Abstract
African primates are naturally infected with over 40 different simian immunodeficiency viruses (SIVs), two of which have crossed the species barrier and generated human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2)1,2. Unlike the human viruses, however, SIVs do not generally cause acquired immunodeficiency syndrome (AIDS) in their natural hosts3. Here we show that SIVcpz, the immediate precursor of HIV-1, is pathogenic in free-ranging chimpanzees. By following 94 members of two habituated chimpanzee communities in Gombe National Park, Tanzania, for over 9 years, we found a 10- to 16-fold higher age-corrected death hazard for SIVcpz-infected (n = 17) compared to uninfected (n = 77) chimpanzees. We also found that SIVcpz-infected females were less likely to give birth and had a higher infant mortality rate than uninfected females. Immunohistochemistry and in situ hybridization of post-mortem spleen and lymph node samples from three infected and two uninfected chimpanzees revealed significant CD4+ T-cell depletion in all infected individuals, with evidence of high viral replication and extensive follicular dendritic cell virus trapping in one of them. One female, who died within 3 years of acquiring SIVcpz, had histopathological findings consistent with end-stage AIDS. These results indicate that SIVcpz, like HIV-1, is associated with progressive CD4+ T-cell loss, lymphatic tissue destruction and premature death. These findings challenge the prevailing view that all natural SIV infections are non-pathogenic and suggest that SIVcpz has a substantial negative impact on the health, reproduction and lifespan of chimpanzees in the wild.
Little is known about the in vivo pathogenicity of SIVcpz because, until recently, it has been impossible to identify and monitor infected apes in the wild2. Since the first description of SIVcpz in wild-caught chimpanzees, only seven naturally infected apes have been studied in captivity, five of whom died as infants of unknown causes shortly after they were placed in sanctuaries2. Only one naturally infected chimpanzee was subjected to virological and immunological analyses, and in this ape, SIVcpz infection was not associated with CD4+ T-cell decline, loss of T-cell function, or degenerative changes in lymph node architecture4–6. Similar findings were also reported for two other chimpanzees inoculated with SIVcpz in captivity6. On the basis of these data, it has been assumed that SIVcpz resembles other natural SIV infections of sooty mangabeys and African green monkeys in its non-pathogenic phenotype. However, field and molecular studies suggested otherwise7,8. Epidemiological surveys showed that SIVcpz is less prevalent and much less evenly distributed among wild communities than are SIVs infecting sooty mangabeys and African green monkeys2,7,8. Moreover, SIVcpz was found to be one of very few SIVs to have lost a highly conserved Nef function (that is, the ability to down-modulate the T-cell receptor from the surface of infected CD4+ T cells) that correlates with CD4+ T-cell preservation in naturally infected primates9,10. Finally, evolutionary analyses revealed that chimpanzees, like humans, acquired SIVcpz relatively more recently by cross-species transmission of SIVs through infected monkeys on which chimpanzees prey11. Collectively these data suggested that the natural history of SIVcpz infection differs from that of other primate lentiviruses. To examine this, we initiated a prospective study in Gombe National Park, Tanzania, the only field site where SIVcpz can be studied in wild-living, yet habituated, chimpanzee communities2,7.
Gombe National Park is located on the shores of Lake Tanganyika and is home to three chimpanzee communities, termed Kasekela (∼65 members), Mitumba (∼25 members) and Kalande (10–20 members) (Supplementary Fig. 1). The Kasekela and Mitumba chimpanzees have been under continuous observation since the 1960s and 1980s, respectively, and their demography, social structure, reproductive behaviour and individual life histories are well known12,13. The Kalande chimpanzees are not habituated and thus much less well studied. Although the three communities have distinct ranges, interactions between members occur in the form of territorial fights and the migration of adolescent females who typically leave their natal group before having their first offspring. Owing to extensive habitat destruction surrounding the park (Supplementary Fig. 1a), the Gombe chimpanzees have become isolated from other east African ape communities in recent decades. SIVcpz infection has been documented in all three Gombe communities7, but only the Mitumba and Kasekela chimpanzees have been studied systematically.
