Abstract
OBJECTIVES
Changes in mucosal serotonin (5-HT) signaling have been detected in a number of functional and inflammatory disorders of the gastrointestinal tract. This study was undertaken to determine whether chronic constipation (CC) is associated with disordered 5-HT signaling and to evaluate whether constipation caused by opiate use causes such changes.
METHODS
Human rectal biopsy samples were obtained from healthy volunteers, individuals with idiopathic CC, and individuals taking opiate medication with or without constipation. EC cells were identified by 5-HT immunohistochemistry. 5-HT content and 5-HT release levels were determined by enzyme immunoassay, and mRNA levels for the synthetic enzyme, tryptophan hydroxylase 1 (TpH1) and the serotonin transporter (SERT) were assessed by quantitative real-time RT-PCR.
RESULTS
CC was associated with increases in TpH1 transcript, 5-HT content, and 5-HT release under basal and stimulated conditions, whereas EC cell numbers and SERT transcript levels were not altered. No changes in these elements of 5-HT signaling were detected in opiate-induced constipation.
CONCLUSIONS
These findings demonstrate that CC is associated with a pattern of altered 5-HT signaling that leads to increased 5-HT availability, but does not involve a decrease in SERT expression. It is possible that increased 5-HT availability due to increased synthesis and release contributes to constipation due to receptor desensitization. Furthermore, the finding that the elements of 5-HT signaling were not altered in mucosal from individuals with opiate-induced constipation indicates that constipation as a condition does not lead to compensatory changes in 5-HT synthesis, release or signal termination.
Keywords: SERT, tryptophan hydroxylase, enterochromaffin cell
INTRODUCTION
The original name for serotonin (5-hydroxytryptamine; 5-HT) was enteramine because it was initially discovered in the gastrointestinal tract, and the gut is the major source of 5-HT in vertebrates (1). A subset of enteroendocrine cells called enterochromaffin (EC) cells use the enzyme tryptophan hydroxylase 1 (TpH-1) to synthesize 5-HT, and these cells release 5-HT from their basal surface into the insterstitial space of the lamina propria in response to chemical and mechanical stimuli (2, 3). Once released into the lamina propria, 5-HT can activate receptors on nearby intrinsic nerve fibers to stimulate motility, secretory and vasodilatory reflexes, as well as receptors on extrinsic afferent nerves to send signals to the brain and spinal cord. Serotonergic signaling in the lamina propria is terminated by removal from the interstitial space via the serotonin selective reuptake transporter (SERT), which is expressed by epithelial cells. The 5-HT that is not transported into epithelial cells enters the blood stream where is it taken up via SERT into platelets, which, when activated, release 5-HT to assist in hemostasis.
Given the importance of 5-HT in the regulation of gut functions and in sensory signaling from the gut, a number of studies have been conducted in humans and animal models to test whether 5-HT signaling is altered in disorders associated with changes in gut function and sensation. While a clear pattern has not yet emerged, most of these studies have detected changes in various elements of gut serotoninergic physiology including 5-HT content, numbers of EC cells, TpH-1 expression, mucosal 5-HT release, SERT expression, and postprandial circulating 5-HT levels (4, 5). Moreover, it is likely that alterations in 5-HT signaling do exist in functional GI disorders and/or in the presence of inflammation; however, the pattern of changes and the cause and effect relationship of these changes with regard to diarrhea, constipation, and/or visceral hypersensitivity have not been resolved.
Chronic constipation (CC) is a condition in which pain and discomfort are not primary symptoms. Multiple mechanisms are known to affect colonic motility; from opiate receptor activation, to neurostructural abnormalities, as well as alterations in gut neurochemicals, but it is unclear if altered serotonin signaling plays a role in its’ causality. This is a very common disorder affecting up to 19% of the US population (6), and it has a high degree of symptom overlap with constipation-predominant irritable bowel syndrome (IBS-C). In fact, it is possible that CC and IBS-C are actually the same disorder with different individuals situated at different locations along a continuum of pain and discomfort (7).
The purpose of this investigation was to test the hypothesis that 5-HT synthesis, release, and/or SERT transcript levels are altered in the rectal mucosa of individuals with CC in a manner similar to the changes previously reported in IBS-C. To accomplish this, rectal biopsy samples were obtained from individuals with CC and healthy controls, and levels of 5-HT content, EC cell numbers, 5-HT release, and TpH-1 and SERT transcript levels were compared. We also tested the hypothesis that these elements of 5-HT signaling are not altered in tissue from individuals with opiate-induced constipation to address the issue of whether constipation itself can lead to compensatory alterations 5-HT availability in the intestinal mucosa.
