Abstract
Alzheimer disease (AD) and related tauopathies are histopathologically characterized by a specific type of slow and progressive neurodegeneration, which involves the abnormal hyperphosphorylation of the microtubule associated protein (MAP) tau. This hallmark, called neurofibrillary degeneration, is seen as neurofibrillary tangles, neuropil threads, and dystrophic neurites and is apparently required for the clinical expression of AD, and in related tauopathies it leads to dementia in the absence of amyloid plaques. While normal tau promotes assembly and stabilizes microtubules, the non-fibrillized, abnormally hyperphosphorylated tau sequesters normal tau, MAP1 and MAP2, and disrupts microtubules. The abnormal hyperphosphorylation of tau, which can be generated by catalysis of several different combinations of protein kinases, also promotes its misfolding, decrease in turnover, and self-assembly into tangles of paired helical and or straight filaments. Some of the abnormally hyperphosphorylated tau ends up both amino and C-terminally truncated. Disruption of microtubules by the non-fibrillized abnormally hyperphosphorylated tau as well as its aggregation as neurofibrillary tangles probably impair axoplasmic flow and lead to slow progressive retrograde degeneration and loss of connectivity of the affected neurons. Among the phosphatases, which regulate the phosphorylation of tau, protein phosphatase-2A (PP2A), the activity of which is down-regulated in AD brain, is by far the major enzyme. The two inhibitors of PP-2A, and , which are overexpressed in AD, might be responsible for the decreased phosphatase activity. AD is multifactorial and heterogeneous and involves more than one etiopathogenic mechanism.
Keywords: Alzheimer disease, Tauopathies, Microtubule associated proteins, Abnormally hyperphosphorylated tau, Protein phosphatase-2A, Neurofibrillary pathology
Introduction
Alzheimer disease (AD) and related tauopathies are histopathologically characterized by a specific type of neurodegeneration, the neurofibrillary degeneration. Neurofibrillary degeneration in AD brain is seen as intra-neuronal neurofibrillary tangles, neuropil threads, and as dystrophic neurites surrounding the β-amyloid core in neuritic (senile) plaques. The number of neurofibrillary tangles directly correlates with the presence and the degree of dementia in AD [1, 15, 187].
Microtubule associated protein tau is abnormally hyperphosphorylated in AD brain and in this form, it is the major protein subunit of the paired helical filaments (PHF) and straight filaments (SF) forming neurofibrillary tangles, neuropil threads, and plaque dystrophic neurites in AD [56, 57, 75, 76, 101].
Neurofibrillary degeneration of abnormally hyperphosphorylated tau not only occurs in AD brain but is also seen in a family of related neurodegenerative diseases, called tauopathies (see Table 1). In every one of these tauopathies, the neurofibrillary changes are made up of abnormally hyperphosphorylated tau and their occurrence in the neocortex is associated with dementia. In frontotemporal dementia with Parkinsonism-linked to chromosome 17 and tau pathology (FTDP-17-tau), several missense mutations in tau co-segregate with the disease [71, 156, 179]. Four of these missense mutations, G272V, P301L, V337M, and R406W, which have been most studied to date, make tau a preferable substrate for abnormal hyperphosphorylation in vitro [7].
Table 1.
Diseases characterized by abnormal hyperphosphorylation of tau
| Alzheimer disease, including tangle-only form of the diseasea |
| Down syndrome, adult casesa |
| Guam parkinsonism dementia complexb |
| Dementia pugilisticab |
| Pick diseaseb |
| Dementia with argyrophilic grainsb |
| Fronto-temporal dementia with tau mutationsb |
| Cortico-basal degenerationb |
| Pallido-ponto-nigral degenerationb |
| Progressive supranuclear palsyb |
| Gerstmann-Sträussler-Scheinker disease with tanglesb |
Characterized by both tangles and plaques
Neurofibrillary pathology of abnormally hyperphosphorylated tau in the absence of β-amyloid plaques
The neurofibrillary degeneration of the Alzheimer type is primarily seen in human neurodegenerative disorders. To date, in aged and in cognitively impaired animals, the neurofibrillary degeneration of abnormally hyperphosphorylated tau has been scarcely found.
Understanding of the molecular mechanisms of neurofibrillary degeneration is critical to identification of early biomarkers, additional disease subgroups, and rational therapeutic treatment of AD and related tauopathies.
Structure and function of tau
Tau is the major neuronal microtubule associated protein (MAP) [205]; the other two known MAPs in neurons are the high molecular weight MAPs, MAP1 and MAP2 [133, 178]. Tau is coded by a single gene on chromosome 17 but is expressed in several molecular isoforms that are generated by alternative splicing of its mRNA [67]. In human brain, the alternative splicing of the mRNA results in six molecular isoforms [46]. These six tau isoforms differ in containing three (3R taus) or four (4R taus) microtubule binding repeats (R) of 31–32 amino acids in the carboxy terminal half and one (1N), two (2N), or zero (0N) amino terminal inserts of 29 amino acids each; the extra repeat in 4R tau is the second repeat (R2) of 4R taus. This alternative splicing of tau pre-mRNA results in the expression of three 3R taus (0N3R, 1N3R, and 2N3R) and three 4R taus (0N4R, 1N4R, and 2N4R). The 2N4R tau is the largest size human brain tau with a total of 441 amino acids (tau441) in length. The smallest size tau isoform (0N3R; tau352) is the only form that is expressed in fetal human brain. Tau has little secondary structure; it is mostly random coil with β structure in the second and third microtubule binding repeats.
Tau interacts with tubulin and promotes its assembly into microtubules and helps stabilize their structure [205]. Like MAP1 and MAP2, tau is a phosphoprotein and its biological activity is regulated by the degree of its phosphorylation [3, 94, 114]. Normal brain tau contains 2–3 moles of phosphate per mole of the protein [94], which appears to be optimal for its interaction with tubulin and the promotion of microtubule assembly. In addition to phosphorylation, the alternative splicing also affects the biological activity of tau. Both the extra repeat (Repeat 2) in the 4R taus and the amino terminal inserts (N1 and N2) enhance the binding of tau to tubulin, which makes 2N4R tau (tau441), and 0N3Rtau (tau352, the fetal tau) the most and the least effective relatively in promoting microtubule assembly [7, 8].
Importance of tau pathology in AD and tauopathies
Studies on the correlation of the cognitive impairment to the histopathological changes have consistently demonstrated that the number of neurofibrillary tangles, and not the plaques, correlates best with the presence and or the degree of dementia in AD [1, 15, 187]. Whereas neurofibrillary degeneration appears to be required for the clinical expression of the disease, the dementia, β-amyloidosis alone in the absence of neurofibrillary degeneration does not produce the disease clinically. In fact, some of the normal aged individuals have as much β-amyloid plaque burden in the brain as typical cases of AD, except that, in the former case, plaques lack dystrophic neurites with neurofibrillary changes surrounding the beta-amyloid cores [1, 15, 36, 37, 86]. On the other hand, neurofibrillary degeneration of the AD type, but in the absence of β-amyloidosis, is seen in several tauopathies such as Guam Parkinsonism–dementia complex, dementia pugilistica, corticobasal degeneration and Pick disease. All of these neurodegenerative disorders are clinically characterized by dementia. In the case of the inherited cases of FTDP, the FTDP-17, almost equal numbers are caused by a mutation in tau gene (FTDP-17-tau) or in TAR-DNA binding protein (TDP)-43 gene (FTLD-U). Furthermore, in inherited cases of FTDP-17, certain missense mutations in the tau gene, including those that affect the alternate splicing of its mRNA, favoring the 4-repeat tau isoforms, co-segregate with the disease [71, 156, 179]. These mutated taus and the 4-repeat taus are respectively more favorable substrates for abnormal hyperphosphorylation than wild-type tau and 3-repeat taus [7]. Inclusions of hyperphosphorylated tau have also been observed in small numbers in glial cells in the white matter, especially in frontolobar dementias [95, 96].
Relationship between neurofibrillary degeneration and β-amyloidosis
Currently, the most popular hypothesis on the etiopathogenesis of AD is the Amyloid Cascade Hypothesis, according to which the generation of Aβ is the primary pathological event, which leads to neurofibrillary degeneration and dementia [61, 62]. Consistent with this hypothesis, both intracerebral infusion of Aβ in FTDP-17 tau mutation P301L-expressing transgenic mice, as well as crossing these animals with APPTg2576 mice (APP Swedish plus London mutations), were found to exacerbate neurofibrillary pathology [54, 104] and, in the triple transgenic mice 3XTgAD (APPSWE-PS1M146V-tau P301L), β-amyloid deposition was found to precede the neurofibrillary pathology and these animals showed more neurofibrillary pathology than the double transgenic Tg2X APP/PS1 mice [138, 140]. However, to date, the data from human conditions apparently do not support the amyloid cascade hypothesis—(1) some of the normal aged individuals show similar level and topography of compact Aβ plaques as typical cases of AD, except that plaques in the former lack dystrophic neurites with neurofibrillary pathology [36, 37]; (2) the plaques and neurofibrillary tangles are seen in disproportionate numbers in AD, especially in the plaque-dominant and tangle-dominant AD subgroups [79, 86]; (3) typically, a considerably high brain Aβ burden is seen in hereditary cerebral hemorrhage with amyloidosis, Dutch type (HCHWA-D) but without any accompanying neurofibrillary degeneration [102]; (4) to date, Aβ immunotherapy, both active and passive immunizations, inhibition of Aβ production with a γ-secretase modulating NSAID, Flurizan, as well as treatment with a drug (Alzhmed) that inhibits Aβ aggregation, have all failed to show any significant clinical improvement in clinical trials on AD patients; (5) the clearance of β-amyloid plaques in the brains of patients with AD by Aβ vaccine failed to reduce the number of neurofibrillary tangles [69]; and (6) the tauopathies, such as FTDP-17-tau, Pick disease, corticobasal degeneration, dementia pugilistica and Guam Parkinsonism dementia complex, are characterized by dementia associated with neurofibrillary degeneration of abnormally hyperphosphorylated tau in the absence of β-amyloid deposits. Furthermore, recent studies have shown that PS-1 not only promotes or acts as a γ-secretase activity (the cleavage of APP which produces Aβ), but also activates the phosphatidylinositol 3-kinase (PI3K), which downstream through protein kinase B (Akt) inhibits the glycogen synthase kinase-3 (GSK-3), a major tau kinase. Some of the AD-causing mutations in PS-1 result in loss of its ability to activate PI3K pathway, resulting in a sustained activity of GSK-3 and, consequently, abnormal hyperphosphorylation of tau [16]. Finally, several of the AD-causing PS-1 mutations have been reported to produce either no change or a decrease in Aβ generation in cultured cells [172].
The AD-causing APP mutations might produce neurofibrillary degeneration by altering the molecular topology of the neuronal plasma membrane and or endoplasmic reticulum and consequently of one or more tau kinase signaling. Individuals with reduced membrane fluidity, such as elderly and patients with Niemann-Pick C, may be especially vulnerable to this etiopathogenic mechanism of AD.
