Abstract
The A5 noradrenergic group of the pons projects on the medulla and spinal cord and contributes to control of sympathetic activity and respiration. Because these functions are affected in multiple system atrophy (MSA), we sought to determine whether there was involvement of A5 neurons in this disorder. We counted tyrosine-hydroxylase (TH) immunoreactive neurons in the A5 area and locus ceruleus in the pons obtained from six patients with clinical and neuropathological diagnoses of MSA and six age-matched controls using stereological methods. There was a severe loss of A5 neurons in MSA (total cell number 3,215 ± 258 in controls and 321 ± 62 in MSA, P < 0.001). The severity of cell loss was comparable to that of locus ceruleus neurons in these cases. Our results indicate that A5 neurons are severely affected in MSA and this may contribute to some of the manifestations of this synucleinopathy.
Keywords: Norepinephrine, Cardiorespiratory, Glial cytoplasmic inclusions
Introduction
The A5 neurons of the pontine tegmentum provide noradrenergic innervation to the preganglionic sympathetic neurons of the spinal cord [24] and elicit sympathoexcitation [12]. The A5 neurons are activated during hypotension [4] and hypoxia [6, 9], and their activity is modulated during respiration [9]. Although A5 neurons do not appear to be involved in tonic maintenance of arterial pressure, they may play an important role in sympathoexcitatory responses to hypoxia [14]. They modulate the activity of respiratory neurons [11] and project onto central chemoreceptor areas of the ventral medullary surface [20]. Thus, A5 neurons appear to participate in complex cardiorespiratory interactions.
Multiple system atrophy (MSA) may manifest with prominent impairment of sympathetic cardiovascular and respiratory control [8, 26]. Whereas MSA is associated with loss of noradrenergic neurons in both the locus ceruleus [1, 13] and A1 area of the caudal ventrolateral medulla [2], involvement of neurons of the A5 group has not been systematically explored. In this study, we sought to determine whether the A5 neurons are involved in MSA and to compare their involvement with that of the locus ceruleus neurons in these cases.
Methods
Subjects
Brains were obtained at autopsy from 12 subjects (Table 1). All the patients had signed informed consent for autopsy according to the Institutional Review Board guidelines. Six subjects (five women, one man, age 70 ± 4 years) had no history of neurological or psychiatric disease, and six subjects (four men, two women, age 65 ± 4 years) had the clinical diagnosis of MSA confirmed neuropathologically. The clinical and neuropathological diagnoses of MSA were made according to current consensus criteria [7].
Table 1.
Patient population
| Case | Age/sex | PMD (h) | Motor/cognitive manifestation | Autonomic manifestations | Disease duration (years) | Diagnosis |
|---|---|---|---|---|---|---|
| Control 1 | 85/F | 16 | None | HTN | N/A | MI |
| Control 2 | 73/M | 18 | Mild memory loss | Constipation, HTN | N/A | CHF |
| Control 3 | 66/F | 18 | None | None | N/A | Pulmonary fibrosis |
| Control 4 | 57/F | 8.5 | None | HTN | N/A | Metastatic ovarian carcinoma |
| Control 5 | 77/F | 22 | Mild memory loss | HTN, constipation | N/A | Scleroderma, CAD |
| Control 6 | 62/F | 20 | None | DM, HTN | N/A | COPD |
| MSA 1 | 68/M | 23 | Parkinsonism | OH, NB, impotence, dysphagia | 14 | MSA–SND–OPCA |
| MSA 2 | 67/M | 1 | Parkinsonism, ataxia | OH, NB, HTN, constipation, CVF | 2 | MSA–SND |
| MSA 3 | 53/M | 36 | Parkinsonism ataxia | OH; NB, constipation | 3 | MSA–SND/OPCA |
| MSA 4 | 81/F | 13 | Parkinsonism | OH, NB, constipation, anhidrosis, CVF | 5 | MSA–SND/OPCA LBD (neocortical) |
| MSA 5 | 59/M | 26 | Parkinsonism ataxia | OH, NB, impotence, constipation, anhidrosis, CVF | 5 | MSA–SND–OPCA |
| MSA 6 | 63/F | 6 | Parkinsonism ataxia | OH, NB, HTN, anhidrosis, dysphagia | 6 | MSA–SND–OPCA |
CAD coronary artery disease, CHF congestive heart failure, COPD chronic obstructive pulmonary disease, CVF cardiovagal failure, DM diabetes mellitus, HTN hypertension, MI myocardial infarction, MSA multiple system atrophy, NB neurogenic bladder, OH orthostatic hypotension, OPCA olivopontocerebellar atrophy, PMD postmortem delay, SND striatonigral degeneration
Of the six MSA cases, all had predominant parkinsonism and four also had cerebellar ataxia. All the MSA cases had clinical and laboratory evidence of severe autonomic failure, manifested by orthostatic hypotension and impaired heart rate response to deep breathing indicating impaired vagal control of the sinus node (Table 1). Unfortunately, none of our cases had undergone evaluation for ventilatory or blood pressure responses to hypercapnia or hypoxia.