MATERIALS AND METHODS
Human Tissue Acquisition
Human colonic tissue samples were obtained from individuals who met diagnostic criteria for one of the following groups: healthy controls, CC, individuals undergoing opiate therapy with constipation, and those undergoing opiate therapy without constipation. The University of Vermont Institutional Review Board approved all aspects of this study.
Subjects with CC were recruited from the clinical university based gastroenterology colo-rectal surgery practices (University of Vermont College of Medicine in association with Fletcher Allen Health Care) or through a local printed advertisement. Volunteers with opiate-induced constipation were recruited via posters placed in the local pain management clinic and methadone clinic. A questionnaire was administered by trained healthcare providers to identify subjects who met criteria for chronic constipation or opiate-induced constipation.
Rome II criteria were used to identify subjects included in the chronic constipation group. These subjects reported constipation for at least 12 wks in the previous year. At least 25% of the time, they experienced two or more of the following symptoms: <3 bowel movements/week, straining with defecation, hard and/or lumpy stools, sensation of incomplete evacuation, sensation of anorectal obstruction, use of manual maneuvers to assist with defecation. Patients who met these criteria differ from patients with IBS-C in that abdominal pain or discomfort was not a prominent clinical feature. None of these patients were taking medications that could cause constipation as a primary or secondary effect.
Individuals in the opiate therapy groups had been taking these compounds for at least 3 months, but most had been using opiate analgesics for at least 2 years. The volunteers with opiate-induced constipation (OIC) all experienced <3 bowel movements/week. They were also screened using the same questionnaires as noted above to rule out concurrent diagnoses of IBS, other gastrointestinal disorders or use of other medications that could cause constipation as a primary or secondary effect. The demographic make up of the OIC population is listed in Table 1 of the manuscript.
Table 1.
Characteristics of individuals included in the study
| Group | Control | Chronic Constipation | Control | Opiate-Induced Constipated | Opiate-Induced Non-Constipated |
|---|---|---|---|---|---|
| n | 25 | 21 | 28 | 27 | 16 |
| Sex: Male | M = 12 | M = 5 | M = 15 | M = 17 | M = 10 |
| Female | F = 13 | F= 21 | F = 13 | F= 10 | F= 6 |
| Age | |||||
| Range | 38–77 | 24–74 | 41–76 | 22–56 | 27–62 |
| [Mean] | [61] | [42] | [56.4] | [39.1] | [45.4] |
| (Median) | (60) | (41) | (55) | (40) | (41) |
| # on SSRIs | 3 | 3 | 3 | 3 | 3 |
| # on tegaserod | 0 | 2 | 0 | 0 | 0 |
Healthy controls provided consent at the time they presented for scheduled screening colonoscopy. Following sodium phosphate preparation, five rectal biopsy samples were obtained in succession from each participant using large capacity biopsy forceps, and each sample was immediately processed in one of the manners listed below. Demographic data for the individuals included in the present study are summarized in Table 1.
No significant relationships were observed in any of the populations in regard to age, sex, or use of serotonin-altering medication (selective serotonin reuptake inhibitors, 5-HT4 agonists, or 5-HT3 antagonists) (two-tailed Pearson Correlation Analysis with a confidence interval of 95%).
Immunohistochemistry
Biopsies were fixed in a 10% formalin solution and standard techniques were used to prepare each biopsy sample for immunoreactive staining, as previously described (8). The primary antiserum used to identify EC cells was mouse conjugated anti-5-HT (1:500, Dako Diagnostica). The secondary antibody used was goat anti-mouse AMCA (1:250; Jackson ImmunoResearch). All of the sections were then incubated with 1:30, 000 dilution of the nucleic acid stain, yo-yo (Molecular Probes, Eugene, OR). One slide from each biopsy was stained with H&E to evaluate the degree of inflammation present in the biopsy specimen.
Epithelial cells were quantified by counting the number of stained nuclei of epithelial cells for a given length of colon, including at least 3 crypts, and quantified as the number of epithelial cells per crypt. 5-HT-containing epithelial cells were counted and normalized as functions of the number of EC cells per colonic gland, and as the proportion of epithelial cells that were EC cells. These determinations were made at a magnification of 400x from three random locations in each transverse section while taking care to ensure that the area contained intact colonic glands.