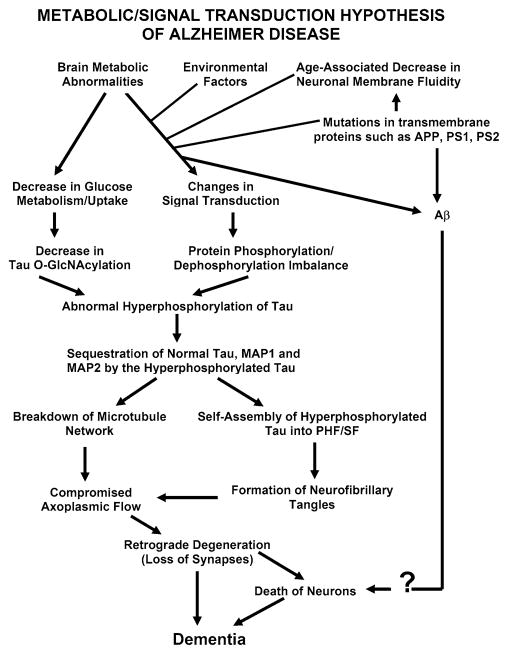
AD may be caused by a number of different factors and the amyloid cascade hypothesis is too simplistic and narrow to explain this multifactorial disease. We have proposed [73] that different signal transduction and metabolic factors, through different disease mechanisms, apparently lead to the same two disease characteristic lesions—neurofibrillary degeneration of abnormally hyperphosphorylated tau and β-amyloidosis (see Fig. 1).
Fig. 1.
A schematic showing different major steps of the “Metabolic/Signal Transduction Hypothesis”. AD and other tauopathies require a genetic predisposition and are triggered by a variety of environmental factors, affecting one or more specific signal transduction pathways which result in a protein phosphorylation/dephosphorylation imbalance and the abnormal hyperphosphorylation of tau that leads to neurofibrillary degeneration and dementia. In AD, the protein phosphorylation/dephosphorylation imbalance in the affected neurons is generated at least in part by a decrease in the activities of tau phosphatases, i.e., PP-2A and PP-1; the activities of tau kinases such as cdk5, GSK-3, CaM kinase II and PKA might also be increased in the affected neurons. This protein phosphorylation/dephosphorylation imbalance probably involves an alteration of a specific signal transduction pathway(s) produced by an increase in the levels of an extracellular signal, e.g., FGF2 or an alteration in the molecular topology of the neuronal cell membrane or both. With age, the molecular topology of the cell membranes is altered due to a decrease in membrane fluidity. The mutations in transmembrane proteins, such as β-APP, PS1 and PS2, increase the vulnerability of the cell membrane to alteration in pathological signal transduction. The increased risk for AD in the carriers of APOE4 allele as opposed to APOE2 or APOE3 alleles might also involve alteration of signal transduction through the interaction of APOE4 with the neuronal cell membrane. Any mutation or posttranslational modification of tau that will make it a better substrate for abnormal hyperphosphorylation will also increase the risk for the disease. High cholesterol such as in Niemann Pick C disease might be involved in decreasing membrane fluidity. Decreased glucose metabolism/uptake might lead to the abnormal hyperphosphorylation of tau through a decrease in its O-GlcNA-cylation (reproduced with permission from Iqbal and Grundke-Iqbal [73])
Abnormal hyperphosphorylation of tau, sequestration of normal MAPs, and disruption of microtubules
Microtubule associated protein tau is highly hydrophilic and is, thus, soluble and heat stable. To date, not only in AD but also in every known human tauopathy, the tau pathology is made up of the abnormally hyperphosphorylated protein. In AD brain, all of the six tau isoforms are hyperphosphorylated and aggregated into PHF [45, 56, 57, 75, 76, 101]. While conformational changes [80–82] and truncation of tau [30, 43, 137] following its hyperphosphorylation [33] have been reported in AD, the most established and the most compelling cause of dysfunctional tau in AD and related tauopathies is the abnormal hyperphosphorylation of this protein [3, 56, 76].
Tau, a phosphoprotein which normally contains 2–3 mol of phosphate/mol of the protein, is at least three to fourfold more hyperphosphorylated in AD brain [76, 94] and, in this state, is the major protein subunit of the PHF/neurofibrillary tangles [56, 57, 75, 101]. Two major known functions of tau are its ability to promote assembly and to maintain structure of microtubules [205]. These functions of tau are regulated by its degree of phosphorylation [3, 74, 90, 114]. In AD brain, there is as much normal tau as in age-matched control human brain, but, in addition, the diseased brain contains four to eightfold of abnormally hyperphosphorylated tau [88, 89]. In situ hybridization studies have revealed no significant change in the expression of tau mRNA in AD brain [128]. Thus, the increase in tau level observed in AD brain is probably mostly due to a decrease in its turnover caused by the hyperphosphorylation [157, 201, 212].
In a normal mature neuron, tubulin is present in over tenfold excess of tau. The neuronal concentration of tau is ~2 μM [88, 89] and it binds to microtubules at a Kd of ~100 nM [53], and thus practically all tau is microtubule bound in the cell. In cultured cells, overexpression of tau can cause microtubule bundling. However, neither in AD nor in any related tauopathy such a situation has been reported.
The tau polymerized into neurofibrillary tangles is apparently inert and neither binds to tubulin nor promotes its assembly into microtubules [5, 74, 90]. As much as 40% of the abnormally hyperphosphorylated tau in AD brain is present in the cytosol and not polymerized into paired helical filaments/neurofibrillary tangles [18, 76, 94]. The AD cytosolic abnormally hyperphosphorylated tau (AD P-tau) does not bind to tubulin and promote microtubule assembly, but instead it inhibits assembly and disrupts microtubules [3, 105, 201]. This toxic property of the pathological tau involves the sequestration of normal tau by the diseased protein [2, 3]. The AD P-tau also sequesters the other two major neuronal MAPs, MAP1 A/B and MAP2 [4]. This toxic behavior of the AD P-tau appears to be solely due to its abnormal hyperphosphorylation because dephosphorylation of diseased tau converts it into a normal-like protein [3, 105, 198, 201].
The inhibitory activity of the non-fibrillized abnormally hyperphosphorylated tau has been confirmed in yeast, drosophila, and in mouse models that express human brain tau. The expression of the longest human brain tau (2N4Rtau) in yeast produces pathological phosphoepitopes, assumes a pathological conformation, and forms aggregates. These processes are modulated by yeast kinases Mds1 and Pho85, orthologues of GSK-3β and cdk5 [192, 193]. In yeast, tau aggregates more when it is more phosphorylated, the mobility in SDS-PAGE is slower with increased phosphorylation, and hyperphosphorylated tau isolated from the stably transfected yeast is able to assemble into filaments, and is able to nucleate the assembly of the normal non-phosphorylated tau. These yeast studies, like those carried out previously using AD P-tau, suggest that the hyperphosphorylated tau works as a nucleation factor that initiates and promotes the aggregation of tau [2, 127]. In wild-type human tau- and mutated human tau-transgenic drosophila, the accumulation of the abnormally phosphorylated tau in the absence of its fibrillization into neurofibrillary tangles leads to neurodegeneration [208]. In a P301L tau inducible transgenic mouse model, cognitive improvement was observed when expression of human tau, which became abnormally hyperphosphorylated, was suppressed although neurofibrillary tangles continued to form, suggesting that the accumulation of the cytosolic abnormally hyperphosphorylated, and not its aggregation, was apparently involved in behavioral impairment in these animals [163]. In a recent study, methylthioninium chloride (methylene blue dye) has been found to disaggregate PHF in vitro, reduce the number of tau aggregates in tau transgenic mice, and shows significant inhibition of cognitive impairment in a PHASE II double blind clinical trial on AD patients [63, 206]. Whether disaggregation of pathological aggregates of tau with methylthioninium chloride results also in its dephosphorylation remains to be studied.
Reduction of soluble Aβ and soluble abnormally hyperphosphorylated tau, but not soluble Aβ alone, was found to ameliorate cognitive decline in 3xTg mice that express both plaque and tangle pathology [139]. Furthermore, in vitro dephosphorylation of neurofibrillary tangles disaggregates filaments and, as a result, the tau released behaves like normal protein in promoting microtubule assembly [201]. Thus, two characteristics of AD abnormally hyperphosphorylated tau is (1) that it sequesters normal MAPs and disrupts microtubules and (2) that it self-assembles into paired helical and or straight filaments.
Transient and reversible abnormal hyperphosphorylation of tau
Hyperphosphorylation of tau, though not to the same level as in AD, is not only associated with the disease as in tauopathies, but is also employed by the neuron to down regulate its activity transiently and reversibly where required. For instance, during development, the level of tubulin in the brain is at its highest, i.e., almost 33% of total cytosolic protein, which is almost 1.5-fold the critical concentration of 4 mg/ml tubulin required for its polymerization into microtubules [165]. Probably to avoid microtubule bundling, the fetal tau is transiently hyperphosphorylated during development. However, the level of abnormal hyperphosphorylation of tau in fetal brain is far less than that seen in AD brain. Similarly, anesthesia and hypothermia induced by anesthesia or by hibernation in hibernating animals induces transient abnormal hyperphosphorylation of tau [12, 155, 161, 183]. The molecular mechanism of the transient abnormal hyperphosphorylation of tau observed during development is, at present, not understood. However, during hypothermia, the activity of protein phosphatase-2A (PP-2A), the major brain phosphoseryl/phosphothreonyl phosphatase activity is transiently and reversibly reduced and is believed to cause the abnormal hyperphosphorylation of tau [155, 183]. In AD and Down syndrome (DS), the decrease in brain PP-2A activity apparently involves different molecular mechanisms, and occurs in a non-transient and irreversible manner [49, 51, 111]. It is the non-reversible nature of the abnormal hyperphosphorylation of tau in AD, DS, and related tauopathies, which results in an involuntary slowing down of neuronal activity and a consequent chronic progressive neurodegeneration and its clinical phenotype, the dementia.
