replication in the stalked cell, DivL accumulates at the new cell pole, driving the localization of CckA to that pole and promoting its autophosphorylation and phosphotransfer activity, whereas any CckA accumulated at the stalked pole remains inactive (Fig. 3E). Our findings demonstrate how the enigmatic DivL kinase is integrated into the Caulobacter cell cycle regulatory circuitry.
Experimental Procedures
Growth Conditions and Cell Manipulations.
Caulobacter was grown at 28 °C in PYE-rich media, M2G media, or M5G low-phosphate media supplemented with 1 mM glutamate (M5GG) (23, 24). Unless indicated otherwise, the experiments were performed by growing Caulobacter in M2G, keeping it in log phase. When necessary, the medium was supplemented with 0.3% xylose, 0.2% glucose, or 0.5 mM vanillate. Caulobacter transformation was performed by electroporation as described previously (21). Generalized transduction was performed with the phage ΦCr30 as described previously (23). Immunoblot analysis and in vivo phosphorylation experiments are described in SI Experimental Procedures.
CckA/FixL PfixK-lacZ Reporter Activity Assays and G1-Phase Cell Synchrony.
β-galactosidase assays were conducted as described previously (25). LacZ synthesis was measured by performing [35S]-methionine pulse experiments as described previously (26). Before G1-phase cell synchrony [performed as described previously (27)], the culture was incubated with 0.3% xylose overnight, and xylose was present during the [35S]-methionine pulse experiment.
Fluorescence Microscopy.
Strains were grown in M2G minimal media and immobilized onto 1.0% agar in M2G before imaging by phase and epifluorescent microscopy with a Leica DM6000 microscope using KAMS v0.8 software (28). Adobe Photoshop CS2 was used to scale channel intensity, maintaining a gamma value of 1 to maximize the dynamic display range. Histograms of monopolar CckA-CFP or DivL-mCherry partitioning between new and old poles were created by observing and quantifying monopolar fluorescence dots in cells presenting with a stalk. The transposon-mediated conditional mutagenesis and the fluorescence microscopy screen are described in SI Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants GM51426, GM32506, and GM073011 (to L.S.) and Department of Energy Grant DE-FG-02-05ER64136 (to L.S.). N.H. was supported by Damon Runyon Cancer Research Foundation fellowship DRG-1880-05. We thank J. C. Chen and L. Britos for providing plasmid pJC343 and pLB07 and J. L. Boyd-Kozdon for assistance with the construction of plasmid pNJH221.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1001767107/DCSupplemental.
References
- 1.Laub MT, Shapiro L, McAdams HH. Systems biology of Caulobacter. Annu Rev Genet. 2007;41:429–441. doi: 10.1146/annurev.genet.41.110306.130346. [DOI] [PubMed] [Google Scholar]
- 2.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 3.Crosson S, McGrath PT, Stephens C, McAdams HH, Shapiro L. Conserved modular design of an oxygen sensory/signaling network with species-specific output. Proc Natl Acad Sci USA. 2005;102:8018–8023. doi: 10.1073/pnas.0503022102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabret C, Feher VA, Hoch JA. Two-component signal transduction in Bacillus subtilis: How one organism sees its world. J Bacteriol. 1999;181:1975–1983. doi: 10.1128/jb.181.7.1975-1983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura Y, Nakano H, Terasaka H, Takegawa K. Myxococcus xanthus mokA encodes a histidine kinase–response regulator hybrid sensor required for development and osmotic tolerance. J Bacteriol. 2001;183:1140–1146. doi: 10.1128/JB.183.4.1140-1146.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby JR. Chemotaxis-like regulatory systems: Unique roles in diverse bacteria. Annu Rev Microbiol. 2009;63:45–59. doi: 10.1146/annurev.micro.091208.073221. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro L, Losick R. Protein localization and cell fate in bacteria. Science. 1997;276:712–718. doi: 10.1126/science.276.5313.712. [DOI] [PubMed] [Google Scholar]