Histological Assessment
H&E stained tissue sections from each individual in the study were blindly assigned an inflammatory activity score, on a scale of 0–3, by an experienced gastrointestinal pathologist. The scoring system was as follows: (0), normal numbers of inflammatory cells in the lamina propria with no active inflammation; (1), normal numbers of inflammatory cells in the lamina propria with rare neutrophils within the crypt epithelium or minimally increased numbers of inflammatory cells in the lamina propria and no neutrophils within the crypt epithelium; (2), increased numbers of inflammatory cells in the lamina propria and mild active inflammation; (3), increased numbers of inflammatory cells in the lamina propria and moderate/severe active inflammation. Scores of 0 and 1 were considered to be within the range of normal. Scores of 2 or 3 were considered to be histopathologically inflamed and usually reflected an inactive/mildly active colitis or a moderately/severely active chronic colitis, respectively. Individual samples that were evaluated to have an inflammatory score of 2 or higher were to be eliminated from further analysis and would not be included in the results. All of the sections evaluated in the current study had scores of 0 or 1; therefore, none of the samples were excluded for inflammation.
Measuring 5-HT Content and Release
The 5-HT content in homogenized biopsy specimens, or released into buffer solution from each individual, was analyzed with an enzyme immunoassay kit according to the manufacturer’s instructions (Beckman Coulter, Fullerton, CA). Two biopsy specimens from each individual were used to assess 5-HT release. Specimens were maintained at 37°C under one of two experimental conditions: “Basal Release” - 5 minutes in 500 mL of a 10 mM Hepe’s buffer solution at 37°C (pH = 7.4) (Sigma Chemical, St. Louis, MO) or “Stimulated Release” - 2 minutes in 500 mL of Hepe’s buffer at 37°C followed by 3 minutes mild agitation on a vortex machine. After the five minutes, 400 mL of solution was removed and placed in a new Eppendorf tube. The 5-HT released into the bathing solution was subsequently measured by the same enzyme immunoassay described above.
Measurement of mRNA encoding TpH-1 and SERT
Transcripts encoding TpH-1 and SERT were quantified by using real time reverse transcriptase and the polymerase chain reaction (RT-PCR). RNA was extracted from the samples using RNeasy mini-kits (Qiagen Sciences, Valencia, CA), and cDNA was generated by reverse transcription (Promega, Madison, WI). For real-time PCR, Fast Universal PCR Master Mix and validated fluorogenic TaqMan Gene Expression Assays-on-Demand for TpH-1 (Hs00188220_m1), SERT (Hs00169010_m1), phosphmannomutase 1 (PMM1; Hs00160195_m1), and hypoxanthine-guanine phosphoribosyl transferase (HPRT; Hs99999909_m1) were used in conjunction with an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Data were calculated using the relative standard curve method and the level of SERT or TpH-1 RNA in each sample was normalized to PMM1 and HPRT. Amplification efficiencies ranged from 98.9 – 100.4%. PMM1 and HPRT transcript levels in the populations studied were comparable (p>0.05). Here we are presenting data normalized to PMM1 because the span of cycle thresholds was tighter for this gene than for HPRT.
Data Analysis
The data presented are means ± S.E.M. for n subjects. Statistical analyses were performed using Prism software (v 4; GraphPad Software, San Diego, CA). Comparisons between two groups were made parametrically with a Student’s t-test to compare control versus CC data or by a one-way ANOVA with a Tukey post-hoc test to compare values from healthy subjects and individuals undergoing opiate therapy with or without constipation. Only differences of p < 0.05 were considered to be significant.
RESULTS
5-HT content
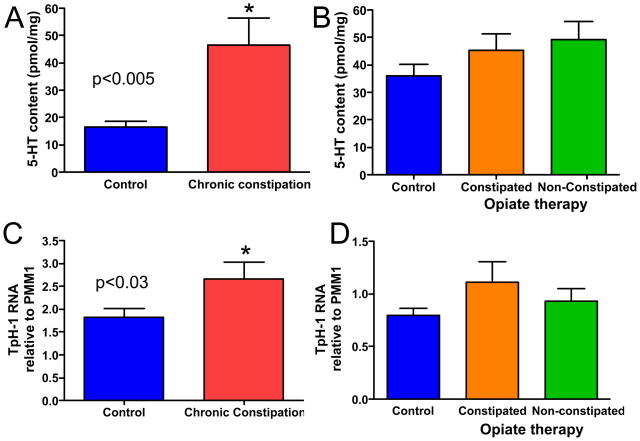
Enzyme immunoassay was used to determine the amount of 5-HT in individual biopsy specimens. As demonstrated in figure 1A, 5-HT content of the samples from CC subjects was significantly higher than that of healthy subjects. However, no significant differences in 5-HT content were detected amongst samples from healthy controls and samples from individuals undergoing opiate therapy with or without constipation (Fig 1B).
Figure 1.
