SUMMARY
Objective
Conidia derived from a small number of common fungal genera are widely accepted as the etiological agents responsible for fungal allergic sensitization. The contribution of fungal conidia, spores, airborne hyphae, and subcellular fragments from other uncharacterized fungal genera remains unclear. In this proof-of-concept study, we examined the composition of mycoaerosols that atopic women were exposed and sensitized to in their own indoor environment using the fluorescent halogen immunoassay (fHIA).
Patients and Methods
Mycoaerosols were collected onto mixed cellulose ester protein binding membranes (PBMs) for 30 minutes with volumetric air sampling pumps. The PBMs were laminated with an adhesive cover slip and indirectly immunostained with individual patient serum IgE using the fHIA. Samples were examined using confocal laser scanning microscopy and immunostained particles were expressed as a percentage of total particles.
Results
All air samples contained a broad spectrum of fungal spores, conidia, hyphae, and other fungal particulates. Airborne concentrations varied between individual study participant environments. Positively immunostained conidia belonging to moniliaceous amerospores, Cladosporium, Alternaria, and many unknown species were observed in the majority of air samples. Other fungal genera including Bipolaris, Curvularia, Pithomyces, and Stachybotrys, in addition to, ascospore genera and dematiaceous hyphal fragments released detectable allergen. Twelve percent of all fHIA haloes quantified in the analysis were directed towards fungal particles. No immunostaining was detected to conidia belonging to Epicoccum, Fusarium, and Spegazzinia species.
Conclusion
In addition to characterized fungal aeroallergens, we observed a wider composition of fungi that bound human IgE. Field surveillance studies that utilize immunodiagnostic techniques such as the fHIA will provide further insight into the diversity of fungi that function as aeroallergen sources in individual study participant environments.
Keywords: allergen, Alternaria, Cladosporium, Conidia, Fungi, Hyphae, Immunoassay, Mold
INTRODUCTION
Exposure assessment scientists characterize personal fungal exposure as the inhalation of fungal conidia derived from environmentally abundant and morphologically discernible fungal genera, such as Alternaria, Aspergillus, Cladosporium, and Penicillium. These fungal genera are the most widely recognized etiological agents associated with fungal allergic sensitization [11], and respiratory morbidity [5, 29, 49]. However, the contribution of fungal conidia, hyphae, and subcellular fragments from uncharacterized fungal genera remains unclear because of the methodological challenges associated with immunodiagnosis, as well as identifying fungi in environmental samples [20].
Compared to other perennial and seasonal allergens, fungal bioaerosols are viable heterotrophic microorganisms. Some fungal conidia possess unique virulence factors that facilitate colonization by reducing respiratory cilia beat frequency [4, 6, 10, 50]. Depending on the site of deposition, some fungi may also germinate and release a complex assortment of mycotoxins, antigens, and other immunostimulatory macromolecules [16]. Germination is required for fungal colonization (chronic rhinosinusitis and invasive aspergillosis), and may result in further individual exposure to fungal allergens [16, 32, 48]. Because of these aspects, the characterization of fungal allergens has lagged behind other aeroallergen sources. Complex life cycles, rich biodiversity, and variability of fungal allergens have all hindered understanding fungal allergic sensitization. These confounding factors have also influenced the standardization of commercial extracts available for diagnosis and have limited the availability of diagnostic reagents for many uncharacterized fungi [52].
New developments in immunodiagnostic methodologies, such as the Halogen Immunoassay (HIA), have enhanced the ability to collect, and detect allergic sensitization in patients exposed to mycoaerosols [15, 21, 32, 37, 38, 51]. The uniqueness of this method is that it provides a method to match the spectrum of an individual’s allergic responses with the inherent diversity of bioaerosols collected from the patient’s own environment. Compared to other commercially available immunodiagnostic tests, the HIA correlates with Phadia ImmunoCap technologies but less well to skin prick testing [21]. Specifically, eluted surface antigens bind in close proximity to the particle and are immunostained with the patient’s serum immunoglobulin E (IgE). IgE from sensitized patients binds to the immobilized bioaerosol antigens and following secondary detection, a halo of immunostaining will illuminate the particle.
Studies utilizing HIA have provided insight into the contribution of fungal particulates to the aeroallergen load, particularly discrete dematiaceous hyphal fragments <100 μm long, partial multicellular conidia, and subcellular fragments [19, 20]. However, several limitations have hindered the performance of the HIA as a field surveillance methodology. Alkaline phosphatase-based enzymatic methodologies can detect nanogram quantities of antigen. Given the picogram quantities of antigen released from the smaller fungal spores and fragments, this had limited the ability to immunostain these particles in air samples [20]. Recent improvements utilizing Alexa Fluor® (Molecular Probes, Inc., Eugene, OR) secondary detection and confocal laser scanning microscopy have mitigated these limitations and enabled the detection of moniliaceous amerospores (colorless unicellular conidia) and fungal fragments as small as 0.5 μm [15, 20]. The development of the fluorescent HIA (fHIA) is an important immunodiagnostic development given the recent observations of elevated concentrations of smaller fungal particulates in indoor and occupational environments [14, 22, 30].
In this observational study, we explored the feasibility of the fHIA as a field surveillance methodology to detect individual fungal allergic sensitization profiles in a cohort of atopic women. Compared to traditional immunodiagnostic methods, it is possible using the fHIA to understand the complete composition of mycoaerosols to the aeroallergen load.
