Abstract
The surface specific technique vibrational sum frequency spectroscopy has been applied to in situ studies of the degradation of Langmuir monolayers of 1,2-diacyl-phosphocholines with various degrees of unsaturation in the aliphatic chains. To monitor the degradation of the phospholipids, the time-dependent change of the monolayer area at constant surface pressure and the sum frequency intensity of the vinyl CH stretch at the carbon-carbon double bonds were measured. The data show a rapid degradation of monolayers of phospholipids carrying unsaturated aliphatic chains compared to the stable lipids carrying fully saturated chains when exposed to the ambient laboratory air. In addition, the degradation of the phospholipids can be inhibited by purging the ambient air with nitrogen. This instability may be attributed to spontaneous degradation by oxidation mediated by various reactive species in the air. To further elucidate the process of lipid oxidation in biological membranes artificial Langmuir monolayers probed by a surface specific spectroscopic technique as in this study can serve as a model system for studying the degradation/oxidation of cell membrane constituents.
Main Text
Phospholipids are the main constituents of all biological membranes and are thus vital components of the cell. One of the most important molecular processes damaging membrane structures is lipid oxidation, which significantly increases when an imbalance between pro-oxidant and antioxidant systems occurs. Free radical reactions play a central role in such oxidative stress. The propagation of their peroxidation products provokes severe perturbations of the membranes' physical properties and biological functions (1). Thus, it is of great importance to fully understand the processes of lipid oxidation in biological membranes. Due to the high complexity of living cells, lipid oxidation is difficult to study in vivo and model systems like artificial phospholipid monolayers may be used as an alternative.
The oxidation of lipids is a well-known phenomenon that has been studied for centuries. However, the majority of this work has focused on oxidation of bulk lipids and bilayers and, to the best of our knowledge, Langmuir monolayers of unsaturated lipids exposed to the atmosphere remain unexplored in this context. The combination of the Langmuir technique for preparing a monolayer with the surface specific spectroscopic technique used in this study offers a way to investigate the oxidation of lipids in situ (2,3).
Oxidation of C=C double bonds in lipids can be mediated by a variety of reactive species in the atmosphere, such as singlet oxygen, ozone, or the OH• radical (4,5). After the initial reaction step, which causes the vinyl CH stretch to disappear, further decomposition results in the insertion of a variety of oxygen containing polar groups. These increase the solubility and restrict the free movement of the aliphatic chains by forming hydrogen bonds with the surrounding water (6). Another outcome is breakage of the chains, which generates more soluble short chain aldehydes or alcohols (7).
We have investigated the degradation in situ of Langmuir monolayers of four 1,2-diacyl-phosphocholines with identical fully saturated, single, double, and triple unsaturated aliphatic chains respectively, using vibrational sum frequency spectroscopy (VSFS) (8,9). VSFS is a surface specific technique, which obviates the need for background subtraction and provides submonolayer sensitivity. Essentially, two high intensity pulsed laser beams, one tunable in the infrared (IR) and one fixed in the visible range, are spatially and temporally overlapped on the surface of the sample. Provided that the inversion symmetry is broken, as is the case for an interface, a sum frequency signal is generated which is enhanced when the IR frequency is in resonance with a vibrational frequency of a molecule at the interface. All spectra shown in this article are of the polarization combination ssp, where the p-polarization is parallel and the s-polarization is perpendicular to the plane of incidence. The order refers to the polarizations of the sum frequency, visible, and IR beams respectively. A detailed description of the VSFS setup used has been presented elsewhere (10). Briefly, it features a 24 picoseconds laser, an OPG/OPA to generate the visible and tunable IR-beams, a photomultiplier tube connected to a monochromator, and a gated integrator to acquire the signal.
1,2-distearoyl-sn-glycero-3-phosphocholine (18:0 PC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (18:1 PC), 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (18:2 PC), and 1,2-dilinolenoyl-sn-glycero-3-phosphocholine (18:3 PC) were purchased from Avanti Polar Lipids (Alabaster, AL). All water used was purified using a Millipore (Billerica, MA) system featuring constant monitoring of the conductivity (>18.2 MΩ·cm) and the organic content (<4 ppb). To prepare the monolayers, a 1 mg/ml solution of lipid in CHCl3 (≥99.8%, stabilized with amylene, used as received, Sigma Aldrich, St. Louis, MO) was spread at the pure water surface (21 ± 0.5°C) of a Langmuir trough (Minimicro 1S, KSV Instruments, Helsinki, Finland). The monolayer was immediately compressed to a surface pressure of 4 mN/m using a speed of 5 mm/min and the recording of the vibrational sum frequency spectrum was started as soon as the desired surface pressure was reached. To lower the content of reactive species in the atmosphere adjacent to the film, the trough was placed in a sealed box that was purged with filtered nitrogen in some of the experiments. The rate of the degradation varied from day to day, but the general trends were the same. The data for the unsaturated lipids exposed to the laboratory air presented in this article were recorded during the same day and are a representative selection of many repeats.