Serotonin content and TpH1 transcript levels are increased in CC, but are not altered in opiate-induced constipation. (A) 5-HT content is significantly increased in CC (p=0.003). (B) No significant differences were detected in 5-HT levels of samples from individuals undergoing opiate therapy, with or without constipation (p>0.05 for each group vs control). (C) The transcript level for TpH-1 was significantly higher in RNA from CC samples as compared to healthy controls (p=0.028). (D) No significant differences were detected in TpH-1 RNA levels of samples from individuals in the opiate therapy groups as compared to controls (p>0.05 for each group vs control). n-values: A, 19, 16; B, 24, 20, 14; C, 22, 12; D, 13, 9, 13.
TpH-1 RNA levels
Two isoforms exist for TpH, which is the rate-limiting enzyme for 5-HT synthesis (9). Isoform 1, or TpH-1, is used by EC cells in the intestinal mucosa whereas TpH-2 is expressed by neurons in the central and peripheral nervous systems. We therefore used real time quantitative RT-PCR to evaluate TpH-1 transcript levels in RNA extracted from biopsy samples. Data were evaluated relative to transcript levels for phosphomannomutase 1 (PMM1), which did not differ amongst the populations investigated in this study. Consistent with the finding of higher 5-HT content samples from individuals with CC as compared to healthy control samples, TpH-1 RNA levels were higher in samples from CC subjects than in samples from healthy control volunteers (Fig 1C). No differences were detected amongst the TpH-1 RNA levels from the two opiate therapy groups and healthy controls (Fig. 1D). Comparable findings were obtained when hypoxanthine-guanine phosphoribosyl transferase (HPRT) was used as an endogenous control. We chose to present data relative to PMM1 because the cycle thresholds were tighter amongst the samples for PMM1 than for HPRT.
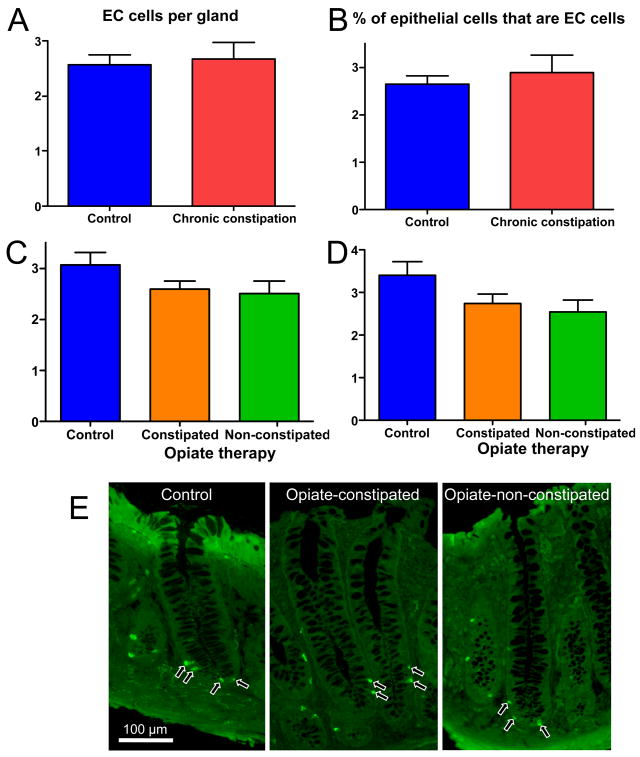
EC cell numbers
The data described above indicate that 5-HT synthesis and content are increased in the mucosa of individuals with CC, but that these elements of 5-HT signaling are unchanged in opiate-induced constipation. As EC cells are the predominant source of 5-HT in the mucosal layer of the gut, antiserum directed against 5-HT was used to label and quantify EC cells in the colonic glands. No differences were detected in either the CC group or the opiate treatment group as compared to healthy controls with regard to number of EC cells per colonic gland or the proportion of epithelial cells that were EC cells (Fig. 3).
Figure 3.
No significant differences in the numbers of EC cells per gland (A, C) or the percentage of epithelial cells that were EC cells (B, D) were detected in the CC samples or in biopsies from individuals undergoing opiate therapy, as compared to healthy controls (p>0.05 for each group vs control). E: photomicrographs showing representative colonic glands that were used to quantify the EC cells in these preparations. n-values: A, 19, 11; B, 19, 11; C, 23, 25, 15; D, 23, 25, 15.