MATERIALS AND METHODS
Human Subjects
Serum samples were collected from 24 women living in New York City. Each subject was enrolled in a prospective longitudinal study of an existing birth cohort of Puerto Rican ethnicity, where the subject (mother of newborn infant of Puerto Rican ethnicity) had a clinical history of asthma, allergic rhinitis, and/or inhalant allergy [1]. Following serum collection, all samples were stored in aliquots for future use at -70°C. The Columbia University Medical Center Institutional Review Board approved the study (AAAA-9880), and the subjects gave written informed consent following a full explanation of the study.
Personal Air Sampling
The sampling train consisted of a button sampling head (SKC Ltd, Eighty Four, PA) connected to a SKC Airchek 2000 sampling pump (SKC Ltd, Eighty Four, PA) that provided a constant 3.0 liters per minute (lpm) air flow through a mixed cellulose ester protein binding membrane (PBM). The button sampling head was sterilized prior to use and fitted with a 0.8 μm pore size mixed cellulose ester PBM (Millipore Corp., Bedford, MA) for use in the fHIA. Compared to other samplers, the button sampling head allows better dispersion of particles across the PBM especially when air currents are turbulent [2]. Air samples were collected from twenty-four homes located in New York City for a period of 30 minutes, in the room identified as the study participant’s bedroom as previously described [1]. These were homes of participants in a birth cohort study designed to characterize the factors that contribute to allergen exposure and allergic sensitization. During the air sampling, dust samples were vacuumed (Eureka Mighty Mite, Bloomington, IL) from the subjects bed for a period of 3 minutes and from the surrounding floor also for 3 minutes; thus the air sample was not collected during quiescent conditions. At the completion of each sampling interval, the PBM was removed from the button sampler and stored at room temperature until fHIA analysis. The sampling pumps were post-calibrated, and measurements ranged from 2.8-3.02 lpm.
Fluorescent Halogen Immunoassay
The PBM was removed from the button sampling head, placed in a humid chamber overnight to enable conidia germination and immunostained using the fHIA as described previously [19]. The germination of fungal conidia increases the detection thresholds of the immunoassay [16, 32]. Impacted fungal particles were permanently laminated to the mixed cellulose ester PBM by overlaying the sample with an optically clear adhesive/glass coverslip [15]. The laminated samples were immersed in borate buffer (pH 8.2) at room temperature for three hours to enable antigens and other macromolecules to elute from the fungal particles and bind in close proximity to the PBM. Membranes were blocked in 5% bovine serum albumin (BSA) in phosphate buffered saline (PBS) for 90 minutes. PBMs were incubated overnight at 4°C with individual patient serum diluted 1:3 in 5% BSA/PBS/0.05% Tween 20 [15]. After the primary antibody incubation, the membranes were rinsed three times in PBS/0.05% Tween 20 and incubated for 1.5 h with biotinylated goat anti-human IgE (Kirkegaard & Perry Laboratories, Gaithersburg, MD) diluted 1:500. Following incubation, the membranes were rinsed three times in PBS/0.05% Tween 20 and then incubated for 3 h with streptavidin, Alexa Fluor® 488 conjugate (green fluorescence; Molecular Probes, Inc., Eugene, OR) diluted 1:500 in 5% BSA/PBS/0.05% Tween 20. This procedure was followed by rinsing the membranes three times in dH2O. Each sample was then mounted on a microscope slide in ProLong® Gold (Molecular Probes, Inc., Eugene, OR) antifade reagent. The principle of the fHIA is outlined in Figure 1.
Figure 1.
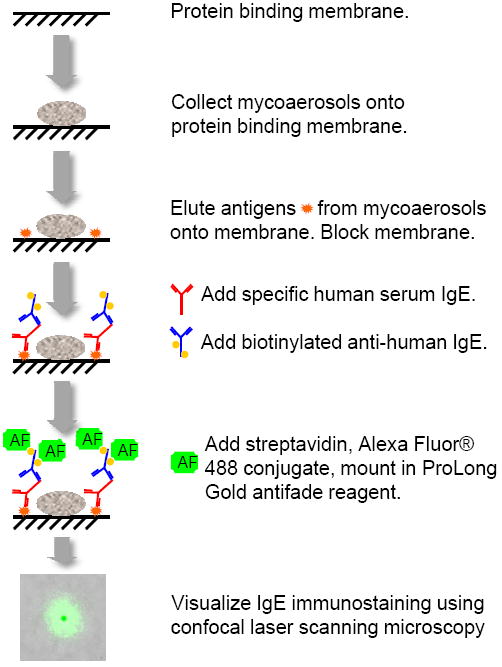
Experimental flow chart of the fHIA
Negative control treatments consisted of the collection of culture-derived Alternaria alternata (ATCC 11612), Cladosporium herbarum (ATCC 6506), Epicoccum nigrum (ATCC 34929), and Paecilomyces variotii (ATCC 66705) conidia and hyphae onto PBMs as previously described [15]. Each negative control PBM was processed in parallel by substituting human atopic IgE with (1) non-atopic human IgE, biotinylated goat anti-human IgE, and streptavidin, Alexa Fluor® 488 conjugate (2) biotinylated goat anti-human IgE, and streptavidin, Alexa Fluor® 488 conjugate, or (3) streptavidin, Alexa Fluor® 488 conjugate.
Confocal Laser Scanning Microscopy Images
Confocal laser scanning images were captured using a Zeiss LSM 510 laser scanning confocal system (Carl Zeiss Inc., Thornwood, NY) with an Axioplan 2 microscope, 40x C-Apochromat water immersion objective lens, and Argon and HeNe lasers. The images of human IgE-immunostained fungal particles were captured using 488 nm excitation and a narrow emission filter bandwidth (505-550 nm). Fluorescent and differential interference contrast images (DIC) were captured using Zeiss software version 3.2 (Carl Zeiss Inc., Thornwood, NY). All settings on the confocal microscope remained constant in the analysis of positive samples and negative controls.
