Abstract
Nanogels are swollen nano-sized networks composed of hydrophilic or amphiphilic polymer chains, which can be non-ionic or ionic. They are developed as carriers for drug delivery and can be designed to spontaneously absorb biologically active molecules through formation of salt bonds, hydrogen bonds, or hydrophobic interactions. Polyelectrolyte nanogels can readily incorporate oppositely charged low molecular mass drugs or biomacromolecules such as oligonucleotides, siRNA, DNA and proteins, which bind with the nanogel ionic chains and phase separate within the finite nanogel volume. As a result, the loading capacity of such nanogels is superior to most other drug carriers. Binding of the drugs induces collapse of nanogel, which usually decreases the volume by at least one order of magnitude. However, the drug-nanogel particles remain dispersed due to the lyophilizing effect of nanogel’s hydrophilic polymer chains, such as poly(ethylene glycol) exposed at the particle surface. Multiple chemical functionalities of nanogels can be employed for introduction of imaging labels, targeting molecules and triggered drug release capabilities such as stimuli-responsive and degradable cross-links. Recent studies suggested several promising biomedical applications of nanogels, including drug delivery of phosphorylated nucleoside analogs, oligonucleotides or siRNA for anticancer or antiviral treatment, encapsulation of bioactive proteins, fabrication of nanometallic or nanoceramic composites, imaging agents, oral and CNS drug delivery. The research of different functional nanogels as novel pharmaceutical carriers for diagnosis and therapy shows promise and is rapidly developing.
1. Introduction
The term “nanogels” usually defines aqueous dispersions of hydrogel particles formed by physically or chemically cross-linked polymer networks of nanoscale size. We introduced this term to define the swollen chemically cross-linked networks of cationic and neutral polymers, e.g., branched polyethylenimine (PEI) and poly(ethylene glycol) (PEG) (PEG-cl-PEI), initially designed for delivery of antisense oligonucleotides.[1, 2] However, prior to our work the group of J. Sunamoto described the phenomenon of physical cross-linking (self-assembly) of cholesterol-modified polysaccharides (e.g., pullulan, mannan, amilopectin and dextran), which resulted in formation of swollen hydrogels of nanoscale size;[3] a review on this type of systems was recently published.[4] Technically, these systems are also nanogels and we will refer to them as nanogels below. All together, nanogels are very promising as drug delivery carriers due to their high loading capacity, high stability, and responsiveness to environmental factors, such as ionic strength, pH, and temperature that are unprecedented for common pharmaceutical nanocarriers. Since the first review on synthesis and application of nanogels was published in 2002,[5] this novel family of nanoscale materials has attracted increasing attention for the delivery of drugs, biomacromolecules and imaging agents. The present paper provides an updated overview of pharmaceutical use and prospective of nanogels. We also recommend recent reviews for additional reference on synthesis and applications of nanogels.[6, 7]
Unloaded nanogels in a swollen state contain considerable amount of water. Loading of biological agent(s) is usually achieved spontaneously through electrostatic, van-der Waals and/or hydrophobic interactions between the agent and the polymer matrix. As a result, nanogels collapse forming stable nanoparticles, in which biological agent becomes entrapped. The aggregation of nanogels can be prevented by introducing dispersing hydrophilic polymers, such as poly(ethylnene glycol) (PEG) in their structure. During the collapse of the drug-nanogel complex such polymers become exposed to the surface and form a protective hydrophilic layer around the nanogel that prevents phase-separation. The functional groups at the nanogel surface can be additionally modified with various targeting moieties for site specific drug delivery in the body. Various nanogels were shown to deliver their payload inside cells and across biological barriers. Such nanogels exhibit high stability and protect biological agents from degradation by cell’s metabolic systems. Overall nanogels demonstrate excellent potential for systemic drug delivery and enhancing oral and brain bioavailability of low molecular drugs and biomacromolecules.
2. Manufacturing of Nanogel Networks
Current approaches used for preparation of nanogels can be divided in (i) physical self-assembly of interactive polymers; (ii) polymerization of monomers in homogeneous phase or micro- or nano-heterogeneous environment; (iii) chemical cross-linking of preformed polymers and (iv) template-assisted nanofabrication of nanogels particles. These methods are illustrated in Figures 1-4 and described below.
Figure 1.

Physical self-assembly of nanogels in aqueous media. (a) Aggregation of hydrophobically-modified polymer, cholesterol-pullulan, in the presence of insulin molecules results in nanogels containing entrapped protein. (b) Mixing of lauryl-modified dextran and β-cyclodextrin polymer results in formation of nanogels stabilized through the guest-host binding of the lauryl and β-cyclodextrin moieties.
Figure 4.

Photolithographic technique PRINT uses non-wetting elastomeric perfluoropolyether molds to produce monodisperse, shape-specific nanoparticles from various organic precursors.
The physical self-assembly of polymers was used by several groups to produce various nanogels. This method usually involves controlled aggregation of hydrophilic polymers capable of hydrophobic or electrostatic interactions and/or hydrogen bonding with each other. The preparation of nanogels is conducted in mild conditions and in aqueous media. Self-associating hydrophilic polymers allow encapsulation of biomacromolecules and are useful for preparation of protein-loaded nanogels. For example, Akiyoshi et al. prepared hydrogels by hydrophobic association of cholesterol-modified pullulan in presence of insulin (Figure 1 a).[8] The nanogels formed in a narrow range of cholesterol to sugar units ratio (1:40-1:100). They had a diameter of 20-30 nm and contained up to five insulin molecules per one particle. The sizes of self-assembled nanogels are controlled by proper selection of the concentration of polymers and environmental parameters, such as pH, ionic strength, and temperature. For example, Yu et al. prepared protein nanogels by temperature-induced gelation of oppositely charged proteins, such as ovalbumin and lysozyme or ovotransferrin.[9] Similarly, nanogels were obtained by pH- and temperature-induced gelation of chitosan and ovalbumin.[10] A work by Gref et al. described self-assembly of nanogels of various sizes by association of a lauryl-modified dextran and β-cyclodextrin polymer in aqueous media (Figure 1 b).[11] Nanogels of about 120-150 nm were obtained using a broad range of the concentration of the two polymers. The resulting nanogels were stable and withstood freeze-drying, which was found to be a convenient method for their long-time storage.