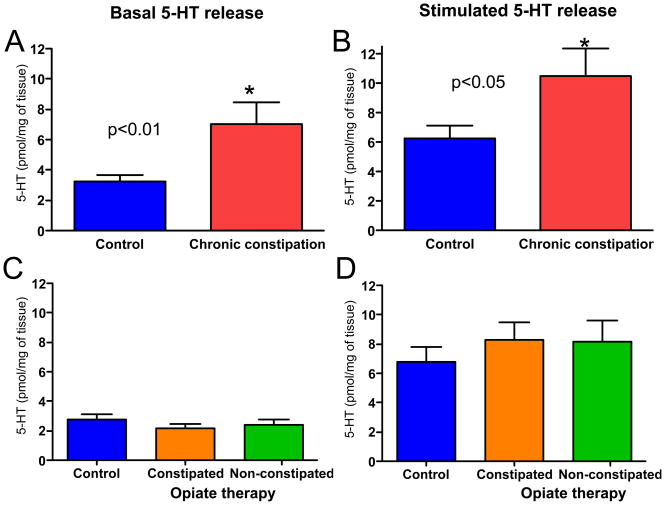
5-HT release
Serotonin is released in the intestinal mucosa under basal conditions, and at higher levels in response to mechanical stimulation (8, 10, 11). In the current study, enzyme immunoassay was used to evaluate 5-HT released from mucosal biopsy specimens into HEPES buffer in samples that were simply heated to 37 °C and in samples that were mechanically stimulated for 3 minutes by mild vibration on a vortex machine. Histological evaluation of sections that underwent mechanical stimulation confirmed that the tissue remained intact following this procedure.
Under both basal and stimulated conditions, 5-HT concentrations were higher in buffer that contained CC biopsy samples than those that contained healthy control samples (Figs 4A and 4B). No differences were detected in 5-HT concentrations in buffer samples from opiate versus control groups under basal or stimulated conditions (Figs 4C and 4D).
Figure 4.
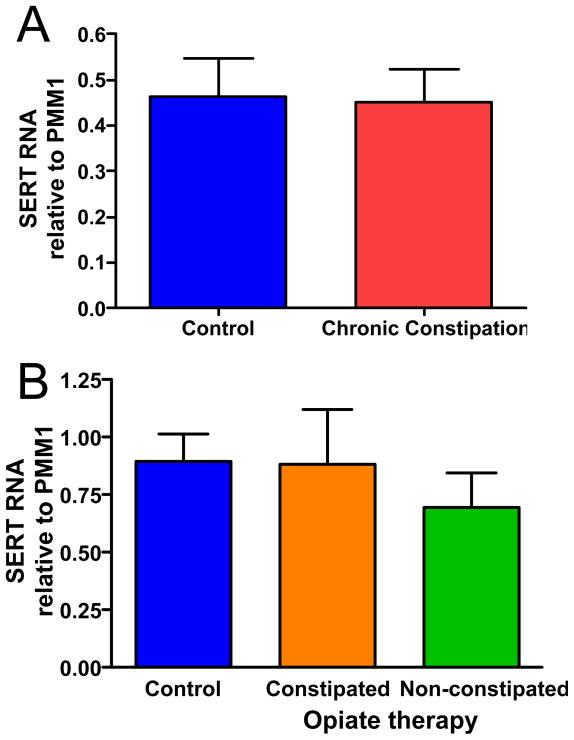
No significant differences in the SERT transcript levels were detected for CC samples (A) or opiate therapy samples as compared to healthy controls (p>0.05 for each group vs control). n-values: A, 22, 13; B, 13, 9, 13.
SERT RNA levels
In addition to content and release, another factor that can influence 5-HT availability in the lamina propria of the intestinal mucosa is the capacity to remove 5-HT from the interstitial space. Intestinal epithelial cells express the serotonin transporter, SERT, and the amount of SERT expressed by intestinal epithelial cells can be altered under certain conditions including inflammation (8, 12), celiac disease (13), and IBS (8). Therefore, real time quantitative RT-PCR was used to assess whether SERT production might be different in the intestinal mucosa of individuals with CC and/or opiate-induced constipation as compared to healthy controls. No differences were detected in SERT RNA levels in samples from CC subjects or the opiate treatment groups as compared to healthy controls (Fig. 5).
Figure 5.