Self-assembly of abnormally hyperphosphorylated tau into filaments
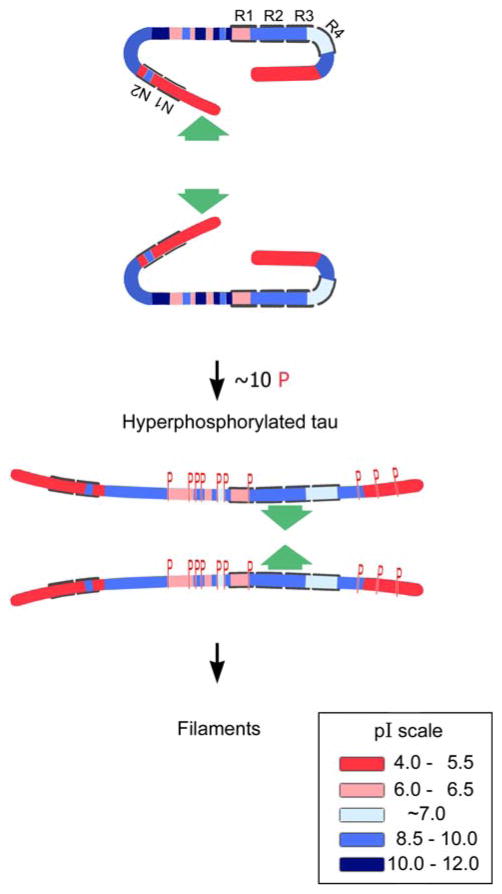
Tau has long stretches of positively or negatively charged regions that are not conducive for intermolecular hydrophobic association [160]. The β-structure in tau is concentrated only in repeats R2 and R3, which can self-assemble into filaments [194] and co-assemble with heparin [14]. Both the amino terminal and the carboxy terminal flanking regions to the microtubule binding repeats in normal tau appear to inhibit its self-aggregation into filaments and on AD type abnormal hyperphosphorylation, i.e., the phosphorylation of the amino terminal and the carboxy terminal flanking regions, this inhibition is eliminated, resulting in the formation of tangles of PHF/SF (Fig. 2) [6, 7]. The co-assembly of tau with polyanions such as heparin, heparin sulfate [47, 66, 150, 173], tRNA [84], or polyglutamate [85] appears to involve a mechanism different from what is seen in AD and in tauopathies. The polyanion-induced assembly of tau is very slow and does not result either in the lateral association of filaments into tangles or in the formation of any protofilaments seen in AD PHF. Furthermore, unlike AD and related tauopathies and transgenic animal models, the in vitro polyanions-induced assembly of tau into filaments is inhibited and not promoted by phosphorylation [166].
Fig. 2.
A hypothetical scheme of the phosphorylation-induced self-assembly of tau. Tau self-assembles mainly through the microtubule binding domain/repeat R3 in 3R tau proteins and through R3 and R2 in 4R tau proteins (R2 and R3 have β-structure). Regions of tau molecule both N-terminal and C-terminal to the repeats are inhibitory. Hyperphosphorylation of tau neutralizes these basic inhibitory domains, enabling tau-tau interaction (phosphorylation sites indicated by red Ps). In the case of the C-terminal region beyond Pro-397 (398–441), a highly acidic segment masks the repeats. Phosphorylation (red Ps) of tau at Ser-396 and/or 404 opens this segment, allowing tau–tau interaction through the repeats. The highly basic segments and the C terminus interfere with polymerization. Upon hyperphosphorylation (phosphorylation positions indicated in red Ps), tau proteins adopt the conformation needed to polymerize into filaments (adapted from Alonso et al. [7])
Hyperphosphorylation dependency of tau fibrillization
Dephosphorylation of PHF/neurofibrillary tangles isolated from AD brain results in their dissociation and disaggregation, and the dephosphorylated tau released behaves like normal tau in promoting microtubule assembly in vitro [201]. Similarly, dephosphorylation of AD cytosolic abnormally hyperphosphorylated tau with PP-2A inhibits its ability to self-aggregate into PHF/SF, sequester normal tau, and inhibit microtubule assembly in vitro, and rephosphorylation of the PP-2A-AD Ptau by several combinations of protein kinases restores all of its above pathological properties [198, 200].
Association of abnormally hyperphosphorylated tau with rough endoplasmic reticulum
There is approximately as much tau in somato-dendritic compartment/cerebral gray matter as in the axons/cerebral white matter [89]. In the somato-dendritic compartment, tau is associated with rough endoplasmic reticulum and Golgi apparatus [76, 94, 112]. The abnormal hyperphosphorylation of tau and its accumulation in the somato-dendritic compartment in AD might have been responsible for the morphological alterations of the RER and the Golgi apparatus and the abnormal N-glycosylation of tau in AD [55, 113, 199]. In AD brain, abnormally hyperphosphorylated tau is associated with granulovacuolar changes [44, 56, 72, 98]. Overexpression of tau, which results in its hyperphosphorylation, has been found to induce fragmentation of Golgi both in neuronal cultures and in neurons in JNPL3 P301L tau transgenic mice [112]. In P301S tau transgenic mice, a selective decrease in mitochondria and RER has been observed [214]. The chronic accumulation of the hyperphosphorylated tau as a misfolded protein in the ER could cause neurodegeneration due to protracted ER stress [91]. Hyperphosphorylation of tau might also be involved in neurodegeneration through alterations of RER and Golgi and a consequent reduction in RER and mitochondria.
Truncation and conformational changes following abnormal hyperphosphorylation of tau
In addition to abnormal hyperphosphorylation, conformational changes and cleavage of tau have also been implicated in the pathogenesis of AD [43, 82, 126, 137]. The hyperphosphorylation of tau has been found to precede both conformational changes and cleavage of this protein [33]. Truncation of tau might make it a more favorable substrate for abnormal hyperphosphorylation. Transgenic rats expressing human tau truncated both N- and C-terminally tau151–391 show a marked neurofibrillary degeneration of abnormally hyperphosphorylated tau [216]. Hyperphosphorylation is known to produce conformational changes in a protein. The late appearance and low abundance of cleaved tau in neurofibrillary tangles probably represent little more than the unsuccessful attempts of the affected neuron to turn over the pathological aggregates.
Role of proteolysis in tau pathology
The AD abnormal hyperphosphorylation of tau (AD P-tau) makes tau resistant to both calcium activated neutral proteases, calpains, and its degradation by the ubiquitin-proteosome pathway. Unlike normal tau, the AD hyperphosphorylated tau is resistant to proteolysis by calpains [201]. Subsequent to its hyperphosphorylation in AD neurofibrillary tangles, tau becomes polyubiquitinated [17, 18, 31, 58, 94, 129, 130, 152, 153, 211]. However, the ubiquitination of the abnormally hyperphosphorylated tau in neurofibrillary tangles apparently does not lead to its clearance by digestion in the proteasome. This could partly be due to a faster rate of accumulation of the ubiquitinated phosphotau than the ability of the proteosomes of the degenerating neurons to digest it. Inhibition of proteasome by its inhibitor, lactacystin, increases accumulation of both normal and hyperphosphorylated taus in rats [123]. Inhibition of proteasome with its inhibitor, MG-132, in cultured oligodendrocytes causes ubiquitination and aggregation of tau [48]. An in vivo cause of impaired proteasome might be the occurrence, in the tangle-bearing neurons, of the one frame-shift mutation of ubiquitin (UBB + 1) which inhibits the proteasome activity [191]. Another cause of the proteasome inhibition could be the increased level of BAG-1, an Hsp70/Hsc70 binding partner in the degenerating neurons. BAG-1 has been shown to inhibit degradation of tau by the 20S proteosome without affecting the ubiquitination of tau [41].
Overexpression of Hsp70, which interacts with the heat-shock cognate (Hsc) 70-interacting protein (CHIP), a ubiquitin ligase, causes a reduction of tau in transgenic mice [154]. In AD brain, levels of both CHIP and Hsp70 are increased and the level of the former is inversely proportional to that of sarkosyl-insoluble tau [162]. The increase in CHIP might be protective in the early stages of AD. Interestingly, chronic administration of lithium, a known GSK-3β inhibitor, has been reported to decrease the tau lesions by promoting their ubiquitination in a tau transgenic mouse model [134]. Protein kinase B, Akt, which can hyperphosphorylate tau both directly and indirectly through GSK-3β and PAR1/MARK2, has been reported to prevent CHIP-induced tau ubiquitination and its subsequent proteolysis either by regulating Hsp90/CHIP complex directly or by competing as a client protein with tau for binding [35].
Frontotemporal dementia tau mutations and abnormal hyperphosphorylation
Tau mutations, which cause FTDP-17, result either in increase in 4-R:3-R tau ratio or in missense mutations in the protein. Both 4-repeat tau and the mutated protein are more easily abnormally hyperphosphorylated than the normal wild-type protein [7, 20]. Four of these missense mutations, G272V, P301L, V337M, and R406W, which have been most extensively studied to date, make tau a more favorable substrate than the wild-type protein for abnormal hyperphosphorylation by brain protein kinases in vitro [7]. These mutated taus become hyperphosphorylated at a faster rate and self-aggregate into filaments more readily, i.e., at a phosphorylation stoichiometry of 4–6 as compared to 10 or more in the case of the wild-type protein. This faster kinetics of the hyperphosphorylation of the mutated tau might explain a relatively early onset, severity, and autosomal dominance of the disease in the inherited FTDP-17 cases.
The six human brain tau isoforms are differentially sequestered by AD P-tau in vitro [8]. The association of AD P-tau to normal human brain recombinant taus is 2N4Rtau >1N4Rtau >0N4Rtau and 2N3Rtau >1N3R-tau >0N3Rtau, and 2N4Rtau >2N3Rtau. AD P-tau also inhibits the assembly and disrupts microtubules pre-assembled with each tau isoform with an efficiency, which corresponds directly to the degree of interaction with these isoforms. In vitro hyperphosphorylation of recombinant tau converts it into an AD P-tau-like state in sequestering normal tau and inhibiting microtubule assembly. The preferential sequestration of 4R taus and taus with amino terminal inserts explains both (1) why fetal tau (ON3Rtau) is protected from Alzheimer neurofibrillary pathology and (2) why intronic mutations seen in certain inherited cases of FTDP-17, which result in alternate splicing of tau mRNA and consequently an increase in 4R:3R ratio, lead to neurofibrillary degeneration and the disease. In vitro, at a phosphorylation stoichiometry of 4 and above, the hyperphosphorylated tau sequesters normal tau, whereas it requires a stoichiometry of 10 or more to self-aggregate into filaments [5, 7, 105]. On aggregation into filaments, tau loses its ability to sequester normal tau. Furthermore, AD P-tau, but not PHF, inhibits regeneration of microtubule network in detergent-extracted PC12 cells, indicating that the formation of filaments might be initiated as a self-defense response by the affected neurons [5, 8]. Opposite to FTDP-17, in Pick disease and in DS the tau 3R:4R ratio is very much increased [142, 171, 216]. Since the activity of 3R tau is lesser than of 4R tau in binding to tubulin/microtubules, the unbound 3R tau becomes abnormally hyperphosphorylated because free tau is a more favorable substrate than tau on microtubules for phosphorylation [168].
Neurofibrillary degeneration in Down syndrome
DS is caused by partial or complete trisomy 21. Virtually, all DS patients develop AD-type brain lesions, i.e., amyloid plaques and neurofibrillary degeneration, when they reach the fourth decade of life [99, 204, 207], which is 20–30 years earlier than in AD. Accompanying these histopathological changes is the occurrence of age-related cognitive impairment that progresses to dementia resembling AD [68, 164]. The early onset of amyloidosis in DS brain is believed to result from over-expression of APP, the gene for which is located in chromosome 21 [42, 170]. Recent studies shed light into the mechanism by which neurofibrillary pathology is accelerated in DS.