The relative monolayer area, A/A0, where A is the measured trough area and A0 is the area after the initial compression to 4 mN/m was finished, is plotted versus time in Fig. 1. It is evident that the monolayer area decreased rapidly with time when the films of unsaturated lipids were exposed to the laboratory air, while the area remained almost constant for the saturated lipids. At a relative area of ≈ 0.3, the barriers could not move anymore and the experiment was terminated. As shown for 18:2 PC, protecting the monolayer by purging with nitrogen stabilized all three unsaturated films.
Figure 1.
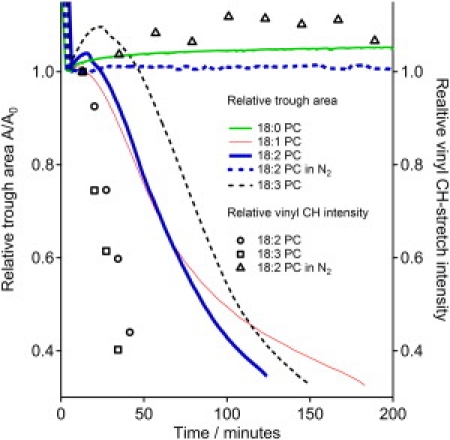
Relative trough area of monolayers of phospholipids with saturated and increasingly unsaturated aliphatic chains at constant surface pressure plotted versus time. The 18:0 PC monolayer was stable, whereas the films of unsaturated lipids degraded. Purging the ambient atmosphere with nitrogen inhibited the degradation. The markers show the amplitude of the vinyl CH stretch.
Fig. 2 shows the corresponding vibrational sum frequency spectra for the four lipids at different times. The resonances at 2850 cm−1 and 2880 cm−1 are the methylene and methyl symmetric stretching vibrations (CH2-ss and CH3-ss) respectively, and their Fermi resonances (CH2-FR and CH3-FR) form a broad peak centered at 2940 cm−1 (11). The cis-carbon-carbon double bonds on the unsaturated lipids give rise to a vinyl CH stretch vibration at 3010 cm−1 (12). This peak is clearly visible only for the two unsaturated lipids with two and three double bonds per aliphatic chain and decays with time, primarily because the C=C double bonds are destroyed, while the signal from the lipid containing one double bond per chain is too weak to be detected. In Fig. 1, the fitted amplitudes (9) of this peak are plotted and normalized so that the amplitude in the first spectrum acquired is set to one. At relative amplitudes lower than ∼0.4 the peaks are too weak to produce a reliable fit.
Figure 2.
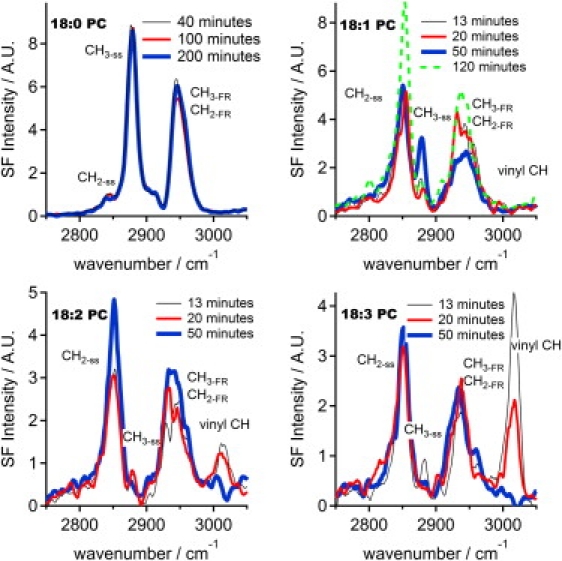
VSFS-spectra showing the changes accompanying the degradation in air. The 18:2 PC and 18:3 PC exhibit a vinyl CH peak that essentially vanishes within 50 min. The ratio of the CH2 to CH3 peaks indicates that 18:0 PC forms a well-ordered, tightly packed monolayer whereas the lipids containing unsaturations form significantly less ordered monolayers.
The decrease in area of the unsaturated lipid monolayers exposed to laboratory air indicates that matter leaves the surface. The sharp decay of the vinyl CH peak, and the fact that it disappears almost completely before the monolayer area starts to decrease, supports the hypothesis that the reduction in monolayer area is caused by oxidation followed by dissolution of the oxidized species. The alternative explanation —that the area decrease is a consequence of dissolution of monomers without preceding oxidation— can be ruled out since this would not result in major changes of any of the spectral features. Additional data not presented here show that similar degradation also takes place at higher surface pressures (20 mN/m).
It is worth noting that, after the initial compression to constant pressure in laboratory air, the area of the monolayers increases during the first few minutes, while at the same time the VSFS spectra show an obvious decrease in the vinyl amplitude, indicating that the decomposition has already begun. This increase in molecular area is more prominent for lipids with many unsaturations and is almost certainly due to a combinaton of two factors. Firstly, the number of species at the surface is likely to be increased upon oxidation due to cleavage and these fragments will mix in the layer with the remaining and unreacted monolayer. Secondly, any polar component associated with oxidation of the chain will be energetically disfavored in the alkyl region and the remaining chain will thus tend to change conformation such that the polar species can sample the more polar environment of the headgroup-water interface. This will necessarily increase the area per molecule (5).