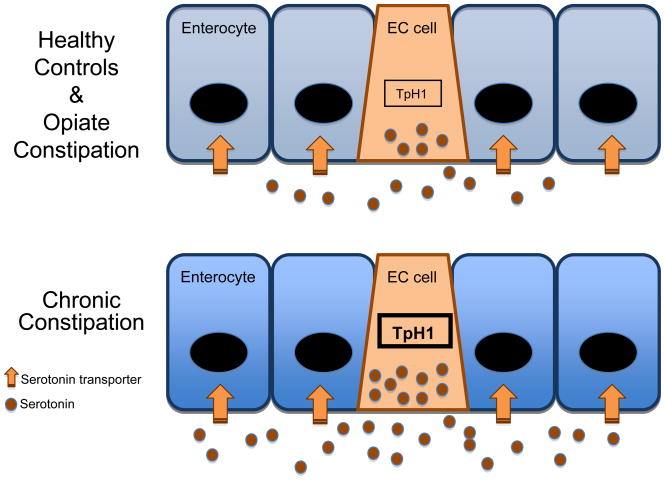
Schematic representation of the findings of this study. No changes were detected relative to control in samples from subjects with opiate induced constipation. Findings from subjects with chronic constipation revealed increased RNA levels in the rate limiting enzyme for 5-HT synthesis, tryptophan hydroxylase 1 (TpH1) in EC cells, increased mucosal 5-HT content indicating higher concentration of 5-HT in the EC cells, and increased 5-HT release under basal and stimulated conditions. Elements of 5-HT signaling that were not altered in chronic constipation included the number of EC cells and RNA levels for the 5-HT transporter.
DISCUSSION
Release of 5-HT from EC cells in the intestinal mucosa is one of the principal triggers of motor and sensory reflex activity in the gut, as well as communication from the gut to the central nervous system (3). Studies of a number of inflammatory and functional disorders indicate that alterations in various aspects of mucosal 5-HT are features of these conditions (4, 5). One aim of this investigation was to determine whether CC is associated with alterations in mucosal 5-HT signaling. Furthermore, we evaluated the status of 5-HT signaling in the rectal mucosa of individuals with opiate-induced constipation to investigate the cause and effect relationship of altered 5-HT signaling by using a condition in which the cause of disordered function is understood. The findings of this study are schematically summarized in figure 5.
Data from the current study indicate that 5-HT availability in the lamina propria of the colonic mucosa is increased in CC. We found that 5-HT content was significantly higher in CC samples as compared to rectal biopsy specimens from healthy controls. This is consistent with the findings of Lincoln and colleagues, who reported that 5-HT levels were significantly elevated in the sigmoid colon in severe idiopathic constipation (14). Additional lines of evidence from the current study supporting increased 5-HT availability in CC include increased levels of TpH-1 RNA, the rate limiting biosynthetic enzyme used by EC cells, and increased 5-HT release under both basal and stimulated conditions in mucosal samples from subjects with CC as compared to those from healthy volunteers.
Changes in the mucosa that could contribute to increased 5-HT synthesis and release in CC include an increase in the number of EC cells that are present in the mucosa and/or an increase in the synthesis and release of 5-HT from each EC cell. Data reported here implicate the latter of these possibilities. In the current study, a blinded quantification of the EC cells involved evaluating the numbers of EC cells per colonic gland and proportion of epithelial cells that were EC cells. No changes in the EC cell population were detected in the CC sections as compared to controls.
These results, demonstrating no change in the density of EC cells in CC, are different from previous reports of alterations in the EC cell population in constipated individuals. El-Salhy and colleagues reported that the number of 5-HT-immunoreactive cells per unit area of epithelial cells is lower in colons removed for treatment of slow transit constipation (15). On the other hand, others have reported that the average number of EC cells per field is increased in surgical specimens (16, 17). One possible explanation for these differences is that great care was taken in the present study to orient the specimens so that sections were cut perpendicular to the luminal surface, and only regions of sections containing full-length colonic glands were quantified. This is because the EC cells are unevenly distributed in the glands, with more of these cells located at their base. The micrographs included in the previous publications indicate that randomly oriented colonic glands were included in those studies, and there is no indication that full-length glands were evaluated. Other possible explanations include the fact that we were looking at rectum and they were looking at the right and left colon. Furthermore, the previous studies were limited to slow transit constipation cases that were severe enough to require surgical intervention, whereas the current study involved endoscopically obtained rectal biopsies from individuals with intact colons.
It is often difficult to distinguish CC from IBS-C, and it has been proposed that they actually represent a single condition with sufferers situated at various sites along a pain and discomfort spectrum (7). In the current study, subjects were excluded if pain and/or discomfort were a predominant symptom. In other words, visceral hypersensitivity was not a common condition in these individuals as it is in IBS-C. Comparison of the results of the current study regarding 5-HT signaling in CC with those from studies of IBS-C indicate that while 5-HT is altered in both disorders, the features of these alterations differ between CC and IBS-C.