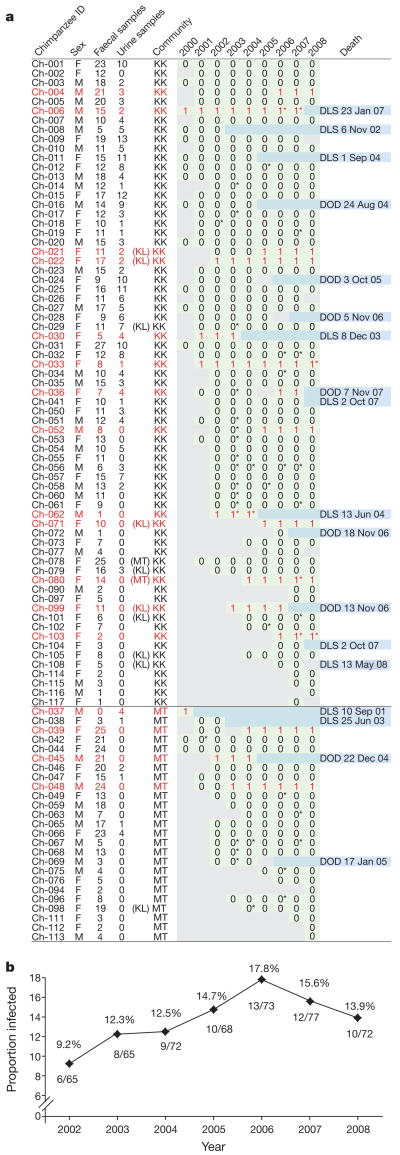
SIVcpz testing of Gombe chimpanzees began in 2000 when non-invasive (faecal- and urine-based) assays for virus-specific antibody and nucleic acid detection were first developed2,7. Since then, 226 urine and 1,153 faecal samples have been collected from 100 chimpanzees (median 11 samples per individual; range 1–70), including 69 from Kasekela and 25 from Mitumba (Supplementary Table 1). Most chimpanzees were sampled at least once every year, and 32 individuals were followed continuously over 7 years. Urine and faecal samples were examined for SIVcpz antibodies by western blot analysis, and a subset of antibody-positive faecal samples was tested for viral RNA by reverse transcription polymerase chain reaction (RT–PCR) amplification7,8. In addition, all chimpanzees were genotyped by amplifying mitochondrial and microsatellite markers from faecal DNA to verify sample identity8. Using these non-invasive approaches, we found 13 Kasekela and 4 Mitumba chimpanzees to be antibody-positive, 14 of whom also had detectable SIVcpz RNA in their faeces (Fig. 1). Nine chimpanzees were infected at first analysis, whereas eight others were identified during the course of the study (Fig. 1a). The latter included two infants of SIVcpz-infected mothers (Ch-062 and Ch-103), as well as six individuals who acquired incident infections after exposure to infected community members (Ch-004, Ch-021, Ch-036, Ch-039, Ch-048 and Ch-052). Figure 1b depicts the proportion of SIVcpz-positive chimpanzees in Mitumba and Kasekela over a 7-year observation period. During this time, SIVcpz prevalence rates ranged between 9% and 18%.
Figure 1. SIVcpz prevalence in Gombe.
a, Prospective epidemiological study of 69 Kasekela (KK) and 25 Mitumba (MT) chimpanzees (F, female; M, male). Green fields denote the presence (‘1’) or absence (‘0’) of SIVcpz infection as determined by faecal and urine testing (grey fields indicate absence of data). Infected chimpanzees are highlighted in red (inferred results are denoted by an asterisk; see Methods). For each deceased chimpanzee, the date of death (DOD) or the date last seen (DLS) is listed. Parentheses indicate the natal community of females who immigrated during the course of the study (KL, Kalande). b, SIVcpz infection rates between 2002 and 2008. Only years with greater than 70% coverage of the combined KK and MT population are shown.