Chemical synthesis in heterogeneous colloidal environments generally can provide broad capabilities for variations in nanogel structure and properties. Few studies used inverse microemulsions (w/o) as a media for polymerization of monomers with bifunctional monomers added as cross-linkers to ensure formation of stable nanoscale networks (Figure 2 a). Speiser et al. was the first to carry out copolymerization of monomers solubilized in reverse micelles.[12] Their approach was extended by Levashov and colleagues to covalently immobilize enzymes in polymeric nanogranules of acrylamide and N,N-methylene bisacrylamide copolymers.[13] Subsequently, DeSimone et al. prepared cationic nanogels by inverse microemulsion polymerization in heptane of 2-acryloxyethyltrimethylammonium chloride and 2-hydroxyethylacrylate using PEG-bisacrylate as a bifunctional cross-linker (this nanogel is designated below as PAETMAC).[14, 15] Anionic nanogels with the particle hydrodynamic diameters as small as 50 nm were also prepared by copolymerization of poly(dimethylacrylamide-co-2-acrylamido-2-methyl-1-propanesulfonic acid) and N,N-methylene bisacrylamide cross-linker in inverse microemulsion.[16] Labile bonds are frequently introduced into nanogels during polymerization to make them degradable and facilitate drug release. For example, Frechet and co-workers reported free radical polymerization in the inverse emulsion to prepare degradable acrylamide-based nanogels containing acid-labile acetal cross-linkers for protein, antigen and DNA delivery.[17] Such acetal cross-linker is stable at neutral pH t1/2 24h), but rapidly hydrolyzes at acidic endosomal pH (t1/2 5 min), which results in nanogel degradation and facilitates the release of the payload.[18, 19] Matyjaszewski group used atom transfer radical polymerization (ATRP) in inverse microemulsion for synthesis of stable cross-linked nanogels of water-soluble polymers.[20] A disulfide-functionalized cross-linker was used in their work to synthesize biodegradable nanogels. The disulfide groups are stable in extracellular environment but cleave inside cells due to presence of glutathione. This also may facilitate the release of the nanogel payload inside the cells. Matyjaszewski and Kataoka further extended this approach to synthesize biodegradable, cross-linked poly[oligo(ethylene oxide)-methyl methacrylate] nanogels.[21]
Figure 2.

Chemical synthesis of nanogels by copolymerization in colloidal environments. (a) Copolymerization of monomers (1) and bifunctional cross-linkers (2) in w/o microemulsions stabilized by surfactants (3) produces nanogels that can be then transferred in aqueous media after removal of surfactants and organic solvent. (b) Copolymerization reactions can be also carried out in o/w emulsions that can be additionally stabilized by surfactants.
Polymerization reactions resulting in formation of nanogels can be also carried out in o/w emulsions or aqueous suspensions (Figure 2 b). Furthermore, the polymerization can be started in a homogeneous aqueous solution of water soluble monomers and result in formation of the colloidal suspension of a growing polymer. For example, Peppas et al. synthesized a nanosphere suspension composed of PEG-grafted poly(methacrylic acid) using a UV-initiated solution/precipitation polymerization method in water.[22]
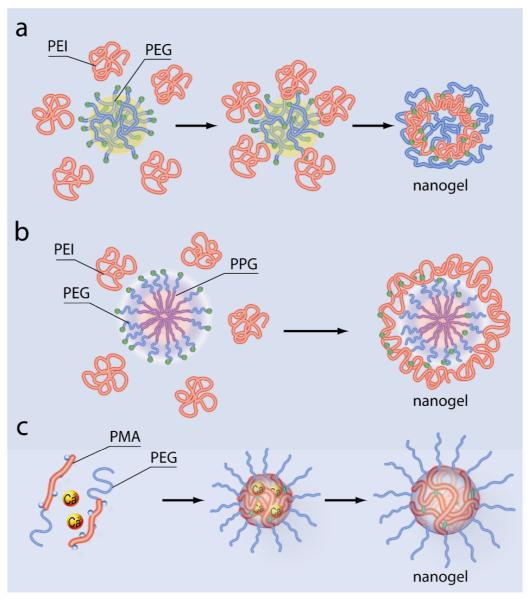
In addition to approaches based on polymerization of monomers the covalent cross-linking of preformed polymer chains provide excellent opportunities for producing nanogels with large pore sizes.[23] The cross-linking method was widely applied to synthesize a variety of functional nanogels for drug delivery. In particular, it was used to synthesize the first cross-linked cationic nanogel for polynucleotide delivery.[1] In this case a bis-activated PEG was conjugated to a branched PEI in an o/w emulsion (dichloromethane-in-water), followed by evaporation of the solvent in vacuo and maturation of the nanogel in aqueous solution (Figure 3 a). Cationic PEI-containing nanogels of 80-200 nm were also obtained by photo-Fenton reaction in aqueous media.[24, 25] Small (40-45 nm) non-toxic cross-linked pullulan nanogels were prepared in the reverse micellar system (Aerosol OT/hexane).[26, 27] Like in the case of polymerization reactions the cross-links connecting polymeric chains in such nanogels can be made degradable. For example, a biodegradable segmented PEI connected by disulfide linkers was used to prepare cationic nanogels for polynucleotide delivery with reduced toxicity.[28, 29] Another study fabricated hyaluronic acid nanogels containing biodegradable disulfide linkages by inverse w/o emulsion method.[30] An interesting type of cross-linked nanogels containing DNA was obtained by mixing thiol-functionalized six-arm branched PEG and DNA in dimethyl sulfoxide, which produced 100 nm particles. Such nanoparticles were then cross-linked by oxidation to obtain DNA-loaded biodegradable nanogels.[31]
Figure 3.