Tissue processing and immunocytochemistry
Postmortem delay was similar in control (17 ± 2 h) and MSA cases (18 ± 5 h). The left half of the brain was examined for routine neuropathological studies. The six control brains, including those from patients with mild memory complaints, were macroscopically normal and were not studied for Braak and Braak staging. Of the six MSA cases, all had evidence of severe striatonigral degeneration. Five also had evidence of olivopontocerebellar atrophy that was severe in two and moderate in three cases. All the MSA cases had evidence of α-synuclein immunostained glial cytoplasmic inclusions (GCIs). The MSA cases had mild Braak and Braak Alzheimer-type pathology (Braak and Braak stages 0–III), none of which satisfied the NIA-Reagan or CERAD criteria for AD [3].
A block containing the pons from 15 to 22 mm rostral to the obex was separated for the purposes of this study. Brains were immersion-fixed in 5% formalin for 24 h at 4°C and cryoprotected in buffered 30% sucrose for 5–7 days prior to processing. Serial 50 μm cryostat sections were obtained and every eighth section was processed for tyrosine hydroxylase (TH) immunoreactivity (mouse monoclonal, 1:3,000, Immunostar, Hudson, WI, USA). Diaminobenzidine/glucose oxidase solution with nickel enhancement (SIGMA, St Louis, MO, USA) was used for the substrate reaction. Immunoreactive neurons were identified under bright-field illumination by a characteristic dark brown to black reaction product that densely fills the perikarya and their processes. The distribution of immunoreactive neurons in the pons identified using this TH antibody was similar to that reported in earlier studies [18]. Omission of the primary antibody or incubation with normal sera resulted in a lack of immunostaining. All sections were co-stained with thionin to identify surrounding structures and to determine whether loss of immunoreactivity reflected neuronal loss or lack of expression of the antigen. Paraffin-embedded 6-μm sections obtained from the contralateral pons at the same level were immunostained for α-synuclein (goat polyclonal, 1:400, Santa Cruz Biotechnologies, Santa Cruz, CA, USA).
Image analysis and quantitation
The sections were examined under bright-field microscopy using the Axiovision 4.2 image analysis system (Carl Zeiss, Inc., Thornwood, NY, USA) and cell numbers were estimated using design-based stereology [16, 21]. Digital images were acquired using the AxioCam MRc, (Carl Zeiss, Inc., Thornwood, NY, USA) attached to a microscope (Lieca, Diaplan; Wetzlar, Germany) fitted with a motorized stage (Ludl Electronic Products, Hawthorne, NY, USA) and stage position encoder. Sections were studied using the Analyze 8.1 three-dimensional stereology software developed in the Biomedical Imaging Resource Department, Mayo Clinic, Rochester, MN, USA [15]. We systematically sampled the whole extent of the pons by obtaining 50 μm sections at intervals of 400 μm apart. The total number of sections studied was 18 ± 1 (average 17.5) per case. The total number of TH immunoreactive neurons was determined using a systematic and uniformly random sampling of the A5 area or locus ceruleus. The A5 and locus ceruleus boundaries were determined as defined on the basis of the atlas by Paxinos and Huang [17]. We only counted the immunoreactive neurons that could be clearly identified by the presence of nucleus, dendritic processes, or both in a single focal plane within the total cross section of each 50 μm section. All immunoreactive neurons that fulfilled this criterion were counted within each cross section. We also measured the diameter and area of the soma (without including the processes) of all highly immunoreactive cells that could be identified by these criteria. For neuronal size measurements, we selected, for each case, five sections obtained 1.5 mm apart. We measured 20 neurons per section in each of the six controls (total 600 neurons) and four neurons per section in each MSA case (total 120 neurons).