To examine the relationship of the newly identified viruses to each other and to previously characterized SIVcpz strains, we constructed phylogenetic trees of available pol (481–854 bp) and env–nef (279–609 bp) sequences (Supplementary Fig. 2). For these analyses, we also included five SIVcpz strains from the non-habituated Kalande community. The results showed that all Gombe viruses formed a monophyletic lineage within the SIVcpzPts (SIVcpz from Pan troglodytes schweinfurthii) radiation. Within this lineage, viruses from Mitumba, Kasekela and Kalande were interspersed, indicating frequent transfer of SIVcpz between communities, most probably via migrating females. Phylogenetic analyses also identified clusters of epidemiologically linked infections, indicating horizontal transmission. One such cluster included four Kasekela chimpanzees (Ch-004, Ch-021, Ch-036 and Ch-052), all of whom became infected within a 20 month time period (Supplementary Table 2). In humans, primary HIV-1 infection is responsible for a substantial portion of new transmissions because acutely infected individuals have higher viral loads and are more infectious than chronically infected individuals14. The finding of nearly identical viruses in four chimpanzees thus suggests that some of them acquired SIVcpz from acutely infected mating partners. Closely related viruses were also observed in a presumed mother/daughter pair (Ch-071 and Ch-099), suggesting vertical transmission, but this conclusion remains tentative because both females were already sexually active at the time of first sampling (Supplementary Table 3). In addition, vertical transmission is suspected in two infants (Ch-062 and Ch-103) who were faecal-antibody-positive (but viral RNA-negative) at the age of 1.8 and 2.6 years, respectively. Interestingly, one of these (Ch-103) was born to a SIVcpz-negative mother (Ch-021) who became infected 10–15 months after giving birth (Supplementary Table 3). Thus, the infant seems to have acquired SIVcpz by breast milk transmission. Together, these data suggest that SIVcpz, like HIV-1, is transmitted by both horizontal and vertical routes.
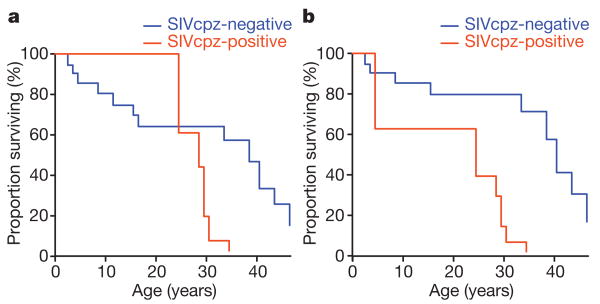
To determine whether SIVcpz infection has an impact on chimpanzee survival, we used discrete event-history methods to model the hazard of death for both infected and uninfected individuals. For this analysis, only Kasekela and Mitumba chimpanzees of known SIVcpz infection status were included. During the 9-year observation period, 7 of 17 infected and 11 of 77 uninfected chimpanzees died or disappeared. Death of missing individuals was defined based on previously reported criteria15,16, and for all but one chimpanzee for whom only urine samples were available (Ch-037), infection was confirmed using genotyped faecal samples. Age, sex, SIVcpz infection and death status were tabulated for each individual in each year, yielding over 550 chimpanzee years of observation for analysis. Figure 2 compares the survival rates of SIVcpz-infected (red) and uninfected (blue) chimpanzees using maximally and minimally conservative event definitions. For a maximally conservative estimate (Fig. 2a), the two faecal antibody-positive but viral RNA-negative infants were classified as SIVcpz negative, and two uninfected females who went missing for more than 1 year were counted as being dead (this resulted in the removal of one potentially SIVcpz-related death and the addition of two SIVcpz-unrelated deaths). For a less conservative estimate (Fig. 2b), the two infants were assumed to be infected, and the two females were censored, that is, assumed to have migrated to Kalande or outside the park (this resulted in the addition of one SIVcpz-related death and the removal of two SIVcpz-unrelated deaths). The results showed that SIVcpz-infected chimpanzees died at a significantly faster rate, even under the most stringent assumptions. The maximally conservative approach yielded a 9.8-fold increased death hazard for SIVcpz-positive chimpanzees (95% confidence interval = 2.8–34.3; P < 0.0001), whereas the less conservative approach indicated a 15.6-fold increased hazard (95% confidence interval = 4.7–51.8; P < 0.0001).