An unblinded examiner examined entire membranes and the number of positively immunostained samples were recorded and expressed as percentages of totals. Fungi were identified to genus level using morphologically discernible phenotypic features including, conidia shape, size, and septation. A mycologist confirmed the identification of several fungal conidia that were unable to be identified during the analysis. Fragments of dematiaceous fungi were classified as hyphal fragments if hyphal septation was present in the fragment.
RESULTS
Fungal conidia and spores were present in all collected air samples. The predominant fungal genera included, moniliaceous amerospores (unicellular colorless conidia belonging to Aspergillus, Penicillium, and other genera), Cladosporium, ascospores, Fusarium, Epicoccum, and Alternaria species (Table 1). Airborne dematiaceous hyphal fragments were also present in all air samples. The quantities of airborne fungal conidia and hyphae collected onto individual PBMs varied substantially between study participants’ environments (Table 1). The greatest variation in airborne counts was observed for moniliaceous amerospores, unknown conidia, Cladosporium conidia, as well as airborne hyphae (Table 1).
Table 1.
Halogen Immunoassay data for each individual study participant (n=24).
| Subject | Total IgEa | Total Halob | Collected fungal genera | %gTotal | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monilaceous amerosporec | Unknown | Cladosporium | Alternaria | Hyphae | Pithomyces | Curvularia | Bipolaris | Ascospore | Fusarium | Epicoccum | Spegazzinia | ||||||||||||||||
| +d | -e | + | - | + | - | + | - | + | - | + | - | + | - | + | - | + | - | + | - | + | - | + | - | ||||
| 1 | 110 | 125 | 4 | 14 | 3 | 15 | nd | 15 | nd | nd | nd | 10 | nd | 1 | 1 | 1 | nd | nd | nd | 7 | nd | nd | nd | nd | nd | nd | 6% |
| 2 | 24.8 | 92 | nd | 67 | nd | 25 | 8 | 19 | 2 | nd | nd | 3 | nd | nd | nd | 1 | nd | nd | nd | 6 | nd | nd | nd | nd | nd | nd | 11% |
| 3 | 942 | 70 | nd | 20 | nd | 28 | nd | 18 | nd | nd | nd | 5 | nd | nd | nd | nd | nd | nd | nd | 3 | nd | nd | nd | nd | nd | nd | 0% |
| 4 | 52.9 | 30 | nd | 23 | nd | 15 | nd | 5 | nd | nd | nd | 11 | nd | nd | nd | nd | nd | nd | nd | 5 | nd | nd | nd | 1 | nd | nd | 0% |
| 5 | 45.3 | 16 | nd | 34 | nd | 5 | nd | 9 | nd | nd | nd | 24 | nd | nd | nd | nd | nd | nd | nd | 4 | nd | nd | nd | 1 | nd | nd | 0% |
| 6 | 327 | 44 | 1 | 46 | 1 | 10 | nd | 2 | 1 | nd | 1 | 23 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1 | nd | nd | 7% |
| 7 | 1147 | 130 | 6 | 21 | 1 | 12 | nd | 4 | nd | nd | nd | 4 | nd | nd | nd | nd | nd | nd | nd | 3 | nd | 1 | nd | nd | nd | nd | 5% |
| 8 | 1469 | 58 | nd | 81 | nd | 6 | nd | 1 | nd | nd | nd | 5 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1 | nd | nd | nd | nd | 0% |
| 9 | 442 | 240 | 66 | 322 | nd | 11 | 2 | 2 | 1 | nd | 1 | 23 | 1 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 30% |
| 10 | 341 | 156 | 10 | 112 | 3 | 4 | nd | 5 | 2 | nd | nd | 28 | nd | nd | nd | nd | 1 | nd | nd | 1 | nd | 3 | nd | nd | nd | nd | 10% |
| 11 | 232 | 201 | 5 | 90 | 2 | 1 | 1 | 1 | nd | nd | 1 | 53 | nd | nd | nd | nd | nd | 1 | nd | 4 | nd | nd | nd | nd | nd | nd | 5% |
| 12 | 201 | 167 | 5 | 102 | nd | 2 | nd | 1 | nd | 1 | nd | 45 | nd | nd | nd | nd | nd | nd | nd | 2 | nd | 2 | nd | nd | nd | nd | 3% |
| 13 | 221 | 18 | nd | 76 | nd | 1 | nd | 4 | nd | nd | nd | 28 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0% |
| 14 | 41.4 | 650 | 60 | 340 | 2 | 28 | nd | 58 | nd | nd | nd | 116 | nd | nd | nd | nd | nd | nd | nd | 8 | nd | 18 | nd | nd | nd | nd | 10% |
| 15 | 34.8 | 115 | 4 | 132 | 1 | 4 | 1 | 16 | nd | nd | nd | 19 | 1 | 1 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 6% |
| 16 | 185 | 39 | 4 | 262 | nd | 3 | 1 | 4 | nd | nd | nd | 26 | nd | nd | nd | nd | nd | nd | nd | 1 | nd | nd | nd | nd | nd | nd | 13% |
| 17 | 10.7 | 46 | 28 | 100 | nd | 4 | nd | 10 | nd | nd | nd | 20 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 61% |
| 18 | 311 | 49 | 9 | 140 | nd | 2 | 1 | 3 | nd | nd | nd | 31 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1 | 20% |
| 19 | 45.4 | 25 | nd | 46 | nd | 1 | nd | nd | nd | nd | nd | 25 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0% |
| 20 | 244 | 515 | 120 | 285 | 1 | 2 | nd | 7 | 3 | nd | nd | 12 | nd | nd | nd | nd | nd | nd | nd | 1 | nd | nd | nd | 1 | nd | nd | 24% |
| 21 | 4.15 | 125 | 34 | 130 | nd | 1 | nd | 4 | nd | nd | nd | 16 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1 | nd | nd | 27% |
| 22 | 12 | 30 | nd | 34 | nd | 1 | nd | nd | nd | nd | nd | 18 | nd | nd | nd | nd | nd | nd | nd | 1 | nd | nd | nd | nd | nd | nd | 0% |
| 23 | 806 | 134 | 79 | 2155 | nd | nd | nd | 15 | nd | nd | nd | 215 | nd | nd | nd | nd | nd | 1 | nd | nd | nd | nd | nd | nd | nd | nd | 59% |
| 24 | 83.7 | 3320 | 3 | 108 | nd | 1 | nd | nd | nd | nd | nd | 42 | nd | nd | nd | nd | nd | nd | 1 | nd | nd | nd | nd | nd | nd | nd | 0.1% |
| Prevalence of HIA stainingf | 67% | 100% | 33% | 96% | 25% | 88% | 17% | 100% | 13% | 21% | 8% | 13% | 4% | 8% | 4% | 12% | 4% | 58% | 0% | 21% | 0% | 21% | 0% | 4% | 12%h | ||
Total IgE concentrations are expressed as a concentration of International Units IgE (kU/L).