One important gene within the DS critical region is DYRK1A that encodes a serine/threonine protein kinase named Dyrk1A (dual-specificity tyrosine-phosphorylated and regulated kinase 1A) [87]. Recent studies suggest that over-expression of this kinase due to trisomy 21 may lead to the accelerated tau pathology and neurofibrillary degeneration in DS by two mechanisms. First, Dyrk1A phosphorylates tau and, more importantly, primes tau to be a better substrate for phosphorylation with GSK-3β at many phosphorylation sites as seen in the hyperphosphorylated tau in DS brain [121, 210]. Not only the expression level, but also the kinase activity was found to be indeed increased in DS brain [121]. In vitro studies have demonstrated that tau phosphorylated with Dyrk1A, especially together with GSK-3β, inhibits its biological activity to stimulate microtubule assembly and leads to its self-assembly into filaments [121]. Second, we found that Dyrk1A phosphorylates the alternative splicing factor ASF at Ser-227, Ser-234 and Ser-238, drives ASF into nuclear speckles, and prevents it from facilitating tau exon 10 inclusion [171]. Thus, the increased Dyrk1A activity in DS brain leads to increased 3R:4R tau ratio, an imbalance that is known to associate with neurofibrillary degeneration in Pick disease. These observations suggest that neurofibrillary degeneration in DS might be caused by Dyrk1A over-expression through phosphorylating tau and disturbing normal 3R:4R tau ratio. Thus, inhibition of Dyrk1A activity is likely to inhibit neurofibrillary degeneration and the consequent dementia in DS.
Defensive role of fibrillization of abnormally hyperphosphorylated tau
The abnormal hyperphosphorylation of tau makes it resistant to proteolysis by the calcium activated neutral protease [198, 201] and turnover of hyperphosphorylated tau is several folds slower than the normal tau [157]. Most likely, it is because of this reason that the levels of tau are severalfold increased in AD [88, 89]. Some increase in tau level in AD brain can also result from the activation of p70 S6 kinase which upregulates the translation of tau [9, 149]. It is likely that to neutralize the ability of AD P-tau to sequester normal MAPs and cause disassembly of microtubules, the affected neurons promote the self-assembly of the abnormal tau into tangles of PHF. The fact that the tangle-bearing neurons seem to survive many years [132] and that in AD brain, the decrease in microtubule density was unrelated to PHFs accumulation [21] is consistent with such a self-defense role of the formation of tangles. Employing an inducible transgenic mouse model that expressed human four-repeat tau with the P301L mutation, Santacruz and colleagues [163] found that the cognitive deficiencies correlate with the appearance of soluble hyperphosphorylated tau. In this model, when tau expression was turned off, there was no clearance of the polymerized tau, soluble phosphotau decreased, and there was improvement in cognition, suggesting that the polymerized tau was not sufficient to cause cognitive decline or neuronal cell death. Andorfer et al. [11] showed that in human tau transgenic mice, while there was widespread neurodegeneration, the PHF-containing neurons, however, appeared “healthy” in terms of nuclear morphology, suggesting that the polymerization of hyperphosphorylated tau into fibrils was probably neuroprotective [11].
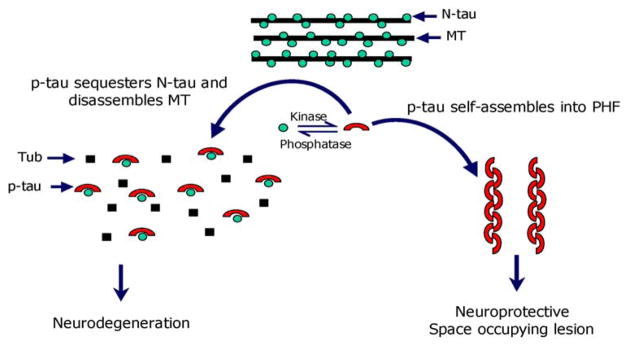
The AD P-tau readily self-assembles into tangles of PHF/SF in vitro under physiological conditions of protein concentration, pH, ionic strength, and reducing conditions [6]. Furthermore, dephosphorylation inhibits the self-assembly of AD P-tau into PHF/SF, and the in vitro abnormal hyperphosphorylation of each of the six recombinant human brain tau isoforms promotes their assembly into tangles of PHF/SF. Thus, all these studies taken together demonstrate the pivotal involvement of abnormal hyperphosphorylation in neurofibrillary degeneration and the disruptive properties to the microtubule network of the cytosolic abnormally hyperphosphorylated tau, whereas AD P-tau polymer remains inert (Fig. 3).
Fig. 3.
Proposed mechanism of tau-induced neurodegeneration in AD and related tauopathies (reproduced with permission from Alonso et al. [5])
Protein kinases involved in the abnormal hyperphosphorylation of tau
The state of phosphorylation of a phosphoprotein is a function of the balance between the activities of the protein kinases and the protein phosphatases that regulate its phosphorylation. Tau, which is phosphorylated at over 38 serine/threonine residues in AD [60, 131], is a substrate for several protein kinases [83, 175]. Among these kinases, glycogen synthase kinase-3 (GSK-3), cyclin dependent protein kinase-5 (cdk5), protein kinase A (PKA), calcium and calmodulin-dependent protein kinase-II (CaMKII), casein kinase-1 (CK-1), mitogen activated protein (MAP) kinase ERK 1/2, and stress-activated protein kinases (SAPKs) have been most implicated in the abnormal hyperphosphorylation of tau [78, 148]. A large number of the abnormally hyperphosphorylated sites in tau are proline-directed, i.e., serine/threonine followed by proline which are canonical sites of the proline-directed protein kinases (PDPKs), GSK3, cdk5, ERK1/2, SAPKs and Dyrk1A.
GSK-3β and cdk5 phosphorylate tau at a large number of sites, most of which are common to the two enzymes [10, 120, 202]. The expressions of GSK-3β and cdk5 are high in the brain [103, 188, 209] and both enzymes have been shown to be associated with all stages of neurofibrillary pathology in AD [145, 146]. Overexpression of GSK-3β in cultured cells and in transgenic mice results in hyperphosphorylation of tau at several of the same sites seen in AD and inhibition of this enzyme by lithium chloride attenuates phosphorylation in these models [70, 124, 125, 151, 180, 181, 186, 196].
Cdk5 requires for its activity interaction with p39 or p35 or, better, their proteolytic products p29 or p25, respectively, which are generated in post mitotic neurons by digestion with calpains [97, 143]. Overexpression of p25 in transgenic mice, which results in an increase in the activity of cdk5, also produces hyperphosphorylation of tau [32, 136].
The MAP kinase family, which includes ERK1, ERK2, p70S6 kinase and the stress-activated kinases JNK and p38 kinase, have been shown to phosphorylate tau at several of the same sites as the abnormally hyperphosphorylated tau and so has been the association of these enzymes with the progression of neurofibrillary degeneration in AD [9, 39, 93, 100, 147, 148, 159].
Dryk1A has been implicated in tau phosphorylation recently. Woods et al. [210] first reported that this kinase phosphorylates tau at Thr212. Our further studies demonstrated that it also phosphorylates tau at several other sites including Thr181, Ser199, Ser202, Thr205, Thr217, Thr231, Ser396, Ser400, Ser404 and Ser422 both in vitro and in cultured cells [121]. More importantly, phosphorylation of tau with Dyrk1A primes tau for further phosphorylation with GSK-3β at multiple sites [121]. Although the role of Dyrk1A in neurofibrillary degeneration in AD is not clear, a genetic association study of late-onset AD results in 17 genetic risk markers, of which DYRK1A gene shows the highest significance in logistic regression [92]. The same study also reported an increased mRNA level of Dryk1A in the hippocampus of patients with AD as compared with pathological controls. In DS, Dyrk1A over-expression due to trisomy 21 appears to underlie neurofibrillary degeneration (see above).
Unlike the PDPKs, the non-PDPKs have been shown to phosphorylate tau at only a few of the sites. CaM Kinase II phosphorylates tau at Ser-262/356 and at Ser-416 [19, 176, 177, 182]. Both PKA and MARK kinase have also been shown to phosphorylate tau at Ser-262 [38, 40, 167]. Phosphorylation of tau by these non-PDPKs markedly increases the phosphorylation of tau by PDPKs, GSK-3β and cdk5 [26, 120, 169, 174, 202]. The priming of tau by PKA appears to be sufficient to promote the abnormal hyperphosphorylation of tau by the basal level of GSK-3β activity in normal adult rat brain and leads to an impairment of spatial memory in these animals [122]. Although, to date, the activities of these protein kinases have not been reproducibly shown to be upregulated in AD brain, transient stimulation of these enzymes, especially the priming kinases such as PKA or CaMKII, might be sufficient to result in the abnormal hyperphosphorylation of tau since the level of GSK-3 is increased [144].
Protein phosphatase activities that regulate the phosphorylation of tau
The activities of protein phosphatase (PP)-2A and PP-1 are compromised by ~20% in AD brain [49, 51]. The phosphorylation of tau that suppresses its microtubule binding and assembly activities in adult mammalian brain is regulated by PP-2A and not by PP-2B [19, 52] and PP-2A accounts for over 70% of tau phosphatase activity in human brain [116]. PP-2A also regulates the activities of several tau kinases in brain. Inhibition of PP-2A activity by okadaic acid in cultured cells and in metabolically active rat brain slices results in abnormal hyperphosphorylation of tau at several of the same sites as in AD, not only directly by a decrease in dephosphorylation but also indirectly by promoting the activities of CaM Kinase II [19], PKA [106, 184], MAP kinase kinase (MEK1/2), extracellular regulated kinase (ERK 1/2) and P70S6 kinase [9, 148]. Thus, barring the fact that tau is not the only neuronal substrate of these protein kinases and phosphatases, it should be possible to inhibit the abnormal hyperphosphorylation of tau by inhibiting the activity of one or more tau kinases and or restoring or upregulating the activity of PP-2A.
Phosphatase inhibitors that regulate the tau phosphatase activities
Although the brain has several tau phosphatase activities [24, 25], PP-2A and PP-1 make more than 90% of the serine/threonine protein phosphatase activity in mammalian cells [141]. The intracellular activities of these enzymes are regulated by endogenous inhibitors. PP-1 activity is regulated mainly by a 18.7 kDa heat stable protein called inhibitor-1 (I-1) [28, 29]. In addition, a structurally related protein, DARPP-32 (dopamine and cAMP-regulated phosphoprotein of apparent molecular weight 32,000) is expressed predominantly in the brain [197]. I-1 and DARPP-32 are activated on phosphorylation by protein kinase A and inactivated by calcineurin, and at basal calcium level by PP-2A [135]. Thus, inhibition of PP-2A activity would keep I-1, DARPP-32 in active form and thereby result in a decrease in PP-1 activity. In AD brain, a reduction in PP-2A activity might have decreased the PP-1 activity by allowing the upregulation of the I-1/DARPP-32 activity. In a subgroup of AD cases and or at moderate to severe stages of the disease, when there is a persistent excitotoxicity and increase in the intraneuronal calcium, DARPP-32 is probably dephosphorylated and thereby inactivated as PP-1 inhibitor by calcineurin.