Figure 2. SIVcpz-associated mortality in Gombe.
a, b, Kaplan–Meier survival curves are shown for SIVcpz-infected (red) and uninfected (blue) chimpanzees using maximally conservative (a) and less conservative (b) event definitions (see text for details). Under both scenarios, SIVcpz-infected chimpanzees die at a significantly faster rate (9.8- and 15.6-fold increased death hazard, respectively; P < 0.0001).
We also examined the impact of SIVcpz infection on female fertility and infant survival. During the 9-year study period, 22 of 30 uninfected females gave birth to 30 infants, whereas 4 of 9 SIVcpz-infected females gave birth to 4 infants (only Kasekela and Mitumba females of known SIVcpz status at the time of delivery were included). For each female, age, SIVcpz status and the risk of becoming pregnant (taking into account adolescent subfecundity, lactational amenorrhea and immigration status; see Methods) were tabulated, and the number of births per total number of risk years was determined. This yielded significantly different (P = 0.034) birth rates of 0.32 (29 births per 90 female years) and 0.13 (4 births per 30 female years) for uninfected and infected females, respectively, indicating that the odds of an infected female giving birth were 3.1 times lower than those of an uninfected female. In addition, there was a significant association between the SIVcpz status of the mother and the survival of the infant. Of the 30 babies born to uninfected mothers, only six died before the age of 1 year. In contrast, all four infants born to SIVcpz-infected mothers died before their first birthday (Supplementary Table 4). Therefore, infants born to SIVcpz-infected mothers had a significantly higher mortality rate than infants born to uninfected mothers (P = 0.005).
One Kasekela female (Ch-036), who became infected during the course of the study (between December 2004 and March 2006), died within 3 years of acquiring SIVcpz (November 2007; Fig. 1a). She had no observable injuries, but exhibited profound weakness and lethargy several days before her death. At necropsy, she had severe cachexia with skeletal muscle and hepatocellular atrophy, as well as multiple abdominal abscesses due to nematode infestation. Histologically, pancreatic and mesenteric lymph nodes had marked depletion of both cortical and paracortical lymphocytes (Fig. 3a). Specific staining of spleen sections with B-cell and T-cell markers (CD79a and CD3, respectively) confirmed severe lymphopenia and showed that over 95% of the remaining lymphocytes were B cells (Fig. 3b and c). The spleen also contained multiple areas of follicular hyalinization, which is a hallmark of lymphatic tissue destruction. These histopathological findings are consistent with end-stage HIV-1 and SIVmac infections17,18, and suggested that Ch-036 died of an AIDS-like illness.
Figure 3. Histopathology of end-stage SIVcpz infection.
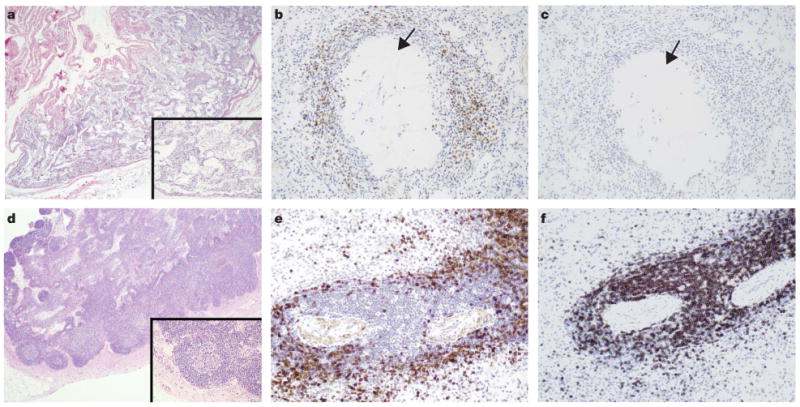
a–c, Severe lymphatic depletion in mesenteric lymph node (a) and splenic tissue (b, c) of a Kasekela female (Ch-036) who died of an AIDS-like illness within 3 years of acquiring SIVcpz. d–f, Normal mesenteric lymph node with multiple primary and secondary lymphoid follicles (d) and normal splenic tissue with PALS (e, f) from an uninfected chimpanzee. a, d, Lymph node sections stained with haematoxylin and eosin (nuclei are blue). b, c, e, f, Spleen sections stained with anti-CD79a (b, e) and anti-CD3 (c, f) antibodies (positive cells are dark brown). Note the severe loss of both B (CD79a) and T (CD3) cells in the PALS of Ch-036. Arrows indicate follicular hyalinization. Original magnification ×4 (a, d) and ×20 (b, c, e, f and insets).