The same investigator who blinded to the clinical and final neuropathological diagnoses performed all counts.
Statistical analysis
The numbers of TH-immunoreactive cells (mean ± SEM) in the A5 or locus ceruleus regions were compared between control and MSA cases. Results were analyzed using the analysis of covariance (ANCOVA) with multivariate and univariate analyses with the SPSS version 10.1 software (SPSS Inc., Chicago, IL, USA). Groups (controls and MSA), age, and disease duration were used as the covariates, and cell count was the dependent variable. Post hoc analyses for a pairwise comparison were also performed using Student’s t test. A P value of less than 0.05 was considered to be significant.
Results
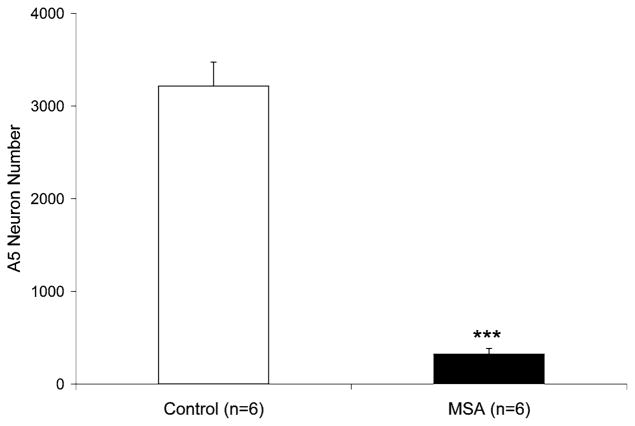
There was severe loss of TH immunoreactive neurons in the A5 region in all the MSA cases compared to controls (Fig. 1). As assessed in Nissl-co-stained sections, this could not be explained by lack of antigen expression in surviving cells. The total number of neurons counted was 414 ± 21 in controls and 41 ± 7 in MSA (P < 0.001 with respect to controls). The total estimated cells was 3,215 ± 258 in controls and 321 ± 62 in MSA (P < 0.001 with respect to controls; Fig. 2). There was no difference in A5 cell size between control and MSA cases. In controls, the cell size was 28.3 ± 0.5 μm in diameter and 545 ± 20 μm2 in area (n = 100 per case, total 600 cells); in MSA, the cell size was 28.8 ± 0.4 μm in diameter and 551 ± 15 μm2 in area (n = 20 per case, total 120 cells). There was no relationship between the degree of A5 neuronal depletion and age (age effect P = 0.09).
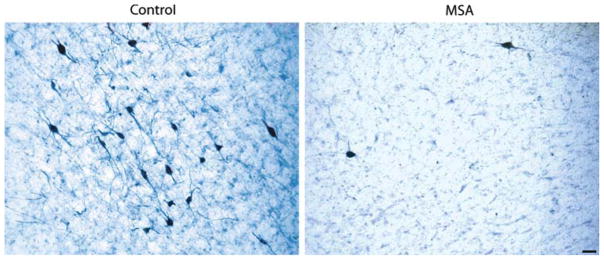
Fig. 1.
Tyrosine hydroxylase (TH) immunoreactive neurons (co-stained with thionin) in the A5 area in 50 μm sections of a 77-year-old woman with no history of neurological disease (postmortem delay 22 h) and a 59-year-old man with pathological diagnosis of multiple system atrophy (MSA, postmortem delay 26 h). Bar 50 μm
Fig. 2.