Synthesis of nanogels by cross-linking of the preformed polymer chains or self-assembled polymeric aggregates. (a) Cross-linking of double-end activated PEG and PEI chains in o/w emulsion followed by evaporation of the organic solvent. (b) Conjugation of PEI to double end activated Pluronic block copolymer (PEG-PPG-PEG), which is self-assembled in polymeric micelles in aqueous solution, results in nanogels containing hydrophobic PPG domains and cross-linked PEI-PEG network. (c) PEG-b-PMA diblock copolymer is condensed in presence of divalent metal cations in aqueous solution into a micelle with a polyion-metal core and PEG corona. This is followed by cross-linking of the micelle core and removal of the condensing metal cations, which results in nanogels with cross-linked polyanion (PMA) core and PEG corona.
Unprecedented opportunities for the control of the spatial distribution of polymeric chains at the nanoscale are provided by combination controlled self-assembly of polymeric micelles and cross-linking techniques. For example, Wooley et al. have chemically linked the shell layers of polymeric micelles to obtain various shell-cross-linked nanoparticles.[32-37] By adjusting factors that affect the morphology, such as solvents and organic counterions, they produced an array of different cross-linked nanostructures including spheres, rods and even toroids. In another study, PEI was cross-linked in aqueous solution to the micelles of bis-activated Pluronic block copolymer (PEG-PPG-PEG, where PPG stands for poly(propylene glycol)) (Figure 3 b).[38] This produced nanogels with hydrophobic PPO core surrounded by swollen cross-linked PEI and PEO shell. Furthermore, Lee et al. photo-cross-linked the polymeric micelles of poly(D,L-lactic acid)-PEG-poly(D,L-lactic acid) end-modified by acrylate.[39] As a result, nanogels were formed, which contained self-assembled hydrophobic domains of micelles with insoluble poly(D,L-lactic acid) cores. These domains additionally loaded with a hydrophobic anticancer drug.
A unique control of spatial distribution of polymer chains in nanogel was achieved by Bronich and coworkers.[40] Their work developed a procedure, in which polyion complex micelles were initially prepared by self-assembly of ionic blocks of double hydrophilic block copolymers with an oppositely charged condensing agent, followed by chemical cross-linking of ionic blocks in the core and removal of the condensing agent (Figure 3 c). The resulting nanogels made of PEG-b-poly(methacrylic acid) (PEG-b-PMA) diblock copolymers contained a hydrophilic PEG shell and a cross-linked hydrophilic PMA ionic core, which swell in water and incorporated hydrophilic drugs.[41] Similar technique, was also used to prepare core-shell nanogels by condensation and cross-linking of PEG-grafted poly(acrylic acid) (PEG-g-PAA).[40]
Finally, DeSimone et al. developed a novel method for fabrication of polymeric particles on the order of tens of nanometers to several microns, which can be used for the synthesis of nanogels.[42] This imprint photolithographic technique called “PRINT” (Particle Replication In Non-wetting Templates) uses non-wetting elastomeric molds of a low surface energy perfluoropolyether network prepared on patterned silicon master templates by photochemical cross-linking of dimethacrylate-functionalized perfluoropolyether oligomers. The non-wetting molds eliminate the formation of a residual interconnecting film between molded objects, thus allowing the production of monodisperse, shape-specific nanoparticles from an extensive array of organic precursors (Figure 4). This method enables strict control over particle size, shape, composition, and surface functionality, and permits the loading of delicate cargos including pharmaceutical drugs and biomacromolecules. For example, monodisperse 200 nm PEG-based swellable particles were fabricated using PRINT methodology by UV-induced copolymerization of several vinyl-monomers such as PEG triacrylate, PEG monomethyl ether monomethacrylate, and p-hydroxystyrene.[43]
Overall, many studies used one of the basic procedures described above to prepare increasingly sophisticated types of nanogels. For example, a nanostructured thermosensitive hydrogel based on interpenetrating network of poly-(N-isopropylacrylamide) (PNIPAAm) was immobilized on porous silicagel and hydroxyapatite.[44, 45] Richtering and co-workers used layer-by-layer deposition of polyelectrolyte multilayers at the surface of an anionic poly-(N-isopropylacrylamide-co-methacrylic acid) nanogel to prepare thermosensitive core-shel materials.[46, 47] The layer-by-layer deposition can be used to introduce various materials such asa magnetic nanoparticles in the surface of the nanogels.[48] Another interesting type of hybrid nanogels covered by a lipid bilayer was recently introduced by K. Levon and coworkers.[49, 50] Their work prepared liposomes loaded with a water-soluble monomer, which was then polymerized by photoinitiation to form a network of poly(acrylamide/N,N-methylene bisacrylamide) within the liposome inner cavity. More recently Yan et al. produced catalytic nanogels encapsulating horse radish peroxidase or bovine carboxyanhydrase by polymerization of poly(acrylamide/N,N-methylene bisacrylamide) on a surface of single enzyme molecule.[51, 52] The enzymes maintained catalytic activity and exhibited high thermal stability due to immobilization within the nanogels particles. In other reports PEGylated nanogels embedded with metal nanoparticles were synthesized by Oishi et al..[53, 54] Such hybrid nanogels containing Pt nanoparticles (<2 nm) have shown catalytic activity which is dependent of pH. They may be used as scavengers of reactive oxygen species in biology and medicine. The nanogels containing Au nanoparticles (ca. 6 nm), exhibited a shift of the surface plasmon resonance (SPR) band in response to pH alteration. Such nanogels may be adopted as SPR probes in various types of sensors. Finally, the photochemical emulsion-free polymerization in aqueous solution on the surface of superparamagnetic ferrous oxide nanoparticles was used for preparation of composite superparamagnetic nanogels[55, 56] Such nanomaterials may be used for microwave ablation therapy.[57] Future developments in preparation of chemically or physically cross-linked nanogels may include the use of micropflidic techniques, which have shown promise in preparation of micron-sized hydrogels and nanomaterials of different sizes, shapes and morphologies.[58, 59]