Previous studies of 5-HT signaling in IBS-C have yielded somewhat contradictory results. Serotonin content in mucosal samples has been reported to be decreased (8), increased (18), or unchanged (19). The number of EC cells is unchanged (8, 19), and the one study of 5-HT release from mucosal samples failed to detect a difference between control and IBS-C samples under basal or stimulated conditions (8). Lines of evidence for a decrease in SERT expression include the finding that SERT RNA levels are lower in IBS-C samples than controls (8), and that platelet 5-HT levels are elevated in IBS-C (20), suggesting that less 5-HT is being taken up into epithelial cells, resulting in more 5-HT entering into the bloodstream and being taken up by platelets. However, another study failed to detect a difference in SERT RNA levels in control versus IBS-C mucosal biopsies (21).
When focusing on data generated by our group, which involved almost identical technical approaches, CC was associated with elevated synthesis and release (5-HT content, TpH-1 RNA, basal release and stimulated release were increased), but SERT RNA levels were unchanged. In IBS-C, 5-HT content and TpH-1 and SERT RNA were decreased, but 5-HT release was not altered under basal or stimulated conditions (8). Both of these scenarios would result in increased 5-HT availability in the lamina propria, with CC involving increased 5-HT release, and IBS-C involving a decreased capacity to remove 5-HT from the interstitial space once it is released.
The findings reported here do not provide direct evidence for a causal relationship between serotonin signaling and constipation in CC; however, it is quite possible that an increase in synthesis and release of 5-HT in the mucosa could contribute to constipation in CC through receptor desensitization. In vitro studies have demonstrated that exposure of guinea pig distal colon preparations to desensitizing concentrations of 5-HT or to the SERT inhibitor, fluoxetine, decrease the rate of propulsive motility (22). Furthermore, in the central nervous system, 5-HT receptor binding is downregulated in response to administration of serotonin selective reuptake inhibitors (23, 24). Despite the in vitro evidence of serotonin induced motility alterations, it is difficult to determine whether the 5-HT alterations measured in bowel disorders are the cause or the result of the motility changes in vivo. This study attempted to delineate cause and effect by taking mucosal samples from subjects experiencing constipation with a known chemical cause, narcotic use, as well as those taking narcotic pain medications who were not constipated. If the colonic samples of those with opiate-induced constipation showed similar alterations in 5-HT to those with chronic constipation, then this would suggest that the 5-HT alterations were a result of the gut dysfunction itself. This study demonstrates that there are no differences in 5-HT content, release or reuptake in opiate-induced constipated, opiate non-constipated or control specimens. Therefore, the 5-HT alterations measured in this study may suggest a causal relationship in CC. It would be interesting to determine whether the features of mucosal 5-HT signaling return to normal in individuals whose symptoms are successfully managed with pharmacological and/or non-pharmacological therapies.
Concluding remarks
The findings from this and other studies demonstrate that various aspects of mucosal 5-HT signaling are altered in disorders involving abnormal intestinal function, raising the question of a cause-and-effect relationship between gut function and 5-HT signaling in the gut. 5-HT content as well as availability in the lamina propria were both increased in subjects with CC when compared with controls. Because the same alterations were not demonstrated in patients with opiate-induced constipation, our findings support the contention that altered 5-HT signaling does not occur in response to altered gut function. Rather, it may be specific to a phenomenon or epiphenomenon associated with functional disorders.
Study Highlights
WHAT IS CURRENT KNOWLEDGE
Serotonin is an important signaling molecule in the intestines.
Serotonin signaling is altered in IBD and IBS.
WHAT IS NEW HERE
Serotonin synthesis and release are increased in chronic constipation
Constipation does not lead to compensatory changes in serotonin signaling
Figure 2.
Serotonin release was elevated in CC samples under basal and stimulated conditions, but was not altered in mucosal samples from individuals with opiate-induced constipation. (A and B) 5-HT release was significantly increased under basal (p=0.009) and stimulated (p=0.03) conditions. (C and D) 5-HT was not significantly altered in samples from individuals undergoing opiate therapy under basal or stimulated conditions (p>0.05 for each group vs control). n-values: A, 23, 18; B, 23, 18 C, 19, 20, 15; D, 19, 20, 15.
Acknowledgments
The authors would like to thank Dr. Cristian Speer, Dr. Magdalana R. Naylor, Dr. Stacey C. Sigmon, and staff members of the Center for Pain Medicine and the Methadone Clinic of Fletcher Allen Healthcare for their assistance in identifying subjects for the study.
Financial Support: Financial support for these studies was provided from Novartis Pharmaceuticals and NIH grant DK62267.