Estimated total number (mean ± SEM) of tyrosine hydroxylase (TH) neurons in the A5 area in six control subjects and six cases with pathological diagnosis of multiple system atrophy (MSA). There was a significant reduction of TH positive A5 neurons in all MSA cases. *** P < 0.001 compared to controls
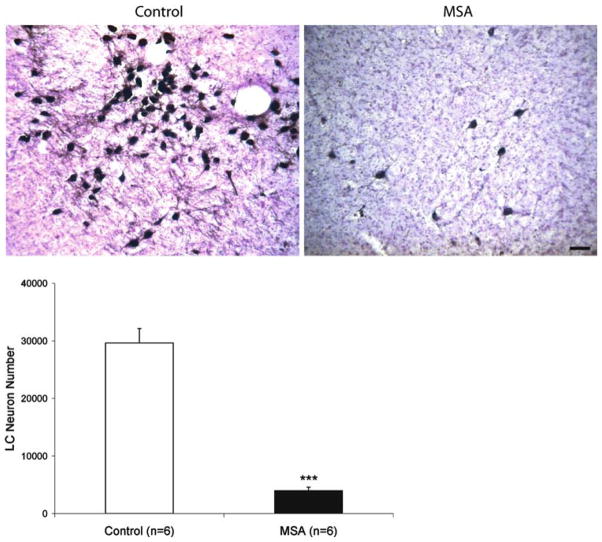
In the A5 region, there were abundant α-synuclein immunoreactive GCIs in all the MSA cases. We did not detect α-synuclein immunoreactive inclusions in the cytoplasm of remaining A5 neurons (Fig. 3). The magnitude of A5 cell loss (approximately 90%) was comparable to that observed in the adjacent locus ceruleus (LC) of the same cases (Fig. 4). The total LC cells counted was 1,590 ± 111 in controls and 220 ± 59 in MSA (P < 0.001 with respect to controls). The total estimated numbers of LC cells was 29,612 ± 2,548 in controls and 3,960 ± 613 in MSA (P < 0.001 with respect to controls). There was no difference in locus ceruleus cell size between controls and MSA cases. In controls, the cell size was 31.8 ± 1 μm in diameter and 703 ± 42 μm2 in area (n = 100 per case, total 600 cells); in MSA, the cell size was 32.3 ± 1 μm in diameter and 706 ± 33 μm2 in area (n = 20 per case, total 120 cells). There was no relationship between the degree of locus ceruleus neuronal depletion and age (age effect P = 0.8). As in the A5 region, there were abundant α-synuclein immunoreactive GCIs in the locus ceruleus of all the MSA cases. We did not detect α-synuclein immunoreactive inclusions in the cytoplasm of locus ceruleus neurons.
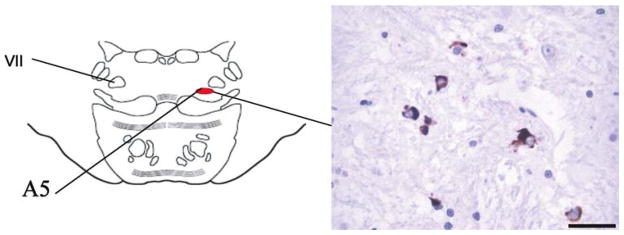
Fig. 3.
Six micrometer paraffin-embedded sections of the pons at the level of the A5 area (left) stained for α-synuclein and showing the distribution of glial cytoplasmic inclusions in a 68-year-old man with pathological diagnosis of multiple system atrophy (right) (MSA, postmortem delay 23 h). Bar 25 μm
Fig. 4.