The ratio of the CH3-ss and CH2-ss intensities can be used to estimate the overall order of the lipid monolayer (13). In a tightly packed, well-ordered monolayer the aliphatic chains are stretched in an all trans conformation and hence the CH2 groups reside in a locally centrosymmetric environment and will not give rise to any signal, leading to an ssp spectrum dominated by the CH3-ss peak and its Fermi resonance. Conversely, the ssp spectrum of a disordered monolayer mainly displays a CH2-ss peak with little or no CH3-ss signal (3) as gauche defects destroy the local centrosymmetry and the ordering of the methyl group is compromised. The observation that the CH2 peak was significantly more intense than the CH3 peak during the degradation of the unsaturated monolayers clearly indicates a disordered monolayer and that its area decrease is not caused by an increasingly dense packing. Instead, the intensity of the CH3 peak decreases, except for 18:1 PC where it temporarily increases during part of the degradation, and the CH2 peak increases indicating that the degradation causes the film to become even more disordered.
The spectrum of 18:0 PC exhibited a strong CH3 signal and only a negligible contribution from the CH2, which is consistent with a well-ordered, tightly packed monolayer; only minor changes were observed within 3.5 h. This observation, together with the constant monolayer area, indicates that no degradation of the fully saturated phospholipid took place.
In summary, we have shown that Langmuir monolayers of unsaturated phospholipids on a water subphase are not stable and quickly degrade when in contact with the ambient atmosphere, whereas fully saturated phospholipids are essentially completely stable. The degradation is due to the reaction of the double bonds with reactive species in the air and the use of a controlled environment inhibits degradation and protects the monolayer.
Acknowledgments
Financial support from the Swedish Centre for Biomimetic Fiber Engineering (Biomime), the Swedish Research Council (V.R.), and the Swedish Foundation for Strategic Research (S.S.F.) is gratefully acknowledged. M.R. is a fellow of the Swedish Research Council.
References and Footnotes
- 1.Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Ma G., Allen H.C. Condensing effect of palmitic acid on DPPC in mixed Langmuir monolayers. Langmuir. 2007;23:589–597. doi: 10.1021/la061870i. [DOI] [PubMed] [Google Scholar]
- 3.Roke S., Schins J., Bonn M. Vibrational spectroscopic investigation of the phase diagram of a biomimetic lipid monolayer. Phys. Rev. Lett. 2003;90:128101. doi: 10.1103/PhysRevLett.90.128101. [DOI] [PubMed] [Google Scholar]
- 4.Dix T.A., Aikens J. Mechanisms and biological relevance of lipid peroxidation initiation. Chem. Res. Toxicol. 1993;6:2–18. doi: 10.1021/tx00031a001. [DOI] [PubMed] [Google Scholar]
- 5.Eliason T.L., Gilman J.B., Vaida V. Oxidation of organic films relevant to atmospheric aerosols. Atmos. Environ. 2004;38:1367–1378. [Google Scholar]
- 6.Wong-Ekkabut J., Xu Z.T., Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys. J. 2007;93:4225–4236. doi: 10.1529/biophysj.107.112565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalá A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int. J. Biochem. Cell Biol. 2006;38:1482–1495. doi: 10.1016/j.biocel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y.R. Surface-properties probed by 2nd-harmonic and sum-frequency generation. Nature. 1989;337:519–525. [Google Scholar]
- 9.Lambert A.G., Davies P.B., Neivandt D.J. Implementing the theory of sum frequency generation vibrational spectroscopy: A tutorial review. Appl. Spectrosc. Rev. 2005;40:103–145. [Google Scholar]
- 10.Johnson C.M., Tyrode E., Leygraf C. A vibrational sum frequency spectroscopy study of the liquid-gas interface of acetic acid-water mixtures: 1. Surface speciation. J. Phys. Chem. B. 2005;109:321–328. doi: 10.1021/jp047338q. [DOI] [PubMed] [Google Scholar]
- 11.Lu R., Gan W., Wang H.F. C-H stretching vibrations of methyl, methylene and methine groups at the vapor/alcohol (N = 1-8) interfaces. J. Phys. Chem. B. 2005;109:14118–14129. doi: 10.1021/jp051565q. [DOI] [PubMed] [Google Scholar]
- 12.Attygalle A.B., Svatos A., Voerman S. Gas-phase infrared-spectroscopy for determination of double-bond configuration of some polyunsaturated pheromones and related-compounds. Anal. Chem. 1995;67:1558–1567. doi: 10.1021/ac00082a016. [DOI] [PubMed] [Google Scholar]
- 13.Guyot-Sionnest P., Hunt J.H., Shen Y.R. Sum-frequency vibrational spectroscopy of a Langmuir film: Study of molecular orientation of a two-dimensional system. Phys. Rev. Lett. 1987;59:1597–1600. doi: 10.1103/PhysRevLett.59.1597. [DOI] [PubMed] [Google Scholar]