Total halo, represents the total number of positive haloes recorded in each sample.
Moniliaceous amerospores refers to unicellular colorless conidia belonging to Aspergillus, Penicillium, and other genera.
Represents the number of particles belonging to the fungal group with positive HIA immunostaining.
Represents the number of particles belonging to the fungal group with no HIA immunostaining.
Percentage of the study participant population with (+) or without (-) HIA immunostaining for each collected fungal genera.
Prevalence of haloes to fungal particulates for each individual study participant.
Average percentage of haloes directed toward fungal conidia, spores, hyphae, and fragments (range 0-61%; n=24).
* 29% of the subject population did not have detectable HIA immunostaining to any collected fungal spores or hyphal fragments.
Individual study participants had total IgE concentrations that exceeded 0.35 International Units IgE (kU/L), however, no relationship was observed between the total number of quantifiable haloes and total IgE (results not shown). Interestingly, we found that 12% (range 0%-61%) of all immunostaining was directed towards fungal conidia and hyphae. Positive immunostaining was observed in the study population to a wide diversity of fungal spores. Sixty-seven percent (16/24), 25% (6/24), and 17% (4/24) of study participants had positive immunostaining to moniliaceous amerospores, Cladosporium, and Alternaria, respectively. A smaller prevalence of positive immunostaining was also observed among the study population to other fungi in particular, Pithomyces (8%), Curvularia (4%), Bipolaris (4%), Ascospores (4%), and Stachybotrys (4%). Interestingly, 13% (3/24) of the study population had positive immunostaining to airborne hyphae (Table 1). No immunostaining was observed with collected Epicoccum, Fusarium, and Spegazzinia conidia, however, many other fungal spores that remained unidentified also released allergen that bound human serum IgE. Approximately 29% of the study subjects did not have detectable HIA immunostaining to any captured fungal spores or fragments.
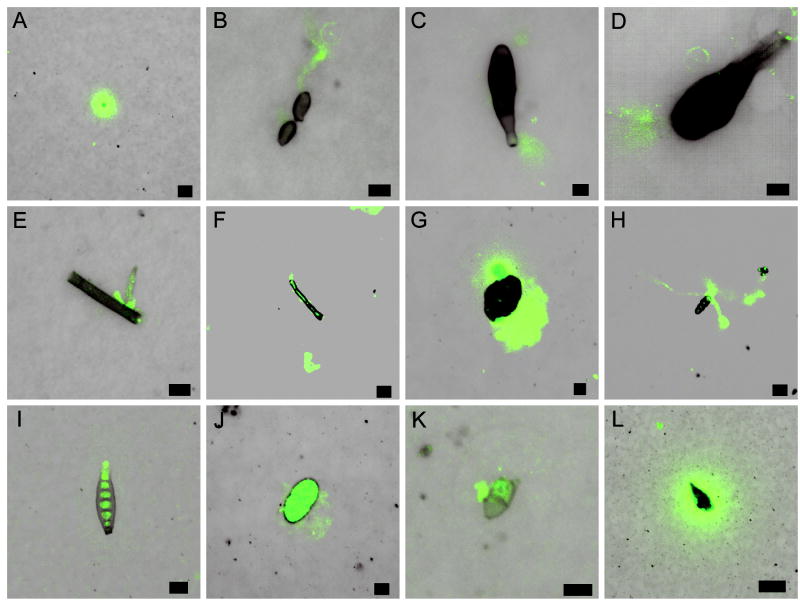
fHIA patterns of immunostaining varied with each individual fungal genus. Moniliaceous amerospore immunostaining was localized around the entire conidia (Figure 2A) or around the tips of emerged Cladosporium hyphae (Figure 2B). Immunostaining of other well-documented fungal aeroallergens belonging to the family Pleosporales, such as Alternaria (Figure 2C and 2D), Pithomyces (Figure 2G), and Bipolaris (Figure 2I) was observed at the beak of Alternaria conidia (Figure 2C), septal junctions (Figure 2G), basal regions (Figure 2C and 2D), and the outer periphery of conidia (Figure 2G). In contrast, the localization of IgE immunostaining in dematiaceous hyphal fragments and ascospore genera was localized to the site of germination (Figure 2E), around ungerminated hyphal tips (Figure 2F), or around the entire length of emerged ascospore hyphae (Figure 2H). For unknown conidia and other amorphous particulates, resultant immunostaining was heterogeneous and restricted to the septal junctions or around the entire particle (Figure 2K and 2L). However, for some fungal conidia such as Stachybotrys, immunostaining was not only localized on the PBM in close proximity to the spore, but was also concentrated on the surface ornamentation of the conidia (Figure 2J). No immunostaining was observed in culture derived negative control samples (data not shown).