3. Chemical Modification of Nanogels for Site-Specific Drug Delivery
Nanocarriers can be delivered to disease-affected sites after injection in the body fluids. Major impediments to this delivery strategy include (i) interaction of nanocarriers with serum proteins resulting in opsonisation or agglutination; (ii) clearance of nanocarriers by reticuloendothelial system (RES) or through kidney glomerules, and (iii) non-specific organ accumulation. To reduce interaction with serum proteins and extend circulation time, nanocarrier surface is often modified with hydrophilic inert polymers, such as PEG.[60] For example, drug-loaded PEG-cl-PEI nanogels have core-shell architecture with the core surrounded by PEG chains.[61] Similarly, PEG chains can be tethered to polymethacrylate nanogels during the emulsion polymerization procedure.[62] Nanogels with cross-linked cores and PEG corona can be produced by polyion core micelles self-assembly followed by the cross-linking of the cores.[40] A recent study characterized in vivo pharmacokinetics and biodistribution of PEG-based cross-linked nanogel cylinders (approx. 200 nm) obtained by PRINT technique.[43] The particles were relatively rapidly cleared from the blood (t1/2 = 17 min) and accumulated in the liver. This result was not surprising since nanogels contained relatively short PEG chains (ca. 1 kDa), while the optimal coating for long circulating nanoparticles should include PEG of 2 to 5 kDa.[63]
Nanogel “surface” can be also decorated with biospecific targeting groups, which can enhance site-specific delivery of the nanogels in the body. For example, we described biotinylated PEG-cl-PEI nanogels that were vectorized via (strepta)avidin by biotinylated ligands (transferrin or insulin).[64] Biotin groups were also attached to OH-functionalized poly[oligo(ethylene oxide)-methyl methacrylate] nanogels.[21] However, biotin-(strepta)avidin conjugation is not practical because of biological activities of biotin. Hence, it is preferable to directly conjugate targeting groups to nanogels. For example, 1% to 5% of primary amino groups in PEG-cl-PEI nanogels were modified with folic acid using carbodiimide activation.[61] These folate-modified nanogels exhibited a noticeable increase of the transport across an in vitro gastrointestinal model barrier (Caco-2 cell monolayers). In another study, polymethacrylate microgels modified with folate demonstrated increased and selective cellular uptake in cancer cells overexpressing folate receptors (FR).[65] To reduce problems associated with folate accessibility to its cellular receptors, several authors recommended insertion of a polymer linker, e.g. PEG between the folate moiety and drug carrier.[66] For example, poly(aminoPEG-cyanoacrylate-co-hexadecyl cyanoacrylate) nanogel was modified with folic acid via terminal amino groups of PEG.[67] Such folate decorated nanogels also demonstrated an enhanced accumulation in FR-overexpressing cancer cells.
Nanogels were also conjugated with human transferrin (hTf), one of the tumor-targeting proteins.[68] In this method, amino groups in hTf were first reacted with a heterofunctional reagent, sulfosuccinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate in aqueous medium, to obtain a maleimide derivative of hTf. Second, thiol groups were introduced into PEG-cl-PEI nanogels by the reaction with 2-iminothiolane (Trout’s reagent). Finally, reaction between maleimide-hTf and thiol-nanogels led to formation of hTf-decorated nanogels with a controlled number of hTf molecules (from 4 to 12) per particle. Because of its large size conjugated hTf was exposed at the nanogel surface, which facilitated its interaction with cellular transferrin receptors. Additionally, peptide ligands can be attached to nanogels through a bifunctional PEG linker.[28] For example, a peptide with terminal cystein was conjugated to maleimide-PEG-N-hydroxysuccinimide linker. Isolated product was then reacted with amino groups of PEI to obtain a decorated nanogel with a required peptide density. Finally, a mono-N-acetylcystamine-PEG linker was introduced into nanogels via 1,1’-carbonyldiimidazole activation; the thiol groups on nanogels were unmasked by dithiothreitol and reacted with the thiol-specific (e.g. maleimide) derivatives to yield the protein/peptide-modified nanogels.[69] All together, nanogels provide multiple possibilities for surface decoration with various targeting groups. Initial evidence suggests that these approaches can be used for delivery of nanogels towards selected cellular receptors.
4. Swelling - the Principal Property of Nanogels
Nanogels are soft nanomaterials. Swelling of nanogels in aqueous environment is controlled by both (i) nanogel structure (chemical nature of polymer chains, degree of cross-linking, charge density for polyelectrolyte gels), and (ii) environmental parameters (for polyelectrolyte gels - pH, ionic strength and chemical nature of low molecular mass ions; for thermo-responsive gels - temperature) (Figure 5). It is well recognized that a balance between the osmotic pressure and the polymer elasticity determines physical dimensions of a hydrogel particle.[70] For polyelectrolyte hydrogels osmotic pressure results from the net difference in concentration of mobile ions between the interior of the gel particle and exterior solution. The ionized groups attract hydrated counterions. This favors swelling of the gel, while the conformational entropy elasticity of the polymer chains opposes this expansion. For weak polyelectrolyte gels ionization depends on pH. Reduction in the total charge and number of counterions due to pH changes results in compression of the gel due to decreased osmotic pressure to an extent when the excluded volume of the polymer chains limits further compression. For example, cross-linked PEG-b-PMA nanogels compressed as pH decreased from 9 to 5 due to protonation of carboxylate groups of PMA.[41] Likewise, PEG-cl-PEI nanogel compressed as pH increased from 8.5 to 10 due to deprotonation of PEI amino groups.[71]
Figure 5.