To examine whether the lymphatic depletion in Ch-036 represented a rare case of immunodeficiency known to occur sporadically in non-pathogenic SIV infections19, we obtained post-mortem spleen samples from two additional infected (Ch-045 and Ch-099) and uninfected (Ch-016 and Ch-069) chimpanzees. Three of these (including both infected chimpanzees) died of trauma-related causes, in one instance following intra-group aggression (Ch-045) and in the two others, following spinal cord injury (Ch-069 and Ch-099); the fourth (uninfected) chimpanzee (Ch-016) died of age-related conditions shortly before reaching his 40th birthday.
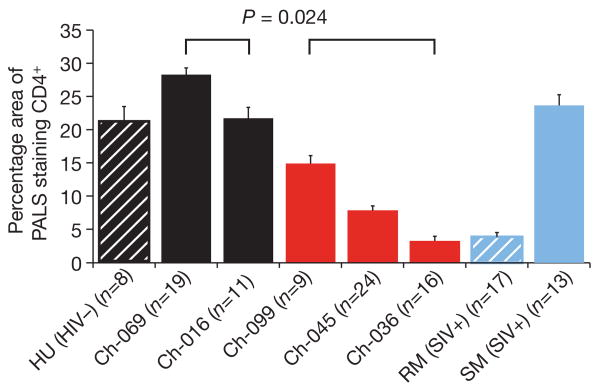
Spleen sections from these four chimpanzees, as well as from Ch-036, were stained with CD4-specific antibodies (as well as CD3 and CD20 antibodies as controls). CD4+ T cells were then quantified by determining the percentage area of periarteriolar lymphoid sheaths (PALS, the T-cell zone equivalent in lymph nodes) that stained positive for CD4. The results revealed that each of the three infected chimpanzees had fewer CD4+ T cells in their spleen than either of the two uninfected controls (Fig. 4). These differences were statistically significant whether chimpanzees were compared individually or as groups. The most profound CD4+T-cell loss was seen in Ch-036, whose spleen was also depleted of CD3+ T cells and CD20+ B cells (Supplementary Fig. 3). A reduction of CD4+ T cells was also apparent in the PALS of Ch-045 and Ch-099; however, this depletion was less pronounced, especially in Ch-099, and occurred in the absence of a concurrent loss of other CD3+ T-cell and B-cell populations (Supplementary Fig. 3). Thus, in the two infected apes who died of trauma-related causes, CD4+ T-cells were selectively depleted whereas other lymphocyte populations remained intact, suggesting that SIVcpz is associated with progressive CD4+ T-cell killing through all stages of infection, including clinical latency.
Figure 4. CD4+ T-cell depletion in SIVcpz-infected chimpanzees.
Quantitative image analysis of splenic CD4+ T cells in SIVcpz-infected (red bars) and uninfected (solid black bars) chimpanzees, as well as in uninfected human (HU, black hatched bar), SIVmac-infected rhesus macaque (RM, blue hatched bar), and SIVsmm-infected sooty mangabey (SM, solid blue bar) controls. Representative PALS (8–24 per individual) were extracted and the percentage of area that stained positive for CD4 was determined20,21. Average values are shown, with error bars indicating s.e.m. All three SIVcpz-infected chimpanzees had significantly lower CD4+ T-cell counts than the two uninfected controls (ANOVA F-test; P = 0.024). The SIVmac-infected rhesus macaque had end-stage AIDS.