Figure 2.
High resolution images of the fHIA immunostaining (green fluorescence) of collected fungal conidia, spores, and hyphal fragments. Each image is a composite of two focal image planes, membrane level and fungus level (8 μm interval) superimposed on the DIC image of the fungal particulates. Moniliaceous amerospore (A); germinated Cladosporium conidia (B); Alternaria conidia (C and D); germinated hyphal fragment (E); hyphal fragment (F); Pithomyces conidia (G), germinated Leptosphaeria-like ascospore (H); Bipolaris conidia (I), Stachybotrys conidia (J); unknown conidia (K); and an amorphous particle (L) labelled with human serum IgE. The photomicrographs depicted represent typical immunostaining patterns of immobilized fungal particles on each membrane. Scale bar, 5 μm (Fig. 2A-E, G, J, and K), 10 μm (Fig. 2F, H, and I), and 20 μm (Fig. 2L).
DISCUSSION
In order to better understand the etiology of fungal allergic sensitization, it is essential to collect precise information on the distribution of fungi in the ambient environment of the patient. Traditional methods of exposure assessment and immunodiagnosis currently fail to achieve this goal. Typically, few fungal extracts are available for skin prick or in vitro testing and there is the inference that these represent the fungal species to which exposure occurs. In addition, positive results to these diagnostic tests can be confounded by the high degree of cross reactivity between fungi, providing a distorted view of the diversity and significance of exposure to individual fungal species. Utilizing innovative immunodiagnostic methodologies such as the fHIA mitigate a number of the limitations associated with traditional diagnostic platforms. This surveillance methodology enables the testing of native antigens derived from mycoaerosols collected from the patient’s own environment.
The results of the present study further demonstrate the contribution of previously overlooked mycoaerosols to the aeroallergen load. Although IgE immunostaining to moniliaceous amerospores, Cladosporium, and Alternaria was predominant, dematiaceous hyphal fragments and other clinically unrecognized fungal conidia also released detectable allergen. These findings confirm previous Australian indoor observations utilizing the HIA [19]. Moreover, the results of the study are in agreement with the observations of Pitkäranta and colleagues [36]. Using molecular techniques, the authors identified a diverse spectrum of fungi within the indoor environment. Basidiomycetous yeasts (Cryptococcus, Malassezia, and Aureobasidium species), ascomycetes (Leptosphaerulina species), and Cladosporium were predominant compared to traditionally cultivable Aspergillus and Penicillium species [36]. Many of these fungi contain allergens and have been associated with allergic sensitization [8, 13, 27, 33]. To date, particulates derived from these mycoaerosols including amorphous fungal wall fragments; intracellular and extracellular structures of spores, conidia, hyphae, chlamydoconidia, and yeast-like cells have also remained uncharacterized as potential aeroallergen sources. In addition, many of the antigens associated with IgE binding in the present study remain uncharacterized and are derived from previously unrecognized aeroallergen sources.
The contribution of aerosolized dematiaceous hyphal fragments to the troposphere was first described following Charles A. Lindbergh’s bioaerosol collection expeditions over the Arctic and Atlantic Ocean in 1933 [31]. Ensuing studies identified hyphal fragments as common atmospheric bioaerosols in numerous locations throughout the world [24, 34, 35, 46]. Hyphal fragments vary in size (7-100 μm) [34, 35] and are characterized in terms of their wall thickness, melanization, septation, and conidiophore features [19, 20, 35]. In particular, fragments derived from dematiaceous fungi belong to allergenic species in the orders Capnodiales, Pleosporales, and Eurotiales [35]. Various immunostimulatory macromolecules including antigens [14], allergens [19], mycotoxins [7, 47], and (1-3)-β-D-glucans [40, 44] have been detected in fungal fragments. Recently, aerosolized hyphal fragments have also been proposed as an indicator of fungal contamination [54] and a potential aeroallergen source in indoor environments [19, 20]. In the present study, dematiaceous hyphal fragments were recovered in all indoor samples, but only a small proportion bound specific human serum IgE; this is comparable with previous observations in Australia [19]. To date, very little is known about the process of hyphal fragmentation, and the species that contribute the greatest concentrations of fungal fragments to the indoor environment. This continues to be the focus of future research.
Fungal conidia derived from Pleosporalean fungi were also frequently identified in the current study. Multi-celled Leptosphaeria-like ascospores as well as Bipolaris, Pithomyces, and Curvularia conidia released antigens that were capable of binding IgE. Clinically, this is an important advancement in the understanding of fungal allergic sensitization. Prior to the development of the fHIA, it was widely accepted that personal exposure to Alternaria conidia was one of the most important etiological agents responsible for fungal induced exacerbations of respiratory morbidity [5, 9, 29, 39, 42, 43]. However, recent studies demonstrated that other closely related Pleosporalean fungi, such as Stemphylium, Curvularia, and Helminthosporium species share many Alternaria allergens, and often in greater concentrations [3, 26, 28, 41]. Moreover, the teleomorphs of prevalent fungal anamorph genera including Alternaria, Phoma, Stemphylium, and Pithomyces species include Didymella, Pleospora, Leptosphaerulina, and Leptosphaeria species. Although ascospores are not clinically recognized aeroallergen sources, epidemiological studies have demonstrated associations between ascospore exposure and respiratory morbidity [8, 12, 23, 25, 53]. Moreover, Pleosporalean hyphal fragments are also abundant bioaerosols [46] that may contain greater concentrations of allergen compared to conidia [16, 32]. Using the HIA, other Pleosporalean fungal conidia belonging to Leptosphaeria, Leptosphaerulina, Sporidesmium, Pleospora, Curvularia, Exserohilum, and Pithomyces species have been shown to release allergen [19]. These studies, in addition to the present findings, further demonstrate that Pleosporalean fungi may make significant contributions to the aeroallergen load of many environments.