PP-2A is inhibited in the mammalian tissue by two heat-stable proteins: (1) the , a 30 kDa cytosolic protein [107] that inhibits PP-2A with a Ki of 30 nM and (2) the , a 39 kDa nuclear protein that inhibits PP-2A with a Ki of 23 nM [107]. Both and have been cloned from human kidney [108, 109] and brain [189]. has been found to be the same protein as the putative histocompatibility leukocyte antigen class II-associated protein-1 (PHAP-1). This protein, which has also been described as mapmodulin, pp32 and LANP [190] is 249 amino acids long and has apparent molecular weight of 30 kDa on SDS-PAGE. , which is the same as TAF-1β or PHAPII, is a nuclear protein that is a homolog of the human SETα protein [195]. In AD brain, there is a shift from nuclear to cytoplasmic localization of and its cleavage into an amino-terminal half, I2NTF, and a C-terminal half, I2CTF, [185]. Both and interact with the catalytic subunit of PP2A, which leads to abnormal hyperphosphorylation of tau [22, 23, 203]. The levels of and are ~20% increased in AD brains as compared with age-matched control brains and probably a cause of decrease in PP-2A activity [185]. Memantine disinhibits the activity of toward PP-2A in dephosphorylation of tau [27]. This effect of memantine might be responsible for the therapeutic effect of this drug in moderate to severe stage AD patients and a reduction in the phosphotau in their CSF [59, 158].
Involvement of more than one kinase and phosphorylation site in abnormal hyperphosphorylation of tau
Abnormally hyperphosphorylated tau from AD brain cytosol, the AD P-tau, self-assembles into bundles of PHF/SF [6, 200]. On treatment with PP-2A, which dephosphorylates most of the known abnormally hyperphosphorylated sites, including Thr231 and Ser262, the AD P-tau loses its ability to both inhibit microtubule assembly and to self-assemble into PHF/SF [198, 200]. Rephosphorylation of the PP-2A dephosphorylated AD P-tau, the PP2A-AD-P-tau, by PKA followed by CaMKinase-II and GSK-3β or cdk5, or cdk5 followed by GSK-3β, results in phosphorylation of Thr231 and Ser262 among several other sites, and restores its ability to inhibit microtubule assembly and self-assemble into PHF/SF. The bundles of filaments formed under these conditions are congophilic and very reminiscent of neurofibrillary tangles seen in AD brain. Rephosphorylation of PP-2A-AD P-tau by none of the above kinases individually, however, results in phosphorylation at both Thr231 and Ser262 and restores its self-assembly into PHF/SF. These studies [200] suggest that more than one specific combination of kinases might be involved in converting normal tau into an AD P-tau-like state, and that PP-2A can alone convert the pathological state of the protein to a normal-like state.
Role of decreased brain glucose metabolism in neurofibrillary degeneration
In addition to abnormal hyperphosphorylation, tau is also glycosylated and the latter appears to precede the former in AD brain [119, 199]. In vitro studies indicate that the abnormal glycosylation promotes tau phosphorylation with PKA, GSK-3β and ckd5, and inhibits dephosphorylation of tau with PP2A and PP5 [115, 117]. In addition, like some other neuronal phosphoproteins, tau is also O-GlcNAcylated [13, 65]. In contrast to classical N- or O-glycosylation, O-GlcNAcylation, which involves the addition of a single sugar at serine/threonine residues of a protein, dynamically post-translationally modifies cytoplasmic and nuclear proteins in a manner analogous to protein phosphorylation [64]. O-GlcNAcylation and phosphorylation often involve the same serine/threonine residues of a substrate protein and reciprocally regulate each other. In AD, probably due to impaired glucose uptake/metabolism, there is a global decrease in O-GlcNAcylation including that of tau and neurofilaments [34, 118]. Decreased glucose metabolism in cultured cells and in mice, which decreases the O-GlcNA-cylation of tau, produces abnormal hyperphosphorylation of this protein [110, 118]. On the basis of these observations, a hypothetical mechanism explaining the pathways through which impaired brain glucose metabolism facilitates abnormal tau hyperphosphorylation and neurofibrillary degeneration in AD has been proposed [50]. Thus, inhibition of O-GlcNAcylase, the enzyme that hydrolyzes the removal of this sugar moiety from proteins, is a promising therapeutic target for AD and related tauopathies. Inhibition of O-GlcNAcylase with PUGNAc or Thiamet-G inhibits hyperphosphorylation of tau by increasing its O-GlcNAcylation [118, 215].
Subgroups of Alzheimer disease
AD is multifactorial and heterogeneous. Based on CSF levels of proteins associated with plaques and tangles, i.e., Aβ1–42, total tau, and ubiquitin, five different subgroups of AD—ATEO, AELO, LEBALO, HARO, and ARTURO—have been identified [77].
Our recent studies have revealed that more than one signaling pathway could be involved in neurofibrillary degeneration. We have found that tau can be abnormally hyperphosphorylated to an AD-like state (i.e., sequester normal tau, inhibit microtubule assembly, and self-assemble into bundles of PHF) with more than one combination of protein kinases and that this phosphorylation of tau can be regulated by PP-2A [200]. Thus, it is likely that in future additional subgroups of AD may be identified from phosphorylation patterns of CSF tau of AD patients, and the disease might be identified preclinically. Identification of specific signal transduction pathways involved selectively in different subgroups of AD should facilitate development of specific and potent therapeutic drugs.
Acknowledgments
We are grateful to Janet Murphy for secretarial assistance. Studies in our laboratories were supported in part by the New York State Office of Mental Retardation and Developmental Disabilities and NIH grants AG019158, AG028538, and AG27429, and Alzheimer’s Association (Chicago, IL) grants IIRG-00-2002, IIRG-05-13095, and NIRG-08-91126.
Abbreviations
- AD
Alzheimer disease
- PHF
Paired helical filaments
- SF
Straight filaments
References
- 1.Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B. Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol. 1987;74:209–225. doi: 10.1007/BF00688184. [DOI] [PubMed] [Google Scholar]
- 2.Alonso AD, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 3.Alonso AD, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso AD, Li B, Grundke-Iqbal I, Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci USA. 2006;23:8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso AD, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso AD, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004;279:34873–34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- 8.Alonso AD, Zaidi T, Novak M, et al. Interaction of tau isoforms with Alzheimer’s disease abnormally hyperphosphorylated tau and in vitro phosphorylation into the disease-like protein. J Biol Chem. 2001;276:37967–37973. doi: 10.1074/jbc.M006497200. [DOI] [PubMed] [Google Scholar]
- 9.An WL, Cowburn RF, Li L, et al. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am J Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderton BH, Betts J, Blackstock WP, et al. Sites of phosphorylation in tau and factors affecting their regulation. Biochem Soc Symp. 2001;67:73–80. doi: 10.1042/bss0670073. [DOI] [PubMed] [Google Scholar]
- 11.Andorfer C, Acker CM, Kress Y, et al. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI. 4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arendt T, Stieler J, Strijkstra AM, et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold CS, Johnson GV, Cole RN, et al. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem. 1996;271:28741–28744. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- 14.Arrasate M, Perez M, Armas-Portela R, Avila J. Polymerization of tau peptides into fibrillar structures The effect of FTDP-17 mutations. FEBS Lett. 1999;446:199–202. doi: 10.1016/S0014-5793(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 15.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 16.Baki L, Shioi J, Wen P, et al. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bancher C, Grundke-Iqbal I, Iqbal K, et al. Abnormal phosphorylation of tau precedes ubiquitination in neurofibrillary pathology of Alzheimer disease. Brain Res. 1991;539:11–18. doi: 10.1016/0006-8993(91)90681-K. [DOI] [PubMed] [Google Scholar]
- 18.Bancher C, Brunner C, Lassmann H, et al. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- 19.Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett. 2001;490:15–22. doi: 10.1016/S0014-5793(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 20.Bhaskar K, Yen SH, Lee G. Disease-related modifications in tau affect the interaction between Fyn and Tau. J Biol Chem. 2005;280:35119–35125. doi: 10.1074/jbc.M505895200. [DOI] [PubMed] [Google Scholar]
- 21.Cash AD, Aliev G, Siedlak SL, et al. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. Am J Pathol. 2003;162:1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Grundke-Iqbal I, Iqbal K. and affect tau phosphorylation via association with the catalytic subunit of protein phosphatase 2A. Alzheimers Dement. 2006;2:S471. doi: 10.1016/j.jalz.2006.05.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Li B, Grundke-Iqbal I, Iqbal K. I1PP2A affects tau phosphorylation via association with the catalytic subunit of protein phosphatase 2A. J Biol Chem. 2008;283:10513–10521. doi: 10.1074/jbc.M709852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng LY, Wang JZ, Gong CX, et al. Multiple forms of phosphatase from human brain: isolation and partial characterization of affi-gel blue binding phosphatases. Neurochem Res. 2000;25:107–120. doi: 10.1023/A:1007547701518. [DOI] [PubMed] [Google Scholar]
- 25.Cheng LY, Wang JZ, Gong CX, et al. Multiple forms of phosphatase from human brain: isolation and partial characterization of affi-gel blue nonbinding phosphatase activities. Neurochem Res. 2001;26:425–438. doi: 10.1023/A:1010963401453. [DOI] [PubMed] [Google Scholar]
- 26.Cho JH, Johnson GV. Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J Biol Chem. 2003;278:187–193. doi: 10.1074/jbc.M206236200. [DOI] [PubMed] [Google Scholar]
- 27.Chohan MO, Khatoon S, Iqbal IG, Iqbal K. Involvement of I2PP2A in the abnormal hyperphosphorylation of tau and its reversal by Memantine. FEBS Lett. 2006;580:3973–3979. doi: 10.1016/j.febslet.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 29.Cohen P, Alemany S, Hemmings BA, et al. Protein phosphatase-1 and protein phosphatase-2A from rabbit skeletal muscle. Methods Enzymol. 1988;159:390–408. doi: 10.1016/0076-6879(88)59039-0. [DOI] [PubMed] [Google Scholar]
- 30.Cotman CW, Poon WW, Rissman RA, Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2005;64:104–112. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- 31.Cripps D, Thomas SN, Jeng Y, et al. Alzheimer disease-specific conformation of hyperphosphorylated paired helical filament-Tau is polyubiquitinated through Lys-48, Lys-11, and Lys-6 ubiquitin conjugation. J Biol Chem. 2006;281:10825–10838. doi: 10.1074/jbc.M512786200. [DOI] [PubMed] [Google Scholar]