Footnotes
Conflict of interest items
1. Guarantor of the manuscript
Gary M. Mawe, PhD
2. Roles of each author
Meagan M Costedio: patient screening, obtaining consent, tissue acquisition, tissue processing, data analysis, and manuscript preparation and editing
Matthew D Coates: patient screening, obtaining consent, tissue acquisition, tissue processing, data analysis, and manuscript preparation and editing
Elice M Brooks: tissue acquisition, data acquisition, and data analysis
Lisa M Glass: data acquisition, and data analysis.
Eric K Ganguly: aided in conception of the project, acquiring IRB approval, obtaining informed consent, and tissue acquisition.
Hagen Blaszyk: evaluation of sections and blind scoring of inflammation levels in the chronic constipation component of the study
Allison L. Ciolino: evaluation of sections and blind scoring of inflammation levels in the opiate constipation component of the study
Michael J Wood: involved in obtaining informed consent, and tissue acquisition.
Doris Strader: involved in obtaining informed consent, and tissue acquisition.
Neil H Hyman: involved in study conceptualization, planning, and identifying potential candidates for the chronic constipation component of the study.
Peter L Moses: involved in study conceptualization, planning, obtaining informed consent, and tissue acquisition, data analysis, and manuscript preparation and editing
Gary M Mawe: involved in study conceptualization, planning, data analysis, and manuscript preparation and editing.
All authors reviewed and approved the manuscript prior to submission.
4. Potential Competing Interests: None
References
- 1.Erspamer V. Occurence of indolealkylamines in nature. In: Erspamer V, editor. Handbook of experimental pharmacology. Vol. 19. New York: Springer; 1966. pp. 132–181. 5-Hydroxtryptamine and related indolealkylamines. [Google Scholar]
- 2.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–76. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 4.Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50:376–88. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- 5.Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–9. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 7.Frissora CL, Koch KL. Symptom overlap and comorbidity of irritable bowel syndrome with other conditions. Curr Gastroenterol Rep. 2005;7:264–71. doi: 10.1007/s11894-005-0018-9. [DOI] [PubMed] [Google Scholar]
- 8.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 10.Linden DR, Chen JX, Gershon MD, et al. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–16. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand PP, Hu X, Mach J, et al. Serotonin (5-HT) release and uptake measured by real-time electrochemical techniques in the rat ileum. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1228–36. doi: 10.1152/ajpgi.90375.2008. [DOI] [PubMed] [Google Scholar]
- 12.Costedio MM, Coates MD, Danielson AB, et al. Serotonin signaling in diverticular disease. J Gastrointest Surg. 2008;12:1439–45. doi: 10.1007/s11605-008-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman NS, Foley S, Dunlop SP, et al. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874–81. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Lincoln J, Crowe R, Kamm MA, et al. Serotonin and 5-hydroxyindoleacetic acid are increased in the sigmoid colon in severe idiopathic constipation. Gastroenterology. 1990;98:1219–25. doi: 10.1016/0016-5085(90)90336-y. [DOI] [PubMed] [Google Scholar]
- 15.El-Salhy M, Norrgard O, Spinnell S. Abnormal colonic endocrine cells in patients with chronic idiopathic slow-transit constipation. Scand J Gastroenterol. 1999;34:1007–11. doi: 10.1080/003655299750025110. [DOI] [PubMed] [Google Scholar]
- 16.Baig MK, Zhao RH, Woodhouse SL, et al. Variability in serotonin and enterochromaffin cells in patients with colonic inertia and idiopathic diarrhoea as compared to normal controls. Colorectal Dis. 2002;4:348–354. doi: 10.1046/j.1463-1318.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhao R, Baig MK, Wexner SD, et al. Enterochromaffin and serotonin cells are abnormal for patients with colonic inertia. Dis Colon Rectum. 2000;43:858–63. doi: 10.1007/BF02238027. [DOI] [PubMed] [Google Scholar]
- 18.Miwa J, Echizen H, Matsueda K, et al. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–94. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- 19.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–57. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson W, Lockhart S, Whorwell PJ, et al. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Andrews CN, Bharucha AE, et al. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 2007;132:17–25. doi: 10.1053/j.gastro.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wade PR, Chen J, Jaffe B, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–64. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Licht CL, Marcussen AB, Wegener G, et al. The brain 5-HT4 receptor binding is down-regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J Neurochem. 2009;109:1363–74. doi: 10.1111/j.1471-4159.2009.06050.x. [DOI] [PubMed] [Google Scholar]
- 24.Vidal R, Valdizan EM, Mostany R, et al. Long-term treatment with fluoxetine induces desensitization of 5-HT4 receptor-dependent signalling and functionality in rat brain. J Neurochem. 2009;110:1120–7. doi: 10.1111/j.1471-4159.2009.06210.x. [DOI] [PubMed] [Google Scholar]