In addition to the contribution of Pleosporalean conidia, a germinated Leptosphaeria-like ascospore and Cladosporium conidia released hyphal allergen that bound specific human IgE. Conidia germination followed by the release of greater concentrations of hyphal allergens is another emerging paradigm for fungal allergic sensitization. Previous studies have demonstrated that significantly greater quantities of allergen are released following germination from hyphal tips and septal junctions of various fungal species [16, 32, 48]. The presence of germinated fungal conidia and yeast-like spores in the nasal cavity and turbinates of immunotolerant subjects has also been recently confirmed [45]. Using the enzymatic HIA, allergen released from other germinated conidia and ascospores such as Pleospora, Leptosphaeria, and two-celled ascospores have been demonstrated in Australia [19]. To our knowledge, this is the first reported observation of allergen release from environmentally derived ungerminated and germinated conidia, ascospores, and hyphal fragments in North America.
It was possible to detect IgE immunostained moniliaceous amerospores and other species using the fHIA. Compared to previous studies utilizing enzymatic HIAs [17-19], this methodology greatly enhanced the detection of picogram quantities of allergen derived from amerospores, subcellular particulates, and improved particle resolution and contrast. Recent improvements to the fHIA have allowed the enumeration of immunostained fungal fragments as small as 0.5 μm [15, 20]. It was also observed that not all fungal spores, conidia, and hyphal fragments released detectable allergen. Environmental parameters such as prolonged exposure to ultra violet radiation, solubilization of surface antigens, and specificity of individual human serum IgE have been proposed to account for the decreased immunostaining [19]. Future epidemiological studies should consider this observation when interpreting fungal exposure assessment data. Utilizing the fHIA in fungal allergic sensitization surveillance studies will enhance the detection of the complete spectrum of mycoaerosols in the environment.
In summary, the fHIA has several distinct advantages over conventional immunodiagnostic and fungal exposure surveillance methodologies. The fHIA provides unique insight into the diversity of mycoaerosols that patients are exposed and sensitized to in their own environment, in addition to localizing the ultra structural sites associated with native allergen release. Results derived from utilizing this methodology have challenged traditional paradigms of fungal allergic sensitization. Our surveillance approach mitigates many of the potential limitations associated with traditional exposure assessment methodologies. The combination of environmental and serological monitoring will provide patient-specific exposure and sensitization profiles that will help to elucidate adverse health effects associated with personal fungal exposure. Ultimately, these advances will contribute to better patient diagnosis and therapeutic management.
Acknowledgments
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health. This work was supported in part by the Inter-Agency Agreement NIEHS Y1-ES0001-06 and the National Institutes of Health grants: R01 ES 10922 and P30 ES 009089. The authors would like to thank Dr. Michael Muilenberg for his mycological assistance identifying several unknown fungal spores.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acosta LM, Acevedo-Garcia D, Perzanowski MS, Mellins R, Rosenfield L, Cortes D, et al. The New York Puerto Rican asthma project: Study design, methods, and baseline results. J Asthma. 2008;45:51–57. doi: 10.1080/02770900701815784. [DOI] [PubMed] [Google Scholar]
- 2.Adhikari A, Martuzevicius D, Reponen T, Grinshpun SA, Cho SH, Sivasubramani SK, et al. Performance of the Button Personal Inhalable Sampler for the measurement of outdoor aeroallergens. Atmos Environ. 2003;37:4723–4733. [Google Scholar]
- 3.Agarwal MK, Jones RT, Yunginger JW. Shared allergenic and antigenic determinants in Alternaria and Stemphylium extracts. J Allergy Clin Immunol. 1982;70:437–444. doi: 10.1016/0091-6749(82)90006-9. [DOI] [PubMed] [Google Scholar]
- 4.Amitani R, Taylor G, Elezis EN, Llewellyn-Jones C, Mitchell J, Kuze F, et al. Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect Immun. 1995;63:3266–3271. doi: 10.1128/iai.63.9.3266-3271.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson M, Downs S, Mitakakis T, Leuppi J, Marks G. Natural exposure to Alternaria spores induces allergic rhinitis symptoms in sensitized children. Pediatr Allergy Immunol. 2003;14:100–105. doi: 10.1034/j.1399-3038.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 6.Arruda LK, Mann BJ, Chapman MD. Selective expression of a major allergen and cytotoxin, Asp f I, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J Immunol. 1992;149:3354–3359. [PubMed] [Google Scholar]