Splenic tissues were also examined for evidence of collagen deposition in the PALS, which is a non-specific marker of chronic immune activation20,21. Although some collagen was detected in the PALS of Ch-099 and the two uninfected chimpanzees, this amount was substantially less than that detected in the PALS of Ch-045, and in particular Ch-036 (Supplementary Fig. 3). In HIV-1-infected humans, collagen deposition in T-cell zones and PALS correlates with CD4+ T-cell depletion, altered CD4 T-cell homeostasis and overall immune dysfunction20,21. Consistent with this, fibrotic scarring of the PALS was most severe in Ch-036, intermediate in Ch-045, and minimal in Ch-099 (also see Fig. 4). Thus, SIVcpz seems to cause immune deficiency by mechanisms that are very similar to those of HIV-1. Indeed, in situ hybridization using SIVcpz-specific RNA probes revealed numerous productively infected lymphocytes in the spleen of Ch-045, documenting active viral replication and extensive trapping of virus particles by follicular dendritic cells (Supplementary Fig. 4).
We show here that SIVcpz-infected chimpanzees in Gombe have a 10–16-fold increased death hazard compared to uninfected chimpanzees, and that infected females are less likely to give birth and have fewer surviving offspring. We also show that SIVcpz infection is associated with progressive CD4+ T-cell loss and immune system destruction, which are hallmarks of pathogenic HIV-1 infection. Although based on a limited sample size, these results suggest that SIVcpz, like HIV-1 in humans22–25, has a substantial negative impact on the health, reproduction and lifespan of wild chimpanzees. What is less certain is the magnitude of this effect and its contribution to the population decline of chimpanzees in Gombe16,26 and elsewhere. Before the introduction of highly active antiretroviral therapy, HIV-1-infected humans had a 18–60-fold higher risk of death than uninfected individuals23–25, whereas HIV-2-infected humans had a 2–5-fold higher risk25,27. In contrast, SIVsmm, the immediate precursor of HIV-2 (ref. 1), does not seem to affect the lifespan of its host, as evidenced by nearly identical survival curves of infected (n = 167) and uninfected (n = 62) sooty mangabeys at the Yerkes primate centre (Supplementary Fig. 5). On the basis of these mortality data, SIVcpz seems to be less pathogenic than HIV-1, but more pathogenic than HIV-2 and SIVsmm. Although the generality of these results remains to be confirmed in a larger number of chimpanzees the finding that SIVcpz is pathogenic in Gombe provides a unique opportunity to compare disease-causing mechanisms of two closely related viruses in two closely related species. Such an approach is likely to accelerate the identification of viral and host factors responsible for HIV/SIV disease progression, and could lead to novel therapeutic and preventive measures that will benefit both humans and chimpanzees.
Methods Summary
Observational data
Kasekela and Mitumba chimpanzees have been observed daily since the 1960s and 1980s, respectively, providing a continuous long-term record of community demography and individual behaviour12,13,16,26.
Non-invasive SIVcpz testing
Chimpanzee faecal and urine samples were tested for virus-specific antibodies by western blot analysis, and SIVcpz infection was confirmed by RT–PCR (GenBank accession numbers FJ895381–FJ895405)7,8. All faecal samples used for SIVcpz status determination were genotyped (Supplementary Table 1).
Phylogenetic analyses
Phylogenetic trees of SIVcpz pol and env–nef sequences were inferred by Bayesian methods28 using a general time-reversible (GTR) likelihood model.
Mortality analyses
SIVcpz-associated mortality hazards were estimated using discrete event history methods as a function of measured covariates (age, sex and SIVcpz status)29. SIVsmm-associated survival differences were examined using a Cox proportional hazards model.