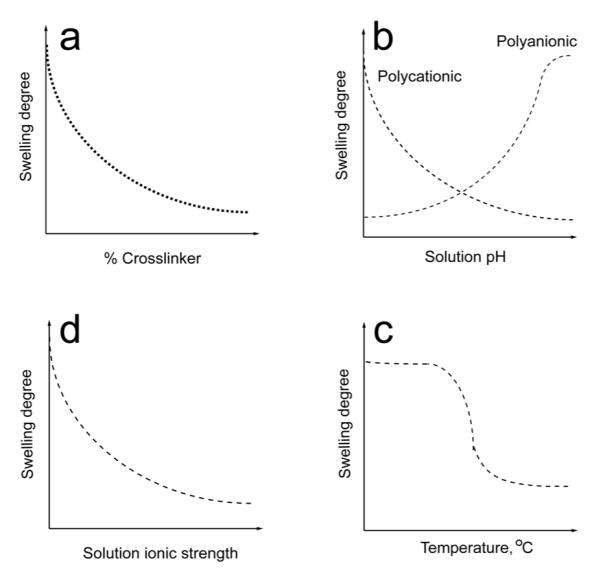
Factors affecting nanogel swelling. (a) Increase of the content of a cross-linker decreases swelling of nanogels composed of a hydrophilic polymer. (b) Increase of pH results in collapse of a nanogel composed of weak polybase chains and swelling of a nanogels composed of a weak polyacid due to deionization/ionization of these polymers. (c) Increase in the ionic strength decreases swelling of a polyelectrolyte nanogel. (d) Nanogels composed of polymers exhibiting LCST behavior collapse as the temperature increases above the LCST.
Swelling of polyelectrolyte hydrogels also depends on ionic strength. For example, at high ionic strengths, the swelling of cationic PAETMAC nanogels was governed by the cross-linker concentration, while at low ionic strengths the swelling was influenced by both the cross-linker and charge concentration.[14] For cross-linked hydrogels, as a general rule, the swelling ratio decreases as the number of cross-links increases.[14, 41] This was shown using cross-linked PEG-b-PMA nanogels as an example.[32]
Temperature, in selected cases, can affect the quality of the solvent, and, therefore, the nanogel particle size. For example, since PPG chains in Pluronic exhibit lower critical solution temperature (LCST) the Pluronic-based nanogels are temperature-responsive. For example, upon a rapid decrease of temperature below LCST small 120 nm nanogels particles were shown to swell drastically to over 400 nm.[72] Temperature sensitivity of swelling was also observed for nanogels of copolymers of N-isopropylacrylamide (NIPAAm).[45, 73] This property can be used for engineering of stimuli-responsive drug carriers. One of recent examples is glucose-sensitive phenylboronic acid-conjugated NIPAAm nanogels with swelling behavior depending on the glucose concentration.[74] One of the advantages of highly dispersed hydrogels is that they usually very rapidly respond to changing environmental conditions,[75] which facilitates incorporation and release of biological agents in pharmaceutical applications. In contrast, the swelling equilibrium for bulk hydrogels requires periods on the order of days.
In selected cases drug loading in the hydrogel can additionally reduce the volume. One reason for that is the drug interaction with the hydrogel chains via electrostatic binding, hydrophobic interactions and/or hydrogen bond formation, which decreases “solubility” of the hydrogel chains and results in the gel condensation and collapse. All together, the swelling and collapse properties of nanogels are unique and important for optimizing the drug loading and release described in the following section.
5. Nanogel Loading with Drug and Drug Release
Biological agents can be incorporated in nanogels by (i) physical entrapment, (ii) covalent conjugation, or (iii) controlled self-assembly. Physical entrapment was employed for incorporation of insulin in cholesterol-modified pullulan nanogels[8] and siRNA in thiol-conjugated hyaluronic acid nanogels.[30]. Physical drug entrapment can be also achieved by complexation of poly-L-lactide- and poly-D-lactide-grafted dextrans into monodisperse biodegradable nanogel particles with an average diameter of 70 nm.[76] Such nanogel contains hydrophilic dextran chains joined by partially crystallized hydrophobic regions of non-covalently bound chains of polylactide stereisomers. In addition, hydrophobic molecules can be solubilized into hydrophobic domains presented in some nanogels. For example, prostaglandin E2 was solubilized in nanogels of cholesterol-modified pullulan.[77] Doxorubicin was also loaded in amphiphilic cross-linked nanogels based on Pluronic F127[78] or poly[oligo(ethylene oxide)-methyl methacrylate].[21] Notably, in most cases loading due to hydrophobic interactions alone results in relatively low loading capacities.
An example of a covalent attachment of biological agent to nanogels is the loading of cisplatin in carboxyl group containing core-shell nanogels made of cross-linked PEG-b-PMA.[79, 80] Such nanogels have a cross-linked polyanion (PMA) core and nonionic polymer (PEG) corona. In aqueous solutions cisplatin reacts with the carboxylic groups in the nanogels core, which leads to formation a condensed drug-loaded core. Modification of enzyme molecules with N-hydroxysuccinimidoacrylate followed by polymerization of acrylamide in dilute aqueous solutions was used to obtain protein-encapsulating polyacrylamide nanogels.[51, 52] Alternatively, polyacrylamide nanogels incorporating modified alpha-chymotrypsin were prepared by copolymerization in inverse microemulsion.[13] Such nanogels containing covalently bound proteins can increase protein thermostability, and shelf life.[56]
A different approach based on controlled self-assembly of polyelectrolyte-based nanogels with oppositely charged solutes can produce nanomaterials with high content of biological agents. Various polyelectrolyte-based nanogels were shown to interact with oppositely charged surfactants, synthetic polyions, polynucleotides and proteins in aqueous solutions.[1, 69, 71, 81, 82] The self-assembly of such materials is usually characterized by high binding cooperativity and efficiency. For example, binding of an anionic surfactant, such as sodium tetradecyl sulfate, with cationic PEG-cl-PEI nanogels has an onset at a “critical association concentration” (cac), which is 2 orders of magnitude lower than the critical micelle concentration (cmc) of this surfactant alone.[71] Such drastic decrease in cac compared to cmc is explained by cooperative stabilization of surfactant aggregates as a result of electrostatic interactions of the surfactant head groups with PEI chains of the nanogel. Charged and amphiphilic biological active molecules such as sodium oleate, indomethacin, and retinoic acid were also incorporated into PEG-cl-PEI nanogels.[71] For example, retinoic acid-loaded nanogels formed nanosized dispersions stable at physiological pH and ionic strength, which can be lyophilized, stored, and then re-dispersed. Similar formulation of an antiepileptic drug, valproic acid in PEG-cl-PEI nanogels was also obtained and investigated in vitro.[69] Furthermore, hydrophobic regions of PEG-cl-PEI/surfactant complexes can serve as non-aqueous reservoirs for solubilizing water-insoluble molecules. Since a combination of polyionic and hydrophobic interactions stabilizes drug-nanogel formulations, anionic compounds bearing only few charges, e.g. 5′-triphosphates of nucleoside analogs, such as fludarabine, zidovudine, cytarabine and floxuridine, can be also efficiently loaded into cationic nanogels[29, 38, 61]. Recently, properties and prospective applications of this novel type of nanoformulations were reviewed.[83] In summary, polyelectrolyte nanogels can be a versatile platform technology for incorporation of various small drug molecules via combinations of electrostatic and hydrophobic interactions as well as hydrogen bond formation.