- 32.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/S0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 33.Delobel P, Lavenir I, Fraser G, et al. Analysis of tau phosphorylation and truncation in a mouse model of human tauopathy. Am J Pathol. 2008;172:123–131. doi: 10.2353/ajpath.2008.070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Y, Li B, Liu F, et al. Regulation between O-Glc-NAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. FASEB J. 2008;22:138–145. doi: 10.1096/fj.07-8309com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickey CA, Koren J, Zhang YJ, et al. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci USA. 2008;105:3622–3627. doi: 10.1073/pnas. 0709180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson DW, Farlo J, Davies P, et al. Alzheimer’s disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol. 1988;132:86–101. [PMC free article] [PubMed] [Google Scholar]
- 37.Dickson DW, Crystal HA, Mattiace LA, et al. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-U. [DOI] [PubMed] [Google Scholar]
- 38.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/S0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 39.Drewes G, Lichtenberg-Kraag B, Doring F, et al. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992;11:2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drewes G, Trinczek B, Illenberger S, et al. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem. 1995;270:7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- 41.Elliott E, Tsvetkov P, Ginzburg I. BAG-1 associates with Hsc70 tau complex and regulates the proteasomal degradation of Tau protein. J Biol Chem. 2007;282:37276–37284. doi: 10.1074/jbc.M706379200. [DOI] [PubMed] [Google Scholar]
- 42.Engidawork E, Lubec G. Protein expression in Down syndrome brain. Amino Acids. 2001;21:331–361. doi: 10.1007/s007260170001. [DOI] [PubMed] [Google Scholar]
- 43.Gamblin TC, Chen F, Zambrano A, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghoshal N, Smiley JF, DeMaggio AJ, et al. A new molecular link between the fibrillar and granulovacuolar lesions of Alzheimer’s disease. Am J Pathol. 1999;155:1163–1172. doi: 10.1016/S0002-9440(10)65219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-V. [DOI] [PubMed] [Google Scholar]
- 46.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 47.Goedert M, Jakes R, Spillantini MG, et al. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 48.Goldbaum O, Richter-Landsberg C. Proteolytic stress causes heat shock protein induction, tau ubiquitination, and the recruitment of ubiquitin to tau-positive aggregates in oligodendrocytes in culture. J Neurosci. 2004;24:5748–5757. doi: 10.1523/JNEUROSCI.1307-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 50.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Impaired brain glucose metabolism leads to Alzheimer neurofibrillary degeneration through a decrease in tau O-GlcNAcylation. J Alzheimers Dis. 2006;9:1–12. doi: 10.3233/jad-2006-9101. [DOI] [PubMed] [Google Scholar]
- 51.Gong CX, Shaikh S, Wang JZ, et al. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 52.Gong CX, Lidsky T, Wegiel J, et al. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem. 2000;275:5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 53.Goode BL, Denis PE, Panda D, et al. Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol Biol Cell. 1997;8:353–365. doi: 10.1091/mbc.8.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301 l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 55.Gray EG, Paula-Barbosa M, Roher A. Alzheimer’s disease: paired helical filaments and cytomembranes. Neuropathol Appl Neurobiol. 1987;13:91–110. doi: 10.1111/j.1365-2990.1987.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 56.Grundke-Iqbal I, Iqbal K, Tung YC, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grundke-Iqbal I, Iqbal K, Quinlan M, et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 58.Grundke-Iqbal I, Vorbrodt AW, Iqbal K, et al. Microtubule-associated polypeptides tau are altered in Alzheimer paired helical filaments. Brain Res. 1988;464:43–52. doi: 10.1016/0169-328x(88)90017-4. [DOI] [PubMed] [Google Scholar]
- 59.Gunnarsson MDLK, Sudelof J, Basun H, Lannfelt L. Reduction of hyperphosphorylated tau during memantine treatment of Alzheimer’s disease. Alzheimers Dement. 2006;2:S63–S64. doi: 10.1016/j.jalz.2006.05.229. [DOI] [Google Scholar]
- 60.Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer’s disease brain using nanoelectrospray mass spectrometry. J Neurochem. 1998;71:2465–2476. doi: 10.1046/j.1471-4159.1998.71062465.x. [DOI] [PubMed] [Google Scholar]
- 61.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 62.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 63.Harrington C, Rickard JE, Horsley D, et al. Methylthioninium chloride (MTC) acts as a tau aggregation inhibitor (TAI) in a cellular model and reverses tau pathology in transgenic mouse model of Alzheimer’s disease. Alzheimers Dement. 2008;4:120. doi: 10.1016/j.jalz.2008.05.259. [DOI] [Google Scholar]
- 64.Hart GW. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- 65.Hart GW, Kreppel LK, Comer FI, et al. O-GlcNAcylation of key nuclear and cytoskeletal proteins: reciprocity with O-phosphorylation and putative roles in protein multimerization. Glycobiology. 1996;6:711–716. doi: 10.1093/glycob/6.7.711. [DOI] [PubMed] [Google Scholar]
- 66.Hasegawa M, Crowther RA, Jakes R, Goedert M. Alzheimer-like changes in microtubule-associated protein Tau induced by sulfated glycosaminoglycans. Inhibition of microtubule binding, stimulation of phosphorylation, and filament assembly depend on the degree of sulfation. J Biol Chem. 1997;272:33118–33124. doi: 10.1074/jbc.272.52.33118. [DOI] [PubMed] [Google Scholar]
- 67.Himmler A, Drechsel D, Kirschner MW, Martin DW., Jr Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol. 1989;9:1381–1388. doi: 10.1128/mcb.9.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holland AJ, Hon J, Huppert FA, Stevens F, Watson P. Population-based study of the prevalence and presentation of dementia in adults with Down’s syndrome. Br J Psychiatry. 1998;172:493–498. doi: 10.1192/bjp.172.6.493. [DOI] [PubMed] [Google Scholar]
- 69.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 70.Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 71.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 72.Ikegami K, Kimura T, Katsuragi S, et al. Immunohistochemical examination of phosphorylated tau in granulovacuolar degeneration granules. Psychiatry Clin Neurosci. 1996;50:137–140. doi: 10.1111/j.1440-1819.1996.tb01678.x. [DOI] [PubMed] [Google Scholar]
- 73.Iqbal K, Grundke-Iqbal I. Metabolic/signal transduction hypothesis of Alzheimer’s disease and other tauopathies. Acta Neuropathol. 2005;109:25–31. doi: 10.1007/s00401-004-0951-y. [DOI] [PubMed] [Google Scholar]
- 74.Iqbal K, Zaidi T, Bancher C, Grundke-Iqbal I. Alzheimer paired helical filaments. Restoration of the biological activity by dephosphorylation. FEBS Lett. 1994;349:104–108. doi: 10.1016/0014-5793(94)00650-4. [DOI] [PubMed] [Google Scholar]
- 75.Iqbal K, Grundke-Iqbal I, Smith AJ, et al. Identification and localization of a tau peptide to paired helical filaments of Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:5646–5650. doi: 10.1073/pnas.86.14.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iqbal K, Grundke-Iqbal I, Zaidi T, et al. Defective brain microtubule assembly in Alzheimer’s disease. Lancet. 1986;2:421–426. doi: 10.1016/S0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- 77.Iqbal K, Flory M, Khatoon S, et al. Subgroups of Alzheimer’s disease based on cerebrospinal fluid molecular markers. Ann Neurol. 2005;58:748–757. doi: 10.1002/ana.20639. [DOI] [PubMed] [Google Scholar]
- 78.Iqbal K, Alonso Adel C, Chen S, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 79.Jellinger KA, Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 2007;113:107–117. doi: 10.1007/s00401-006-0156-7. [DOI] [PubMed] [Google Scholar]
- 80.Jicha GA, Berenfeld B, Davies P. Sequence requirements for formation of conformational variants of tau similar to those found in Alzheimer’s disease. J Neurosci Res. 1999;55:713–723. doi: 10.1002/(SICI)1097-4547(19990315)55:6<713::AID-JNR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 81.Jicha GA, Rockwood JM, Berenfeld B, Hutton M, Davies P. Altered conformation of recombinant frontotemporal dementia-17 mutant tau proteins. Neurosci Lett. 1999;260:153–156. doi: 10.1016/S0304-3940(98)00980-X. [DOI] [PubMed] [Google Scholar]
- 82.Jicha GA, Lane E, Vincent I, et al. A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer’s disease. J Neurochem. 1997;69:2087–2095. doi: 10.1046/j.1471-4159.1997.69052087.x. [DOI] [PubMed] [Google Scholar]
- 83.Johnson GV, Hartigan JA. Tau protein in normal and Alzheimer’s disease brain: an update. J Alzheimers Dis. 1999;1:329–351. doi: 10.3233/jad-1999-14-512. [DOI] [PubMed] [Google Scholar]
- 84.Kampers T, Friedhoff P, Biernat J, Mandelkow EM, Mandelkow E. RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett. 1996;399:344–349. doi: 10.1016/S0014-5793(96)01386-5. [DOI] [PubMed] [Google Scholar]
- 85.Kampers T, Pangalos M, Geerts H, Wiech H, Mandelkow E. Assembly of paired helical filaments from mouse tau: implications for the neurofibrillary pathology in transgenic mouse models for Alzheimer’s disease. FEBS Lett. 1999;451:39–44. doi: 10.1016/S0014-5793(99)00522-0. [DOI] [PubMed] [Google Scholar]
- 86.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 87.Kentrup H, Becker W, Heukelbach J, et al. Dyrk, a dual specificity protein kinase with unique structural features whose activity is dependent on tyrosine residues between subdomains VII and VIII. J Biol Chem. 1996;271:3488–3495. doi: 10.1074/jbc.271.7.3488. [DOI] [PubMed] [Google Scholar]
- 88.Khatoon S, Grundke-Iqbal I, Iqbal K. Brain levels of microtubule-associated protein tau are elevated in Alzheimer’s disease: a radioimmuno-slot-blot assay for nanograms of the protein. J Neurochem. 1992;59:750–753. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 89.Khatoon S, Grundke-Iqbal I, Iqbal K. Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett. 1994;351:80–84. doi: 10.1016/0014-5793(94)00829-9. [DOI] [PubMed] [Google Scholar]
- 90.Khatoon S, Grundke-Iqbal I, Iqbal K. Guanosine triphosphate binding to beta-subunit of tubulin in Alzheimer’s disease brain: role of microtubule-associated protein tau. J Neurochem. 1995;64:777–787. doi: 10.1046/j.1471-4159.1995.64020777.x. [DOI] [PubMed] [Google Scholar]
- 91.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 92.Kimura R, Kamino K, Yamamoto M, et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease. Hum Mol Genet. 2007;16:15–23. doi: 10.1093/hmg/ddl437. [DOI] [PubMed] [Google Scholar]
- 93.Kins S, Kurosinski P, Nitsch RM, Gotz J. Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP2A in transgenic mice. Am J Pathol. 2003;163:833–843. doi: 10.1016/S0002-9440(10)63444-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kopke E, Tung YC, Shaikh S, et al. Microtubule-associated protein tau Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem. 1993;268:24374–24384. [PubMed] [Google Scholar]