Necropsy
Complete necropsies were performed on five chimpanzees whose bodies were recovered within 6–18 h of death. Pathological and histological examinations were performed on all organs.
Immunohistochemistry, in situ hybridization and quantitative image analysis
Post-mortem spleen and lymph node samples were subjected to in situ hybridization, immunohistochemical staining and quantitative image analysis as described previously20,21. Splenic CD4+ T cells were quantified by manually extracting the images of representative PALS into Photoshop (Adobe), and by determining the percentage of area that stained positive for CD4 (refs 20,21).
Statistical methods
Splenic CD4+ T-cell counts of three infected and two uninfected chimpanzees were compared using ANOVA F-tests. Fertility and infant mortality data for infected and uninfected females were compared using Fisher's exact test.
Supplementary Material
Acknowledgments
We thank the field staff at the Gombe Stream Research Centre for collecting behavioural data as well as urine and faecal samples from wild chimpanzees; E. Kaaya for help with necropsies; A. Collins for logistical support; the Tanzania Commission for Science and Technology, the Tanzania Wildlife Research Institute, and the Tanzania National Parks for permission to conduct research in Gombe; I. White for discussions; L. Lowenstine for histological consultation; M. Salazar and Y. Chen for technical assistance; and J. C. White for artwork and manuscript preparation. This work was supported by grants from the National Institutes of Health (R01 AI50529, R01 AI58715, U19 AI067854, T32 GM008111), the National Cancer Institute (contract HHSN266200400088C), the UAB Center for AIDS Research (P30 AI 27767), the Yerkes National Primate Research Center (RR-00165), the National Science Foundation (DBS-9021946, SBR-9319909, BSC-0452315, IIS-0431141, BSC-0648481), the Jane Goodall Institute, the Harris Steel Group, the University of Minnesota, the University of Illinois, the US Fish and Wildlife Service Great Ape Conservation Fund, the Windibrow, Arcus, Guthman and Davee Foundations, and the Lincoln Park Zoo. R.S.R. was funded by a Howard Hughes Medical Institute Med-into-Grad Fellowship. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions All authors contributed to the acquisition, analysis and interpretation of the data; B.F.K., A.E.P., J.G., G.M.S., P.M.S. and B.H.H. initiated and conceived the study; B.F.K., R.S.R., Y.L. and E.W. performed non-invasive SIVcpz screening and genetic analyses; J.H.J., J.S.-S., M.L.W. and A.E.P. analysed chimpanzee fertility and mortality; K.A.T., M.J.K., J.R., D.A.T. and T.M. performed necropsies; K.A.T. and J.D.E performed immunohistochemistry and in situ hybridization studies; G.H.L. and P.M.S. performed phylogenetic analyses; J.H.J., T.M.B. and P.M.S. performed statistical analyses; M.L.W., A.M., S.K., E.V.L. and D.A.T. conducted and supervised all fieldwork. J.G.E. and G.S. provided data on captive sooty mangabeys; and B.F.K., A.E.P., P.M.S., G.M.S. and B.H.H. coordinated the contributions of all authors and wrote the paper. B.F.K., J.H.J., K.A.T., J.D.E., R.S.R. and M.L.W. contributed equally to this paper.
