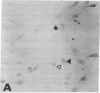
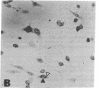
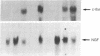
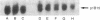Abstract
The chain of events that results in increased production of nerve growth factor (NGF) following beta-adrenergic receptor (BAR) stimulation has been investigated in the C6-2B rat astrocytoma cell line. Exposure of these cells to the BAR agonist isoproterenol elicits the following cascade of events: (i) increase of cAMP content; (ii) increase of c-Fos mRNA content; (iii) accumulation of c-Fos protein immunoreactivity in the nucleus; (iv) increase of NGF mRNA content. The increase in c-Fos mRNA and its translation product are early events (15 and 40 min, respectively) and precede the accumulation of NGF mRNA, which peaks at 3 hr. The increase in the two mRNAs appears interrelated because cycloheximide inhibits the accumulation of c-Fos protein and NGF mRNA elicited by isoproterenol. Moreover, the accumulation of nuclear c-Fos protein and NGF mRNA induced by BAR stimulation is reduced by 2-aminopurine, an inhibitor of c-Fos mRNA induction. These data suggest that, in C6-2B astrocytoma cells, the nuclear accumulation of c-Fos protein is required for the induction of NGF mRNA expression by BAR stimulation.
Full text
PDF



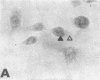
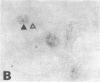
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barka T., Gubits R. M., van der Noen H. M. Beta-adrenergic stimulation of c-fos gene expression in the mouse submandibular gland. Mol Cell Biol. 1986 Aug;6(8):2984–2989. doi: 10.1128/mcb.6.8.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Van Beveren C., Verma I. M. Viral and cellular fos proteins are complexed with a 39,000-dalton cellular protein. Mol Cell Biol. 1985 Jan;5(1):167–172. doi: 10.1128/mcb.5.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Toso R., De Bernardi M. A., Brooker G., Costa E., Mocchetti I. Beta adrenergic and prostaglandin receptor activation increases nerve growth factor mRNA content in C6-2B rat astrocytoma cells. J Pharmacol Exp Ther. 1988 Sep;246(3):1190–1193. [PubMed] [Google Scholar]
- Dal Toso R., De Bernardi M. A., Costa E., Mocchetti I. Beta-adrenergic receptor regulation of NGF-mRNA content in rat C6-2B glioma cells. Neuropharmacology. 1987 Dec;26(12):1783–1786. doi: 10.1016/0028-3908(87)90133-x. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Phosphorylation of initiation factor eIF-2 alpha, binding of mRNA to 48 S complexes, and its reutilization in initiation of protein synthesis. J Biol Chem. 1983 Dec 10;258(23):14556–14562. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G., Nirenberg M. Effect of catecholamines on the adenosine 3':5'-cyclic monophosphate concentrations of clonal satellite cells of neurons. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2165–2168. doi: 10.1073/pnas.68.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington C. A., Lewis E. J., Krzemien D., Chikaraishi D. M. Identification and cell type specificity of the tyrosine hydroxylase gene promoter. Nucleic Acids Res. 1987 Mar 11;15(5):2363–2384. doi: 10.1093/nar/15.5.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa A., Guidotti A., Costa E. Nuclear translocation of cyclic AMP-dependent protein kinase subunits during the transsynaptic activation of gene expression in rat adrenal medulla. Mol Pharmacol. 1979 Jan;15(1):115–130. [PubMed] [Google Scholar]
- Milner R. J., Sutcliffe J. G. Gene expression in rat brain. Nucleic Acids Res. 1983 Aug 25;11(16):5497–5520. doi: 10.1093/nar/11.16.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I., Naranjo J. R., Costa E. Regulation of striatal enkephalin turnover in rats receiving antagonists of specific dopamine receptor subtypes. J Pharmacol Exp Ther. 1987 Jun;241(3):1120–1124. [PubMed] [Google Scholar]
- Montminy M. R., Goodman R. H., Horovitch S. J., Habener J. F. Primary structure of the gene encoding rat preprosomatostatin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3337–3340. doi: 10.1073/pnas.81.11.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols G. A., Brooker G. Temperature sensitivity of cyclic AMP production and catecholamine-induced refractoriness in a rat astrocytoma cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5520–5524. doi: 10.1073/pnas.75.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Cohen D. R., Curran T., Bos T. J., Vogt P. K., Bohmann D., Tjian R., Franza B. R., Jr Fos-associated protein p39 is the product of the jun proto-oncogene. Science. 1988 May 20;240(4855):1010–1016. doi: 10.1126/science.3130660. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Chuang D. M., Costa E. Increase in nerve growth factor content of C6 glioma cells by the activation of a beta-adrenergic receptor. Brain Res. 1977 Dec 2;137(2):369–375. doi: 10.1016/0006-8993(77)90349-3. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Costa E. Regulation of nerve growth factor content in C6 glioma cells by beta-adrenergic receptor stimulation. Naunyn Schmiedebergs Arch Pharmacol. 1977 Nov;300(2):123–129. doi: 10.1007/BF00505042. [DOI] [PubMed] [Google Scholar]
- Szekely A. M., Barbaccia M. L., Costa E. Activation of specific glutamate receptor subtypes increases C-fos proto-oncogene expression in primary cultures of neonatal rat cerebellar granule cells. Neuropharmacology. 1987 Dec;26(12):1779–1782. doi: 10.1016/0028-3908(87)90132-8. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Horovitch S. J., Montminy M. R., Mandel G., Goodman R. H. Structure of the human vasoactive intestinal polypeptide gene. DNA. 1985 Aug;4(4):293–300. doi: 10.1089/dna.1985.4.293. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Berman C., Dull T. J. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983 Jun 30;303(5920):821–825. doi: 10.1038/303821a0. [DOI] [PubMed] [Google Scholar]
- Walsh J. Economists and inflation: which way out of the wilderness? Science. 1974 Oct 11;186(4159):122–125. doi: 10.1126/science.186.4159.122. [DOI] [PubMed] [Google Scholar]
- Waterman M., Murdoch G. H., Evans R. M., Rosenfeld M. G. Cyclic AMP regulation of eukaryotic gene transcription by two discrete molecular mechanisms. Science. 1985 Jul 19;229(4710):267–269. doi: 10.1126/science.2990047. [DOI] [PubMed] [Google Scholar]
- Zinn K., Keller A., Whittemore L. A., Maniatis T. 2-Aminopurine selectively inhibits the induction of beta-interferon, c-fos, and c-myc gene expression. Science. 1988 Apr 8;240(4849):210–213. doi: 10.1126/science.3281258. [DOI] [PubMed] [Google Scholar]