Upper panel tyrosine hydroxylase (TH) immunoreactive neurons of the locus ceruleus in 50 μm sections of a 77-year-old woman with no history of neurological disease (postmortem delay 22 h) and a 59-year-old man with pathological diagnosis of multiple system atrophy (MSA, postmortem delay 26 h). Bar 50 μm. Lower panel estimated total number (mean ± SEM) of tyrosine hydroxylase (TH) immunoreactive neurons in the locus ceruleus (LC) in six control cases, and six cases with pathological diagnosis of multiple system atrophy (MSA). There was a significant reduction of locus ceruleus neurons in all MSA cases. *** P < 0.001 compared to controls
Discussion
Our results indicate, for the first time to our knowledge, that there is severe loss of A5 neurons in MSA. The magnitude of A5 neuronal loss in MSA is comparable to that of locus ceruleus neurons in the same cases. Although the clinical implications of A5 neuronal loss in MSA are uncertain, it is conceivable that A5 cell loss may contribute to some of the manifestations of this disorder.
The A5 neurons provide noradrenergic innervation to preganglionic sympathetic neurons of the spinal cord [24] and elicit sympathoexcitation [12]. The A5 neurons are activated during baroreceptor unloading [4] but do not appear to have a major contribution in maintaining resting sympathetic tone or arterial pressure [10]. However, A5 neurons are also activated by hypoxia and stimulation of the carotid chemoreceptors [6, 9] and their activity is modulated during respiration [9]. It has been proposed that A5 neurons may play an important role in the carotid sympathetic chemoreflex triggered by hypoxia [14]. Respiratory modulation of A5 neurons during hypoxia coincides with episodes of blood pressure drop [19]. Although A5 neurons are activated by hypoxia [6, 9, 19], they do not appear to be a direct component of the hypoxia chemosensitive circuit, as defined by Phox2b gene expression [23]. However, A5 neurons modulate the activity of respiratory neurons [11] and studies in the rat indicate that there is a projection from A5 neurons to central chemoreceptor areas of the ventral medullary surface [20]. The A5 neurons also modulate the cardiorespiratory responses evoked by activation of the parabrachial nucleus [5], which is a critical component of the brainstem respiratory network required for eupnea [22]. Thus, A5 neurons appear to participate in complex cardiorespiratory interactions, including those associated with sympathetic responses to hypoxia. Therefore, it is conceivable that involvement of these neurons may contribute to respiratory [8, 26] or cardiovascular [25] manifestations of MSA. However, given that the role of A5 neurons is not fully characterized by experimental studies, our conclusions regarding their potential contribution to cardiorespiratory manifestations of MSA are speculative at this point.
In summary, our study indicates that there is a severe loss of A5 neurons in MSA. Involvement of A5 neurons may at least have a contributory role in the cardiovascular and respiratory manifestations of this disorder. Unfortunately, none of our cases had undergone assessment of cardiovascular responses to hypoxia. These studies are currently being performed in our laboratory.
Acknowledgments
We thank Jim Tarara from the Department of Molecular Medicine, and Jon Camp of the Biomedical Imaging Resource Department, Mayo Clinic, Rochester, MN, for technical assistance. This study was supported by a grant from the National Institutes of Health (NS32352-P2) and Mayo Funds.
Contributor Information
Eduardo E. Benarroch, Email: benarroch.eduardo@mayo.edu, Department of Neurology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA
Ann M. Schmeichel, Department of Neurology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA
Phillip A. Low, Department of Neurology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA
Paola Sandroni, Department of Neurology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA.