- 95.Kovacs GG, Majtenyi K, Spina S, et al. White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol. 2008;67:963–975. doi: 10.1097/NEN.0b013e318187a80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumaran R, Kingsbury A, Coulter I, et al. DJ-1 (PARK7) is associated with 3R and 4R tau neuronal and glial inclusions in neurodegenerative disorders. Neurobiol Dis. 2007;28:122–132. doi: 10.1016/j.nbd.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 97.Kusakawa G, Saito T, Onuki R, et al. Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem. 2000;275:17166–17172. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- 98.Lagalwar S, Berry RW, Binder LI. Relation of hippocampal phospho-SAPK/JNK granules in Alzheimer’s disease and tauopathies to granulovacuolar degeneration bodies. Acta Neuropathol. 2007;113:63–73. doi: 10.1007/s00401-006-0159-4. [DOI] [PubMed] [Google Scholar]
- 99.Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- 100.Ledesma MD, Correas I, Avila J, Diaz-Nido J. Implication of brain cdc2 and MAP2 kinases in the phosphorylation of tau protein in Alzheimer’s disease. FEBS Lett. 1992;308:218–224. doi: 10.1016/0014-5793(92)81278-T. [DOI] [PubMed] [Google Scholar]
- 101.Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 102.Levy E, Carman MD, Fernandez-Madrid IJ, et al. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 103.Lew J, Huang QQ, Qi Z, et al. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 104.Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 105.Li B, Chohan MO, Grundke-Iqbal I, Iqbal K. Disruption of microtubule network by Alzheimer abnormally hyperphosphorylated tau. Acta Neuropathol. 2007;113:501–511. doi: 10.1007/s00401-007-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li L, Sengupta A, Haque N, Grundke-Iqbal I, Iqbal K. Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett. 2004;566:261–269. doi: 10.1016/j.febslet.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 107.Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 1995;34:1988–1996. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- 108.Li M, Makkinje A, Damuni Z. Molecular identification of I1PP2A, a novel potent heat-stable inhibitor protein of protein phosphatase 2A. Biochemistry. 1996;35:6998–7002. doi: 10.1021/bi960581y. [DOI] [PubMed] [Google Scholar]
- 109.Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 110.Li X, Lu F, Wang JZ, Gong CX. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur J NeuroSci. 2006;23:2078–2086. doi: 10.1111/j.1460-9568.2006.04735.x. [DOI] [PubMed] [Google Scholar]
- 111.Liang Z, Liu F, Iqbal K, et al. Decrease of protein phosphatase 2A and its association with accumulation and hyperphosphorylation of tau in Down syndrome. J Alzheimers Dis. 2008;13:295–302. doi: 10.3233/jad-2008-13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liazoghli D, Perreault S, Micheva KD, Desjardins M, Leclerc N. Fragmentation of the Golgi apparatus induced by the overexpression of wild-type and mutant human tau forms in neurons. Am J Pathol. 2005;166:1499–1514. doi: 10.1016/S0002-9440(10)62366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J Neurocytol. 2003;32:1091–1105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- 114.Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984;259:5301–5305. [PubMed] [Google Scholar]
- 115.Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3beta. FEBS Lett. 2002;530:209–214. doi: 10.1016/S0014-5793(02)03487-7. [DOI] [PubMed] [Google Scholar]
- 116.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J NeuroSci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 117.Liu F, Zaidi T, Iqbal K, Grundke-Iqbal I, Gong CX. Aberrant glycosylation modulates phosphorylation of tau by protein kinase A and dephosphorylation of tau by protein phosphatase 2A and 5. Neuroscience. 2002;115:829–837. doi: 10.1016/S0306-4522(02)00510-9. [DOI] [PubMed] [Google Scholar]
- 118.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:10804–10809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu F, Zaidi T, Iqbal K, et al. Role of glycosylation in hyperphosphorylation of tau in Alzheimer’s disease. FEBS Lett. 2002;512:101–106. doi: 10.1016/S0014-5793(02)02228-7. [DOI] [PubMed] [Google Scholar]
- 120.Liu F, Liang Z, Shi J, et al. PKA modulates GSK-3beta-and cdk5-catalyzed phosphorylation of tau in site- and kinase-specific manners. FEBS Lett. 2006;580:6269–6274. doi: 10.1016/j.febslet.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu F, Liang Z, Wegiel J, et al. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. Faseb J. 2008;22:3224–3233. doi: 10.1096/fj.07-104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu SJ, Zhang JY, Li HL, et al. Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem. 2004;279:50078–50088. doi: 10.1074/jbc.M406109200. [DOI] [PubMed] [Google Scholar]
- 123.Liu YH, Wei W, Yin J, et al. Proteasome inhibition increases tau accumulation independent of phosphorylation. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.02.012. (in press) [DOI] [PubMed] [Google Scholar]
- 124.Lovestone S, Hartley CL, Pearce J, Anderton BH. Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience. 1996;73:1145–1157. doi: 10.1016/0306-4522(96)00126-1. [DOI] [PubMed] [Google Scholar]
- 125.Lucas JJ, Hernandez F, Gomez-Ramos P, et al. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurode-generation in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luna-Munoz J, Garcia-Sierra F, Falcon V, et al. Regional conformational change involving phosphorylation of tau protein at the Thr231, precedes the structural change detected by Alz-50 antibody in Alzheimer’s disease. J Alzheimers Dis. 2005;8:29–41. doi: 10.3233/jad-2005-8104. [DOI] [PubMed] [Google Scholar]
- 127.Maeda S, Sahara N, Saito Y, et al. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer’s disease. Neurosci Res. 2006;54:197–201. doi: 10.1016/j.neures.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 128.Mah VH, Eskin TA, Kazee AM, Lapham L, Higgins GA. In situ hybridization of calcium/calmodulin dependent protein kinase II and tau mRNAs: species differences and relative preservation in Alzheimer’s disease. Brain Res Mol Brain Res. 1992;12:85–94. doi: 10.1016/0169-328X(92)90071-I. [DOI] [PubMed] [Google Scholar]
- 129.Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science. 1987;235:1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 130.Morishima-Kawashima M, Hasegawa M, Takio K, et al. Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron. 1993;10:1151–1160. doi: 10.1016/0896-6273(93)90063-W. [DOI] [PubMed] [Google Scholar]
- 131.Morishima-Kawashima M, Hasegawa M, Takio K, et al. Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- 132.Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol. 1999;58:188–197. doi: 10.1097/00005072-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 133.Murphy DB, Borisy GG. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci USA. 1975;72:2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakashima H, Ishihara T, Suguimoto P, et al. Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies. Acta Neuropathol. 2005;110:547–556. doi: 10.1007/s00401-005-1087-4. [DOI] [PubMed] [Google Scholar]
- 135.Nishi A, Snyder GL, Nairn AC, Greengard P. Role of calcineurin and protein phosphatase-2A in the regulation of DARPP-32 dephosphorylation in neostriatal neurons. J Neurochem. 1999;72:2015–2021. doi: 10.1046/j.1471-4159.1999.0722015.x. [DOI] [PubMed] [Google Scholar]
- 136.Noble W, Olm V, Takata K, et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/S0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- 137.Novak M, Jakes R, Edwards PC, Milstein C, Wischik CM. Difference between the tau protein of Alzheimer paired helical filament core and normal tau revealed by epitope analysis of monoclonal antibodies 423 and 7.51. Proc Natl Acad Sci USA. 1991;88:5837–5841. doi: 10.1073/pnas.88.13.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 139.Oddo S, Vasilevko V, Caccamo A, et al. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 140.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 141.Oliver CJ, Shenolikar S. Physiologic importance of protein phosphatase inhibitors. Front Biosci. 1998;3:D961–D972. doi: 10.2741/a336. [DOI] [PubMed] [Google Scholar]
- 142.Oyama F, Cairns NJ, Shimada H, et al. Down’s syndrome: up-regulation of beta-amyloid protein precursor and tau mRNAs and their defective coordination. J Neurochem. 1994;62:1062–1066. doi: 10.1046/j.1471-4159.1994.62031062.x. [DOI] [PubMed] [Google Scholar]
- 143.Patzke H, Tsai LH. Calpain-mediated cleavage of the cyclin-dependent kinase-5 activator p39 to p29. J Biol Chem. 2002;277:8054–8060. doi: 10.1074/jbc.M109645200. [DOI] [PubMed] [Google Scholar]
- 144.Pei JJ, Tanaka T, Tung YC, et al. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol. 1997;56:70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 145.Pei JJ, Grundke-Iqbal I, Iqbal K, et al. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer’s disease neurofibrillary degeneration. Brain Res. 1998;797:267–277. doi: 10.1016/S0006-8993(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 146.Pei JJ, Braak E, Braak H, et al. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 147.Pei JJ, Braak E, Braak H, et al. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer’s disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis. 2001;3:41–48. doi: 10.3233/jad-2001-3107. [DOI] [PubMed] [Google Scholar]
- 148.Pei JJ, Gong CX, An WL, et al. Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer’s disease. Am J Pathol. 2003;163:845–858. doi: 10.1016/S0002-9440(10)63445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pei JJ, An WL, Zhou XW, et al. P70 S6 kinase mediates tau phosphorylation and synthesis. FEBS Lett. 2006;580:107–114. doi: 10.1016/j.febslet.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 150.Perez M, Valpuesta JM, Medina M, Montejo de Garcini E, Avila J. Polymerization of tau into filaments in the presence of heparin: the minimal sequence required for tau-tau interaction. J Neurochem. 1996;67:1183–1190. doi: 10.1046/j.1471-4159.1996.67031183.x. [DOI] [PubMed] [Google Scholar]
- 151.Perez M, Hernandez F, Lim F, Diaz-Nido J, Avila J. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimers Dis. 2003;5:301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- 152.Perry G, Friedman R, Shaw G, Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci USA. 1987;84:3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Perry G, Mulvihill P, Fried VA, et al. Immunochemical properties of ubiquitin conjugates in the paired helical filaments of Alzheimer disease. J Neurochem. 1989;52:1523–1528. doi: 10.1111/j.1471-4159.1989.tb09203.x. [DOI] [PubMed] [Google Scholar]
- 154.Petrucelli L, Dickson D, Kehoe K, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 155.Planel E, Richter KE, Nolan CE, et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci. 2007;27:3090–3097. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Poorkaj P, Bird TD, Wijsman E, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 157.Poppek D, Keck S, Ermak G, et al. Phosphorylation inhibits turnover of the tau protein by the proteasome: influence of RCAN1 and oxidative stress. Biochem J. 2006;400:511–520. doi: 10.1042/BJ20060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 159.Roder HM, Eden PA, Ingram VM. Brain protein kinase PK40erk converts TAU into a PHF-like form as found in Alzheimer’s disease. Biochem Biophys Res Commun. 1993;193:639–647. doi: 10.1006/bbrc.1993.1672. [DOI] [PubMed] [Google Scholar]