One of the most important features of weakly cross-linked polyelectrolyte nanogels is their ability to incorporate biomacromolecules of the opposite charge. Accommodation of biomacromolecules in hydrogels is usually hindered by effects of excluded volume and cross-linking density. However, if biomacromolecules and polymer network have opposite charges they effectively react with each other forming an interpolyelectrolyte complex. In the cases when the polyelectrolyte chains can penetrate nanogels the process develops as a frontal reaction between oppositely charged polyions and spreads from the exterior of the gel to its core.[84] As a result efficient loading of biomacromolecules can be achieved even in the case of a bulk polyelectrolyte network (Figure 6).[85] This principle has been exploited to immobilize polynucleotides in cationic nanogels. For example, cationic nanogels of PEG-cl-PEI or PAETMAC were used for incorporation of antisense oligonucleotides.[1, 14] Addition of oligonucleotide to PEG-cl-PEI nanogel dispersion at physiological pH resulted in rapid formation of polyelectrolyte complexes between the oligonucleotide and PEI. This process was accompanied by approx. 10-fold reduction of the nanogel volume (“collapse”) due to neutralization of the network’s electrostatic charges. Notably, the oligonucleotide binding with nanogel was almost complete and the nanogel loading capacity was 15 to 30 % by weight. Interestingly, the oligonucleotide loaded nanogels remained stable in the presence of negatively charged serum proteins, which can be explained by a higher cooperativity of binding of oligonucleotide chains with the polycation compared to the protein-polyion binding.[86]
Figure 6.

Loading and release of biomacromolecules in a cross-linked polymer hydrogel. A cross-linked gel composed of nonionic PEG and anionic PAA polymer chains is immersed in a solution of a cationic protein cytochrome C. The initial solution has a red color is due to the presence of the cytochrome C. The cytochrome C is spontaneously loaded in a gel due to a polyion complex formation between the protein and PAA chains. The gel collapses and acquires red color of cytochrome C while the external solution becomes clear. Acidification or addition of Ca2+ ion results in the protein release due to either deprotonation of the carboxylic groups of the PAA (acidification) or competitive binding of Ca2+ ions with the carboxylic groups of the PAA. In both cases the external solution acquired the red color of cytochrome C, while the gel further collapses.
Generally, the drug loading capacities that can be expected for hydrophilic nanogels are greater than those normally observed for other nanosized pharmaceutical carriers such as polymeric micelles, liposomes and biodegradable nanoparticles. The main reason for that is that swollen nanogels are mainly comprised of water and therefore provide for a larger cargo space for incorporation of solutes, which is important in the case of low molecular mass drugs and, especially, biomacromolecules. Furthermore, the high loading in nanogels can be achieved by self-assembly and in relatively mild conditions compared to other carriers, which is very important for preservation of biological activity of labile drugs and biomacromolecules, such as proteins and polypeptides.
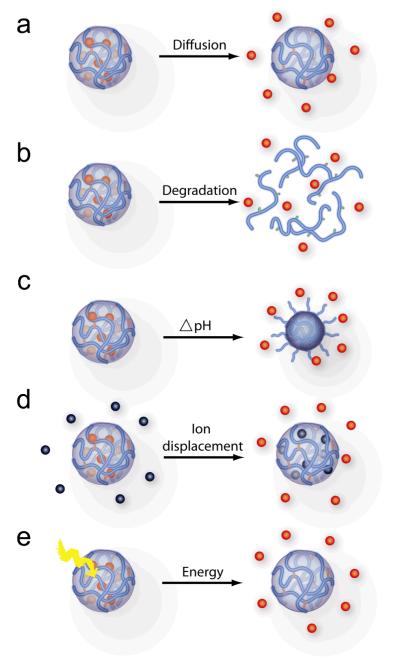
The biological agents can be released from nanogels as a result of (i) simple diffusion, (ii) nanogel degradation; (iii) a pH shift, (iv) displacement by counterions present in the environment, or (v) transitions induced by the external energy source (Figure 7). Examples include diffusion-controlled release of doxorubicin from Pluronic-based hydrogels.[87] Similar release mechanism has been employed for example in polymeric micelles that have already reached a clinical stage.[88] At the same time there is an increased interest in developing nanogels that can release biological agents in response to environmental cues at the disease site. As already discussed a change of pH or presence of reducing environment can serve as chemical signals triggering the release. For example, an acrylamide-based nanogel with acetal cross-links is stable at extracellular pH 7.4 but degrades and releases entrapped protein at pH 5.0.[17] The PEG-cl-PEI nanogels with disulfide cross-links rapidly degraded in presence of a reductive agent.[28, 29] Likewise, a poly[oligo(ethylene oxide)-methyl methacrylate] nanogel with disulfide cross-links degraded in the presence of a glutathione tripeptide commonly found in cells.[21] Degradation of these nanogels was shown to trigger the release of encapsulated solutes, rhodamine 6G and doxorubicin. In another study, dissolution of disulfide cross-linked HA nanogels and release of siRNA was induced by adding glutathione.[30] Clearly, in each case the release kinetics can be fine-tuned by altering the number of the cross-links.