Joseph E. Parisi, Division of Anatomic Pathology, Mayo Clinic, Rochester, MN, USA
References
- 1.Benarroch EE, Schmeichel AM, Parisi JE. Depletion of mesopontine cholinergic and sparing of raphe neurons in multiple system atrophy. Neurology. 2002;59:944–946. doi: 10.1212/wnl.59.6.944. [DOI] [PubMed] [Google Scholar]
- 2.Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Differential involvement of hypothalamic vasopressin neurons in multiple system atrophy. Brain. 2006;129:2688–2696. doi: 10.1093/brain/awl109. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol. 2003;23:597–616. doi: 10.1023/a:1025080314925. [DOI] [PubMed] [Google Scholar]
- 5.Dawid Milner MS, Lara JP, Lopez de Miguel MP, Lopez-Gonzalez MV, Spyer KM, Gonzalez-Baron S. A5 region modulation of the cardiorespiratory responses evoked from parabrachial cell bodies in the anaesthetised rat. Brain Res. 2003;982:108–118. doi: 10.1016/s0006-8993(03)03005-1. [DOI] [PubMed] [Google Scholar]
- 6.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- 7.Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, Litvan I, Mathias CJ, Oliver E, Robertson D, Schatz I, Wenning GK. Consensus statement on the diagnosis of multiple system atrophy. J Auton Nerv Syst. 1998;74:189–192. [PubMed] [Google Scholar]
- 8.Glass GA, Josephs KA, Ahlskog JE. Respiratory insufficiency as the primary presenting symptom of multiple-system atrophy. Arch Neurol. 2006;63:978–981. doi: 10.1001/archneur.63.7.978. [DOI] [PubMed] [Google Scholar]
- 9.Guyenet PG, Koshiya N, Huangfu D, Verberne AJ, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Physiol. 1993;264:R1035–R1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- 10.Guyenet PG, Schreihofer AM, Stornetta RL. Regulation of sympathetic tone and arterial pressure by the rostral ventrolateral medulla after depletion of C1 cells in rats. Ann N Y Acad Sci. 2001;940:259–269. doi: 10.1111/j.1749-6632.2001.tb03682.x. [DOI] [PubMed] [Google Scholar]
- 11.Hilaire G, Viemari JC, Coulon P, Simonneau M, Bevengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Huangfu DH, Koshiya N, Guyenet PG. A5 noradrenergic unit activity and sympathetic nerve discharge in rats. Am J Physiol. 1991;261:R393–R402. doi: 10.1152/ajpregu.1991.261.2.R393. [DOI] [PubMed] [Google Scholar]
- 13.Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153–197. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- 14.Koshiya N, Guyenet PG. A5 noradrenergic neurons and the carotid sympathetic chemoreflex. Am J Physiol. 1994;267:R519–R526. doi: 10.1152/ajpregu.1994.267.2.R519. [DOI] [PubMed] [Google Scholar]
- 15.Miller SM, Farrugia G, Schmalz PF, Ermilov LG, Maines MD, Szurszewski JH. Heme oxygenase 2 is present in interstitial cell networks of the mouse small intestine. Gastroenterology. 1998;114:239–244. doi: 10.1016/s0016-5085(98)70473-1. [DOI] [PubMed] [Google Scholar]
- 16.Ohm TG, Busch C, Bohl J. Unbiased estimation of neuronal numbers in the human nucleus coeruleus during aging. Neurobiol Aging. 1997;18:393–399. doi: 10.1016/s0197-4580(97)00034-1. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Huang XF. Atlas of the human brainstem. Academic Press; San Diego: 1995. [Google Scholar]
- 18.Pearson J, Halliday G, Sakamoto N, Michel J. Catecholaminergic neurons. In: Paxinos G, editor. The human nervous system. Academic Press; San Diego: 1990. [Google Scholar]
- 19.Pyatin VF, Tatarnikov VS, Glazkova EN. Control of respiratory and hypotensive response during hypoxic chemoreflex by A5 region neurons in rats. Bull Exp Biol Med. 2006;142:654–656. doi: 10.1007/s10517-006-0442-3. [DOI] [PubMed] [Google Scholar]
- 20.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 22.St-John WM, Paton JF. Role of pontile mechanisms in the neurogenesis of eupnea. Respir Physiol Neurobiol. 2004;143:321–332. doi: 10.1016/j.resp.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491:156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- 25.Thaisetthawatkul P, Boeve BF, Benarroch EE, Sandroni P, Ferman TJ, Petersen R, Low PA. Autonomic dysfunction in dementia with Lewy bodies. Neurology. 2004;62:1804–1809. doi: 10.1212/01.wnl.0000125192.69777.6d. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda T, Onodera H, Okabe S, Kikuchi Y, Itoyama Y. Impaired chemosensitivity to hypoxia is a marker of multiple system atrophy. Ann Neurol. 2002;52:367–371. doi: 10.1002/ana.10296. [DOI] [PubMed] [Google Scholar]