- 8.Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT. Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: A system-level analysis. PLoS Biol. 2005;3:e334. doi: 10.1371/journal.pbio.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quon KC, Marczynski GT, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs C, Domian IJ, Maddock JR, Shapiro L. Cell cycle–dependent polar localization of an essential bacterial histidine kinase that controls DNA replication and cell division. Cell. 1999;97:111–120. doi: 10.1016/s0092-8674(00)80719-9. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs C, Ausmees N, Cordwell SJ, Shapiro L, Laub MT. Functions of the CckA histidine kinase in Caulobacter cell cycle control. Mol Microbiol. 2003;47:1279–1290. doi: 10.1046/j.1365-2958.2003.03379.x. [DOI] [PubMed] [Google Scholar]
- 12.Biondi EG, et al. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature. 2006;444:899–904. doi: 10.1038/nature05321. [DOI] [PubMed] [Google Scholar]
- 13.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–160. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 14.Laub MT, Chen SL, Shapiro L, McAdams HH. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc Natl Acad Sci USA. 2002;99:4632–4637. doi: 10.1073/pnas.062065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quon KC, Yang B, Domian IJ, Shapiro L, Marczynski GT. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci USA. 1998;95:120–125. doi: 10.1073/pnas.95.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelastro PS, Sliusarenko O, Jacobs-Wagner C. Polar localization of the CckA histidine kinase and cell cycle periodicity of the essential master regulator CtrA in Caulobacter crescentus. J Bacteriol. 2010;192:539–552. doi: 10.1128/JB.00985-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Ohta N, Zhao JL, Newton A. A novel bacterial tyrosine kinase essential for cell division and differentiation. Proc Natl Acad Sci USA. 1999;96:13068–13073. doi: 10.1073/pnas.96.23.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sciochetti SA, Ohta N, Newton A. The role of polar localization in the function of an essential Caulobacter crescentus tyrosine kinase. Mol Microbiol. 2005;56:1467–1480. doi: 10.1111/j.1365-2958.2005.04652.x. [DOI] [PubMed] [Google Scholar]
- 19.Reisinger SJ, Huntwork S, Viollier PH, Ryan KR. DivL performs critical cell cycle functions in Caulobacter crescentus independent of kinase activity. J Bacteriol. 2007;189:8308–8320. doi: 10.1128/JB.00868-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce DL, et al. Mutations in DivL and CckA rescue a divJ null mutant of Caulobacter crescentus by reducing the activity of CtrA. J Bacteriol. 2006;188:2473–2482. doi: 10.1128/JB.188.7.2473-2482.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iniesta AA, Shapiro L. A bacterial control circuit integrates polar localization and proteolysis of key regulatory proteins with a phospho-signaling cascade. Proc Natl Acad Sci USA. 2008;105:16602–16607. doi: 10.1073/pnas.0808807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landt SG, et al. Small non-coding RNAs in Caulobacter crescentus. Mol Microbiol. 2008;68:600–614. doi: 10.1111/j.1365-2958.2008.06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ely B. Genetics of Caulobacter crescentus. Methods Enzymol. 1991;204:372–384. doi: 10.1016/0076-6879(91)04019-k. [DOI] [PubMed] [Google Scholar]
- 24.Domian IJ, Quon KC, Shapiro L. Cell type–specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- 25.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1972. [Google Scholar]
- 26.Collier J, Murray SR, Shapiro L. DnaA couples DNA replication and the expression of two cell cycle master regulators. EMBO J. 2006;25:346–356. doi: 10.1038/sj.emboj.7600927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toro E, Hong SH, McAdams HH, Shapiro L. Caulobacter requires a dedicated mechanism to initiate chromosome segregation. Proc Natl Acad Sci USA. 2008;105:15435–15440. doi: 10.1073/pnas.0807448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christen B, et al. High-throughput identification of protein localization dependency networks. Proc Natl Acad Sci USA. 2010;107:4681–4686. doi: 10.1073/pnas.1000846107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.