Figure 7.
Drug release from nanogels. (a) Diffusion of the drug from nanogels. (b) Drug release due to degradation of biodegradable polymer chains or cross-links. (c) Change in pH results in deionization of polymer network and release of electrostatically bound drug. (d) Multivalent low-molecular cations or polyions of either charge can displace drugs having the same charge sign from electrostatic complexes with ionic nanogel. (e) Drug release can be induced by external energy applied to nanogels that induces degradation or structural transition of the nanogel polymer chains.
Polyelectrolyte hydrogels that incorporate biological agents via electrostatic bonds can also release biological agents in response to environmental changes. For example, pH-sensitive nanogels based on PAA can release an oppositely charged protein upon acidification in tumor sites or endosomal compartments.[10, 73, 89, 90] A different mechanism was proposed for release of nucleotide drugs from cationic PEG-cl-PEI nanogels.[91] In this case negatively charged biomacromolecules bound to nanogels can be displaced by negatively charged cellular components. For example, interaction of cationic nanogels with cellular membranes can trigger release of anionic 5′-triphosphates of nucleoside analogs (NATP) described in the next section.[91]
All together, the combination of the approaches presented in Figure 7 can provide very useful means for the control of the drug release characteristics of the nanogel carriers. For example, in the case of regular non-cross-linked polymeric micelles the drug release in the external environment is controlled by two factors.[92] First, is the strength of binding of the drug in the micelle core (e.g., hydrophobic binding in hydrophobic cores or electrostatic binding in polyion complex cores) characterized by drug partitioning between the external environment and the micelle. Second, is the binding of the polymer chains in the micelles to each other characterized by cmc. Both factors are considered in terms of “thermodynamic” and “kinetic stability”.[88, 93] Due to small sizes of the micelle core and shell the diffusion of drugs from the micelles is usually not rate limiting (at least for low molecular mass drugs). As a result the thermodynamic and kinetic stabilities of drug-micelle constructs are interrelated, i.e. stronger the binding is slower the release is. As a rule, polymeric micelles are less stable than liposomes or nanoparticles made of degradable polymers, hence the rates of release from the micelles are faster. The nanogel technology provides the means to fill the gap between these different carrier types. For example, by cross-linking the polymer chains in the nanogels the drug release can be decreased and by introducing cleavable cross-links it can be adjusted and made responsive to environmental changes. Furthermore, this technology can provide tools for controlling the drug release profiles. Contrary to liposomes and insoluble nanoparticles the hydrophilic nanogels swell as the drug is released, which should sustain the release of the drug from the inner layers of the nanogels as the release conversion increases. This can be used to modify or eliminate the burst release or even to achieve zero-order release kinetics of the drug from nanogels delivered to the disease site.[74, 94]
6. Delivery of Small Therapeutic Agents Using Nanogels
Significant progress was made in applications of nanogels for delivery of small therapeutic agents. For example, retinoic acid was encapsulated into PEG-cl-PEI nanogels.[71] A similar formulation of valproic acid in PEG-cl-PEI nanogels was also prepared.[69] In this case the transport of the nanogels with valproic acid across in vitro blood brain barrier (BBB) model was characterized. The permeability of nanogels loaded with valproic acid across bovine brain microvessel endothelial cells (BBMEC) monolayers was increased by at least 70% compared to a free drug, suggesting that this formulation may be useful for delivery of valproic acid to the brain. In another study, N-hexylcarbamoyl-5-fluorouracil, an anticancer prodrug of 5-fluorouracil (5-FU), was encapsulated in poly(N-isopropylacrylamide)-co-poly(N-vinylpyrrolidone) (PNIPAAm/VP) nanogels coated with polysorbate 80.[95] Drug release from this carrier demonstrated pH- and temperature dependence.[96] These nanogels were shown to accumulate in brain in rabbits. In another work an anti-leishmaniasis drug, arjunglucoside I, was also loaded in PNIPAAm/VP nanogels.[97] This formulation showed enhanced therapeutic efficacy against parasites and reduced hepatotoxicity and nephrotoxicity compared to free drug.
A promising application of nanogels involves delivery of NATPs. The nucleoside-based anticancer and antiviral agents usually enter cells via specific nucleoside transporters and then undergo intracellular activation including (i) phosphorylation into nucleoside 5′-phosphates by intracellular nucleoside kinases, (ii) formation of nucleoside 5′-diphosphates; (iii) conversion of ribonucleotides into deoxyribonucleotides by nucleoside reductases, and, finally, (iv) synthesis of NATP.[98] The latter are actual active molecules, which arrest DNA replication and transcription. Because of low efficiency of conversion of nucleosides in NATP many prospective molecules were withdrawn at preclinical or clinical stages of drug development. However, by using PEG-cl-PEI nanogels as carriers it became possible to directly deliver NATPs into cancer cells.[83] Nanogel-encapsulated NATPs demonstrated enhanced cytotoxic activities in many cancer cell lines and inhibited tumor growth in the mammary carcinoma animal model.[99] Furthermore, an antiviral NATP, 5′-triphosphorylated ribavirin in nanogel formulation exhibited higher activity in MDCK cells infected with influenza A virus compared to a non-phosphorylated analog.[29] Interestingly this formulation also demonstrated a significantly reduced mitochondrial toxicity.