Author Information Newly derived SIVcpz sequences have been deposited in the GenBank database under accession numbers FJ895381–FJ895405. Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 2.Sharp PM, Shaw GM, Hahn BH. Simian immunodeficiency virus infection of chimpanzees. J Virol. 2005;79:3891–3902. doi: 10.1128/JVI.79.7.3891-3902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silvestri G. Immunity in natural SIV infections. J Intern Med. 2008;265:97–109. doi: 10.1111/j.1365-2796.2008.02049.x. [DOI] [PubMed] [Google Scholar]
- 4.Koopman G, Haaksma AG, ten Velden J, Hack CE, Heeney JL. The relative resistance of HIV type 1-infected chimpanzees to AIDS correlates with the maintenance of follicular architecture and the absence of infiltration by CD8+ cytotoxic T lymphocytes. AIDS Res Hum Retroviruses. 1999;15:365–373. doi: 10.1089/088922299311330. [DOI] [PubMed] [Google Scholar]
- 5.Gougeon ML, et al. Lack of chronic immune activation in HIV-infected chimpanzees correlates with the resistance of T cells to Fas/Apo-1 (CD95)-induced apoptosis and preservation of a T helper 1 phenotype. J Immunol. 1997;158:2964–2976. [PubMed] [Google Scholar]
- 6.Heeney JL, et al. Transmission of simian immunodeficiency virus SIVcpz and the evolution of infection in the presence and absence of concurrent human immunodeficiency virus type 1 infection in chimpanzees. J Virol. 2006;80:7208–7218. doi: 10.1128/JVI.00382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santiago ML, et al. Foci of endemic simian immunodeficiency virus infection in wild-living eastern chimpanzees (Pan troglodytes schweinfurthii) J Virol. 2003;77:7545–7562. doi: 10.1128/JVI.77.13.7545-7562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keele BF, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schindler M, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125:1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Schindler M, et al. Inefficient Nef-mediated downmodulation of CD3 and MHC-I correlates with loss of CD4+ T cells in natural SIV infection. PLoS Pathog. 2008;4:e1000107. doi: 10.1371/journal.ppat.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailes E, et al. Hybrid origin of SIV in chimpanzees. Science. 2003;300:1713. doi: 10.1126/science.1080657. [DOI] [PubMed] [Google Scholar]
- 12.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Belknap; 1986. [Google Scholar]
- 13.Pusey AE, Pintea L, Wilson ML, Kamenya S, Goodall J. The contribution of long-term research at Gombe National Park to chimpanzee conservation. Conserv Biol. 2007;21:623–634. doi: 10.1111/j.1523-1739.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 14.Wawer MJ, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 15.Hill K, et al. Mortality rates among wild chimpanzees. J Hum Evol. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- 16.Williams JM, et al. Causes of death in the Kasekela chimpanzees of Gombe National Park, Tanzania. Am J Primatol. 2008;70:766–777. doi: 10.1002/ajp.20573. [DOI] [PubMed] [Google Scholar]
- 17.Pantaleo G, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 18.King NW., Jr . Simian immunodeficiency virus infections. In: Jones TC, Mohr U, Hunt RD, editors. Nonhuman Primates I. Springer; 1993. pp. 5–20. [Google Scholar]
- 19.Pandrea I, Silvestri G, Apetrei C. AIDS in African nonhuman primate hosts of SIVs: a new paradigm of SIV infection. Curr HIV Res. 2009;7:57–72. doi: 10.2174/157016209787048456. [DOI] [PubMed] [Google Scholar]
- 20.Estes JD, et al. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estes JD, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor β1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 22.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105:836–848. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 23.Bhaskaran K, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. J Am Med Assoc. 2008;300:51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 24.Mulder DW, et al. Two-year HIV-1-associated mortality in a Ugandan rural population. Lancet. 1994;343:1021–1023. doi: 10.1016/s0140-6736(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 25.Hansmann A, et al. Baseline plasma viral load and CD4 cell percentage predict survival in HIV-1- and HIV-2-infected women in a community-based cohort in The Gambia. J Acquir Immune Defic Syndr. 2005;38:335–341. [PubMed] [Google Scholar]
- 26.Pusey AE, Wilson ML, Collins DA. Human impacts, disease risk, and population dynamics in the chimpanzees of Gombe National Park, Tanzania. Am J Primatol. 2008;70:738–744. doi: 10.1002/ajp.20567. [DOI] [PubMed] [Google Scholar]
- 27.Poulsen AG, et al. 9-year HIV-2-associated mortality in an urban community in Bissau, west Africa. Lancet. 1997;349:911–914. doi: 10.1016/S0140-6736(96)04402-9. [DOI] [PubMed] [Google Scholar]
- 28.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 29.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford Univ. Press; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.