- 160.Ruben GC, Iqbal K, Grundke-Iqbal I, et al. The microtubule-associated protein tau forms a triple-stranded left-hand helical polymer. J Biol Chem. 1991;266:22019–22027. [PubMed] [Google Scholar]
- 161.Run X, Liang Z, Zhang L, et al. Anesthesia induces phosphorylation of tau. J Alzheimers Dis. 2008 doi: 10.3233/JAD-2009-1003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sahara N, Murayama M, Mizoroki T, et al. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J Neurochem. 2005;94:1254–1263. doi: 10.1111/j.1471-4159.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- 163.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schapiro MB, Haxby JV, Grady CL. Nature of mental retardation and dementia in Down syndrome: study with PET, CT, and neuropsychology. Neurobiol Aging. 1992;13:723–734. doi: 10.1016/0197-4580(92)90096-G. [DOI] [PubMed] [Google Scholar]
- 165.Schmitt H, Gozes I, Littauer UZ. Decrease in levels and rates of synthesis of tubulin and actin in developing rat brain. Brain Res. 1977;121:327–342. doi: 10.1016/0006-8993(77)90155-X. [DOI] [PubMed] [Google Scholar]
- 166.Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- 167.Scott CW, Spreen RC, Herman JL, et al. Phosphorylation of recombinant tau by cAMP-dependent protein kinase Identification of phosphorylation sites and effect on microtubule assembly. J Biol Chem. 1993;268:1166–1173. [PubMed] [Google Scholar]
- 168.Sengupta A, Novak M, Grundke-Iqbal I, Iqbal K. Regulation of phosphorylation of tau by cyclin-dependent kinase 5 and glycogen synthase kinase-3 at substrate level. FEBS Lett. 2006;580:5925–5933. doi: 10.1016/j.febslet.2006.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sengupta A, Wu Q, Grundke-Iqbal I, Iqbal K, Singh TJ. Potentiation of GSK-3-catalyzed Alzheimer-like phosphorylation of human tau by cdk5. Mol Cell Biochem. 1997;167:99–105. doi: 10.1023/A:1006883924775. [DOI] [PubMed] [Google Scholar]
- 170.Shapiro BL. The Down syndrome critical region. J Neural Transm Suppl. 1999;57:41–60. doi: 10.1007/978-3-7091-6380-1_3. [DOI] [PubMed] [Google Scholar]
- 171.Shi J, Zhang T, Zhou C, et al. Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome. J Biol Chem. 2008;283:28660–28669. doi: 10.1074/jbc.M802645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shioi J, Georgakopoulos A, Mehta P, et al. FAD mutants unable to increase neurotoxic Abeta 42 suggest that mutation effects on neurodegeneration may be independent of effects on Abeta. J Neurochem. 2007;101:674–681. doi: 10.1111/j.1471-4159.2006.04391.x. [DOI] [PubMed] [Google Scholar]
- 173.Sibille N, Sillen A, Leroy A, et al. Structural impact of heparin binding to full-length Tau as studied by NMR spectroscopy. Biochemistry. 2006;45:12560–12572. doi: 10.1021/bi060964o. [DOI] [PubMed] [Google Scholar]
- 174.Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau protein by casein kinase-1 converts it to an abnormal Alzheimer-like state. J Neurochem. 1995;64:1420–1423. doi: 10.1046/j.1471-4159.1995.64031420.x. [DOI] [PubMed] [Google Scholar]
- 175.Singh TJ, Grundke-Iqbal I, McDonald B, Iqbal K. Comparison of the phosphorylation of microtubule-associated protein tau by non-proline dependent protein kinases. Mol Cell Biochem. 1994;131:181–189. doi: 10.1007/BF00925955. [DOI] [PubMed] [Google Scholar]
- 176.Singh TJ, Wang JZ, Novak M, et al. Calcium/calmodulin-dependent protein kinase II phosphorylates tau at Ser-262 but only partially inhibits its binding to microtubules. FEBS Lett. 1996;387:145–148. doi: 10.1016/0014-5793(96)00485-1. [DOI] [PubMed] [Google Scholar]
- 177.Sironi JJ, Yen SH, Gondal JA, et al. Ser-262 in human recombinant tau protein is a markedly more favorable site for phosphorylation by CaMKII than PKA or PhK. FEBS Lett. 1998;436:471–475. doi: 10.1016/S0014-5793(98)01185-5. [DOI] [PubMed] [Google Scholar]
- 178.Sloboda RD, Rudolph SA, Rosenbaum JL, Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci USA. 1975;72:177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Spillantini MG, Murrell JR, Goedert M, et al. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Spittaels K, Van den Haute C, Van Dorpe J, et al. Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J Biol Chem. 2000;275:41340–41349. doi: 10.1074/jbc.M006219200. [DOI] [PubMed] [Google Scholar]
- 181.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/S0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 182.Steiner B, Mandelkow EM, Biernat J, et al. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 1990;9:3539–3544. doi: 10.1002/j.1460-2075.1990.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Su B, Wang X, Drew KL, et al. Physiological regulation of tau phosphorylation during hibernation. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05294.x. (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tanaka T, Zhong J, Iqbal K, Trenkner E, Grundke-Iqbal I. The regulation of phosphorylation of tau in SY5Y neuroblastoma cells: the role of protein phosphatases. FEBS Lett. 1998;426:248–254. doi: 10.1016/S0014-5793(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 185.Tanimukai H, Grundke-Iqbal I, Iqbal K. Up-regulation of inhibitors of protein phosphatase-2A in Alzheimer’s disease. Am J Pathol. 2005;166:1761–1771. doi: 10.1016/S0002-9440(10)62486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tatebayashi Y, Haque N, Tung YC, Iqbal K, Grundke-Iqbal I. Role of tau phosphorylation by glycogen synthase kinase-3beta in the regulation of organelle transport. J Cell Sci. 2004;117:1653–1663. doi: 10.1242/jcs.01018. [DOI] [PubMed] [Google Scholar]
- 187.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510X(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 188.Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- 189.Tsujio I, Zaidi T, Xu J, et al. Inhibitors of protein phosphatase-2A from human brain structures, immunocytological localization and activities towards dephosphorylation of the Alzheimer type hyperphosphorylated tau. FEBS Lett. 2005;579:363–372. doi: 10.1016/j.febslet.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 190.Ulitzur N, Rancano C, Pfeffer SR. Biochemical characterization of mapmodulin, a protein that binds microtubule-associated proteins. J Biol Chem. 1997;272:30577–30582. doi: 10.1074/jbc.272.48.30577. [DOI] [PubMed] [Google Scholar]
- 191.van Leeuwen FW, Hol EM, Fischer DF. Frameshift proteins in Alzheimer’s disease and in other conformational disorders: time for the ubiquitin-proteasome system. J Alzheimers Dis. 2006;9:319–325. doi: 10.3233/jad-2006-9s336. [DOI] [PubMed] [Google Scholar]
- 192.Vandebroek T, Terwel D, Vanhelmont T, et al. Microtubule binding and clustering of human Tau-4R and Tau-P301L proteins isolated from yeast deficient in orthologues of glycogen synthase kinase-3beta or cdk5. J Biol Chem. 2006;281:25388–25397. doi: 10.1074/jbc.M602792200. [DOI] [PubMed] [Google Scholar]
- 193.Vandebroek T, Vanhelmont T, Terwel D, et al. Identification and isolation of a hyperphosphorylated, conformationally changed intermediate of human protein tau expressed in yeast. Biochemistry. 2005;44:11466–11475. doi: 10.1021/bi0506775. [DOI] [PubMed] [Google Scholar]
- 194.von Bergen M, Friedhoff P, Biernat J, et al. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc Natl Acad Sci USA. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.von Lindern M, van Baal S, Wiegant J, et al. Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: characterization of the set gene. Mol Cell Biol. 1992;12:3346–3355. doi: 10.1128/mcb.12.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wagner U, Utton M, Gallo JM, Miller CC. Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J Cell Sci. 1996;109(Pt 6):1537–1543. doi: 10.1242/jcs.109.6.1537. [DOI] [PubMed] [Google Scholar]
- 197.Walaas SI, Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev. 1991;43:299–349. [PubMed] [Google Scholar]
- 198.Wang JZ, Grundke-Iqbal I, Iqbal K. Restoration of biological activity of Alzheimer abnormally phosphorylated tau by dephosphorylation with protein phosphatase-2A, -2B and -1. Brain Res Mol Brain Res. 1996;38:200–208. doi: 10.1016/0169-328X (95)00316-K. [DOI] [PubMed] [Google Scholar]
- 199.Wang JZ, Grundke-Iqbal I, Iqbal K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer’s disease. Nat Med. 1996;2:871–875. doi: 10.1038/nm0896-871. [DOI] [PubMed] [Google Scholar]
- 200.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J NeuroSci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 202.Wang JZ, Wu Q, Smith A, Grundke-Iqbal I, Iqbal K. Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett. 1998;436:28–34. doi: 10.1016/S0014-5793(98)01090-4. [DOI] [PubMed] [Google Scholar]
- 203.Wang X, Blanchard J, Li B, Fan G, Liu F, Linden RM, Clement N, Kohlbrenner E, Radu A, Grundke-Iqbal I, Iqbal K. Generation of a non-transgenic rat model of Alzheimer-like abnormal hyperphosphorylation of tau. Abstract presented at the 11th international conference on Alzheimer’s disease (ICAD); Chicago, IL, USA. 26–31 July, 2008.2008. [Google Scholar]
- 204.Wegiel J, Wisniewski HM, Dziewiatkowski J, Popovitch ER, Tarnawski M. Differential susceptibility to neurofibrillary pathology among patients with Down syndrome. Dementia. 1996;7:135–141. doi: 10.1159/000106868. [DOI] [PubMed] [Google Scholar]
- 205.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wischik CM, Bentham P, Wischik DJ, Seng KM. Tau aggregation inhibitor (TAI) therapy with rember arrests disease progression in mild and moderate Alzheimer’s disease over 50 weeks. Alzheimers Dement. 2008;4:167. doi: 10.1016/j.jalz.2008.05.438. [DOI] [Google Scholar]
- 207.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 208.Wittmann CW, Wszolek MF, Shulman JM, et al. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 209.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Woods YL, Cohen P, Becker W, et al. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem J. 2001;355:609–615. doi: 10.1042/bj3550609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang L, Ksiezak-Reding H. Ubiquitin immunoreactivity of paired helical filaments differs in Alzheimer’s disease and corticobasal degeneration. Acta Neuropathol. 1998;96:520–526. doi: 10.1007/s004010050928. [DOI] [PubMed] [Google Scholar]
- 212.Yang LS, Ksiezak-Reding H. Calpain-induced proteolysis of normal human tau and tau associated with paired helical filaments. Eur J Biochem. 1995;233:9–17. doi: 10.1111/j.1432-1033.1995.009_1.x. [DOI] [PubMed] [Google Scholar]
- 213.Yoshida M. Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology. 2006;26:457–470. doi: 10.1111/j.1440-1789.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 214.Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 215.Yuzwa SA, Macauley MS, Heinonen JE, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 216.Zilka N, Filipcik P, Koson P, et al. Truncated tau from sporadic Alzheimer’s disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett. 2006;580:3582–3588. doi: 10.1016/j.febslet.2006.05.029. [DOI] [PubMed] [Google Scholar]