- 7.Brasel TL, Douglas DR, Wilson SC, Straus DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins on particulates smaller than conidia. Appl Environ Microbiol. 2005;71:114–122. doi: 10.1128/AEM.71.1.114-122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burge H, Gold B, Muilenberg M, Solomon W. Allergenicity of airborne ascospores. J Allergy Clin Immunol. 1985;75:118–118. [Google Scholar]
- 9.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Cody DT, McCaffrey TV, Roberts G, Kern EB. Effects of Aspergillus fumigatus and Alternaria alternata on human ciliated epithelium in vitro. Laryngoscope. 1997;107:1511–1514. doi: 10.1097/00005537-199711000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Cruz A, Saenz de Santamaria M, Martinez J, Martinez A, Guisantes J, Palacios R. Fungal allergens from important allergenic fungi imperfecti. Allergol Immunopathol. 1997;25:153–158. [PubMed] [Google Scholar]
- 12.Frankland AW, Gregory PH. Allergenic and agricultural implications of airborne ascospore concentrations from a fungus, Didymella exitialis. Nature. 1973;245:336–337. doi: 10.1038/245336a0. [DOI] [PubMed] [Google Scholar]
- 13.Gandra RF, Melo TA, Matsumoto FE, Pires MFC, Croce J, Gambale W, et al. Allergenic evaluation of Malassezia furfur crude extracts. Mycopathologia. 2001;155:183–189. doi: 10.1023/a:1021181711225. [DOI] [PubMed] [Google Scholar]
- 14.Gòrny RL, Reponen T, Willeke K, Schmechel D, Robine E, Boissier M, et al. Fungal fragments as indoor air biocontaminants. Appl Environ Microbiol. 2002;68:3522–3531. doi: 10.1128/AEM.68.7.3522-3531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green BJ, Millechia L, Blachere FM, Tovey ER, Beezhold DH, Schmechel D. Dual fluorescent Halogen Immunoassay for bioaerosols using confocal microscopy. Anal Biochem. 2006;354:151–153. doi: 10.1016/j.ab.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Green BJ, Mitakakis TZ, Tovey ER. Allergen detection from 11 fungal species before and after germination. J Allergy Clin Immunol. 2003;111:285–289. doi: 10.1067/mai.2003.57. [DOI] [PubMed] [Google Scholar]
- 17.Green BJ, Schmechel D, Sercombe JK, Tovey ER. Enumeration and detection of aerosolized Aspergillus fumigatus and Penicillium chrysogenum conidia and hyphae using a novel double immunostaining technique. Journal of Immunological Methods. 2005;307:127–134. doi: 10.1016/j.jim.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Green BJ, Schmechel D, Tovey ER. Detection of Alternaria alternata conidia and hyphae using a novel double immunostaining technique. Clinical and Diagnostic Laboratory Immunology. 2005;12:1114–1116. doi: 10.1128/CDLI.12.9.1114-1116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green BJ, Sercombe JK, Tovey ER. Fungal fragments and undocumented conidia function as new aeroallergen sources. J Allergy Clin Immunol. 2005;115:1043–1048. doi: 10.1016/j.jaci.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Green BJ, Tovey ER, Sercombe JK, Blachere FM, Beezhold DH, Schmechel D. Airborne fungal fragments and allergenicity. Med Mycol. 2006;44:S245–S255. doi: 10.1080/13693780600776308. [DOI] [PubMed] [Google Scholar]
- 21.Green BJ, Yli-Panula E, Tovey ER. Halogen Immunoassay, a new method for the detection of sensitization to fungal allergens; comparisons with conventional techniques. Allergol Int. 2006;55(2):131–139. doi: 10.2332/allergolint.55.131. [DOI] [PubMed] [Google Scholar]
- 22.Halstensen AS, Nordby KC, Wouters IM, Eduard W. Determinants of microbial exposure in grain farming. Ann Occup Hyg. 2007;51:581–592. doi: 10.1093/annhyg/mem038. [DOI] [PubMed] [Google Scholar]
- 23.Harries MG, Lacey J, Tee RD, Cayley GR, Taylor AJ. Didymella exitialis and late summer asthma. Lancet. 1985;1:1063–1066. doi: 10.1016/s0140-6736(85)92368-2. [DOI] [PubMed] [Google Scholar]
- 24.Harvey R. Air-spora studies at Cardiff. III. Hyphal fragments. Trans Br Mycol Soc. 1970;54:251–254. [Google Scholar]
- 25.Hasnain SM. Allergenic implications of airborne Leptosphaeria ascospores. Grana. 1993;32:315–318. [Google Scholar]
- 26.Hong SG, Cramer RA, Lawrence CB, Pryor BM. Alt a 1 allergen homologs from Alternaria and related taxa: analysis of phylogenetic content and secondary structure. Fungal Genet Biol. 2005;42:119–129. doi: 10.1016/j.fgb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Kato H, Sugita T, Ishibashi Y, Nishikawa A. Evaluation of the levels of specific IgE against Cryptococcus diffluens and Cryptococcus liquefaciens in patients with atopic dermatitis. Microbiol Immunol. 2007;51:945–950. doi: 10.1111/j.1348-0421.2007.tb03991.x. [DOI] [PubMed] [Google Scholar]
- 28.Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EHC, Hyde KD. The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia. 2006;98:571–583. doi: 10.3852/mycologia.98.4.571. [DOI] [PubMed] [Google Scholar]
- 29.Licorish K, Novey HS, Kozak P, Fairshter RD, Wilson AF. Role of Alternaria and Penicillium spores in the pathogenesis of asthma. J Allergy Clin Immunol. 1985;76:819–825. doi: 10.1016/0091-6749(85)90755-9. [DOI] [PubMed] [Google Scholar]
- 30.Madsen AM, Wilkins K, Poulsen OM. Micro-particles from fungi. In: Johanning E, editor. Bioaerosols, Fungi, Bacteria, Mycotoxins and Human Health: Patho-physiology, clinical effects, exposure assessment, prevention and control in indoor environments and work. Albany, New York: Fungal Research Group, Inc; 2005. pp. 276–291. [Google Scholar]
- 31.Meirer FC. Collecting microorganisms in the artic atmosphere with field notes and material by C.A. Lindbergh Sci Mon. 1935;40:5–20. [Google Scholar]
- 32.Mitakakis TZ, Barnes C, Tovey ER. Spore germination increases allergen release from Alternaria. J Allergy Clin Immunol. 2001;107:388–390. doi: 10.1067/mai.2001.112602. [DOI] [PubMed] [Google Scholar]
- 33.Niedoszytko M, Chelminska M, Jassem E, Czestochowska E. Association between sensitization to Auereobasidium pullulans (Pullularia sp) and severity of asthma. Ann Allergy Asthma Immunol. 2007;98:153–156. doi: 10.1016/S1081-1206(10)60688-6. [DOI] [PubMed] [Google Scholar]
- 34.Pady SM, Gregory PH. Numbers and viability of airborne hyphal fragments in England. Trans Br Mycol Soc. 1963;46:609–613. [Google Scholar]
- 35.Pady SM, Kramer CL. Kansas Aeromycology VI: Hyphal fragments. Mycologia. 1960;52:681–687. [Google Scholar]
- 36.Pitkäranta M, Meklin T, Hyvarinen A, Paulin L, Auvinen P, Nevalainen A, et al. Analysis of fungal flora in indoor dust by ribosomal DNA sequence analysis, quantitative PCR, and culture. Appl Environ Microbiol. 2008;74:233–244. doi: 10.1128/AEM.00692-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulos LM, O’Meara TJ, Hamilton RG, Tovey ER. Inhaled latex allergen (Hev b 1) J Allergy Clin Immunol. 2002;109:701–706. doi: 10.1067/mai.2002.122463. [DOI] [PubMed] [Google Scholar]
- 38.Poulos LM, O’Meara TJ, Sporik R, Tovey ER. Detection of inhaled Der p 1. Clin Exp Allergy. 1999;29:1232–1238. doi: 10.1046/j.1365-2222.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- 39.Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 2007;120:610–617. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 40.Reponen T, Seo SC, Grimsley F, Lee T, Crawford C, Grinshpun SA. Fungal fragments in moldy houses: A field study in homes in New Orleans and Southern Ohio. Atmos Environ. 2008;41:8140–8149. doi: 10.1016/j.atmosenv.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saenz de Santamaria M, Postigo I, Gutierrez-Rodriguez A, Cardona G, Guisantes JA, Asturias J. The major allergen of Alternaria alternata (Alt a 1) is expressed in other members of the Pleosporaceae family. Mycoses. 2006;49:91–95. doi: 10.1111/j.1439-0507.2006.01195.x. [DOI] [PubMed] [Google Scholar]
- 42.Salo PM, Arbes SJ, Sever M, Jaramillo R, Cohn RD, London SJ, et al. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salo PM, Yin M, Arbes SJ, Cohn RD, Sever M, Muilenberg M, et al. Dustborne Alternaria alternata antigens in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2005;116:623–629. doi: 10.1016/j.jaci.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo SC, Grinshpun SA, Iossifova Y, Schmechel D, Rao C, Reponen T. A new field-compatible methodology for the collection and analysis of fungal fragments. Aerosol Sci Technol. 2007;41:794–803. [Google Scholar]
- 45.Sercombe JK, Green BJ, Tovey ER. Recovery of germinating fungal conidia from the nasal cavity following environmental exposure. Aerobiologia. 2006;22:295–304. [Google Scholar]
- 46.Sinha RJ, Kramer CL. Identifying hyphal fragments in the atmosphere. Trans Kans Acad Sci. 1971;74:48–51. [PubMed] [Google Scholar]
- 47.Sorenson WG, Frazer DG, Jarvis BB, Simpson J, Robinson VA. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl Environ Microbiol. 1987;53:1370–1375. doi: 10.1128/aem.53.6.1370-1375.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sporik RB, Arruda LK, Woodfolk J, Chapman MD, Platts-Mills TAE. Environmental exposure to Aspergillus fumigatus allergen (Asp f I) Clin Exp Allergy. 1993;23:326–331. doi: 10.1111/j.1365-2222.1993.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 49.Targonski PV, Persky VW, Ramekrishnan V. Effect of environmental molds on risk of death from asthma during pollen season. J Allergy Clin Immunol. 1995;95:955–961. doi: 10.1016/s0091-6749(95)70095-1. [DOI] [PubMed] [Google Scholar]
- 50.Tomee JF, Kauffman HF. Putative virulence factors of Aspergillus fumigatus. Clin Exp Allergy. 2000;30:476–484. doi: 10.1046/j.1365-2222.2000.00796.x. [DOI] [PubMed] [Google Scholar]
- 51.Tovey E, Taylor D, Graham A, O’Meara T, Lovborg U, Jones A, et al. New Immunodiagnostic System. Aerobiologia. 2000;16:113–118. [Google Scholar]
- 52.Tovey ER, Green BJ. Measuring environmental fungal exposure. Med Mycol. 2004;43:S67–S70. doi: 10.1080/13693780400020097. [DOI] [PubMed] [Google Scholar]
- 53.Van Osdol TJ, Hu F, Barnes CS, Portnoy J. The relationship between airborne ascospores, Cladosporium and rainfall events. J Allergy Clin Immunol. 2004;113:S62. [Google Scholar]
- 54.Yang CS, Heinsohn PA. Sampling and analysis of indoor microorganisms. Hoboken, New Jersey: John Wiley and Sons, Inc; 2007. [Google Scholar]