7. Delivery of Biomacromolecules Using Nanogels
There are several examples of delivery of biomacromolecules using nanogels in vitro or in vivo.[1] Perhaps most remarkably, nanogels loaded with oligonucleotides were shown to cross cellular barriers. In particular, incorporation of a phosphorothioate oligonucleotide into cationic PEG-cl-PEI nanogel resulted in a drastic increase in oligonucleotide trans-cellular permeability in polarized Caco-2 cell monolayers used as an in vitro model of gastrointestinal epithelium.[1] At the same time permeability of cell monolayers with respect to 3H-mannitol, a paracellular marker, was not affected. This suggested that oligonucleotide-loaded nanogel was transported across the cells, rather than passively diffused through a paracellular route. Furthermore, in contrast to a free oligonucleotide which was essentially degraded in cells, the oligonucleotide in nanogels was protected against degradation. All together this work indicates that nanogels are promising carriers for oral drug delivery of oligonucleotides.
Moreover, another study evaluated transport of a phosphorothioate oligonucleotide encapsulated in PEG-cl-PEI nanogel across BBB.[64] This work found that oligonucleotide-nanogel complex was effectively transported across the polarized BBMEC monolayers which served an in vitro model of BBB. Permeability was further increased when the surface of the nanogel was modified with bovine transferrin or insulin. These proteins targeted specific receptors at the blood side of the brain epithelium and facilitated transport of the loaded nanogels across BBB to the brain side. Importantly, like in the previous example the oligonucleotide was transported via a transcellular pathway and remained non-degraded and incorporated in the nanogels after release at the brain side. Biodistribution studies in a mouse model further demonstrated that brain accumulation of the oligonucleotide-nanogel complex 1 h after intravenous injection was increased by nearly 15-fold compared to the free oligonucleotide. Overall, this study suggested that cationic nanogel have potential for delivery of oligonucleotides to the brain.
Similarly to oligonucleotides, a plasmid DNA immobilized in cationic nanogels can be protected from enzymatic degradation by extracellular and intracellular nucleases. Furthermore, such nanogels can be used to deliver genes into a cell. For example, transferrin-modified PEG-cl-PEI nanogel loaded with a plasmid DNA was shown to transfect cells in serum-containing medium.[69] Interestingly, the dimensions of a supercoiled DNA are comparable to those of nanogel networks, which in a swollen state have a hydrodynamic diameter between 100 and 200 nm. It is unlikely therefore that the plasmid DNA can percolate deeply into the pores of the cross-linked nanogels. A flexible cationic network is probably capable to “wrap” supercoiled DNA following the initial binding of the DNA with the charged nanogel surface.
There is an emerging interest in the drug delivery field to the pharmacological effects of polymer excipients and nanomaterials in combination with drugs.[100] In particular, synthetic polymers were shown to interact with some drug transport systems and activate selected cell signaling pathways, and this was shown to alter pharmacological, genomic and immune responses to biological agents.[79, 80] Along the similar lines Frechet and colleagues used acid-degradable nanogels for antigen presentation in vitro and vaccination in vivo.[17, 19, 101-103] They have shown that nanogels can be designed to generate immune responses when delivered to phagocytic cells of the immune system for vaccine development. For example, a plasmid DNA incorporated in nanogels with acid-labile cross-linkers induced cytokine IL-6 secretion and immunostimulation of macrophages.[18] In another study, incubation of these nanogels loaded with ovalbumin, as a model antigen, with dendritic cells derived from bone marrow resulted in enhanced presentation of ovalbumin-derived peptides.[101] It was also shown that adjuvant molecules such as CpG oligonucleotides and anti-interleukin-10 oligonucleotides can be co-delivered with the protein antigen for maximized cellular immune response.[103] All together, these studies suggest that nanogels may be useful as delivery and immune response modulating vehicles in development of the DNA and peptide vaccines.
8. Conclusions
In conclusion, nanogels form a distinct class of hydrophilic dispersed drug carriers with promising properties for encapsulation of small biologically active agents and biomacromolecules. The advantages of these systems include simplicity of formulation with the drugs, high loading capacity and stability of the resulting formulation in dispersion. The swelling and collapse properties of the nanogels are unique for the nanoscale pharmaceutical carriers and provide multiple benefits for engineering optimal drug loading and release of drugs. Nanogel networks are responsive to external environmental factors, can undergo rapid volume changes, and allow for stimuli-controlled release of encapsulated biologically active compounds including charged or hydrophobic drugs and biopolymers. Furthermore, nanogels can be chemically modified to incorporate various ligands for targeted drug delivery, triggered drug release or preparation of composite materials. Preclinical studies suggest that nanogels can be used for efficient delivery of biopharmaceuticals in cells as well as for increasing drug delivery across cellular barriers. Clearly, there is no universal delivery modality that can address all needs of current and future drug therapies. In this regard the capabilities of nanogels as well as any other class of pharmaceutical carrier are not infinite. But for human beings “infinite” often means beyond ones imagination. We certainly hope that prospects of nanogels as pharmaceutical carriers will exceed our expectations and believe that many capable scientists across the globe will contribute outstanding work to advance these novel carriers to practical use.
Acknowledgments
The authors are grateful for the support by National Institutes of Health grants CA102791 and NS050660 (to SVV) and NS36229, NS051335, CA89225, CA116591 and RR021937 (to A.V.K) Department of Defense USAMRMC 06108004 (to AVK) and National Science Foundation DMR 0513699 (to AVK). We also would like to thank Daria Alakhova and Zagit Gaymalov, the students at UNMC Pharmaceutical Sciences Graduate Program, for the help in preparation of the illustrations for this paper.
Biography
 Alexander Kabanov obtained his PhD in the chemical kinetics and catalysis at the M.V. Lomonosov State University, Moscow USSR in 1987 and DSC in biochemistry from the same university in 1990. Working in Moscow he further initiated studies using polymeric micelles, DNA/polycation complexes and other nanoscale polymeric systems for the delivery of small drugs and biomacromolecules. In 1994 he moved to the Unites States and took an appointment with University of Nebraska Medical Center (UNMC) College of Pharmacy where he is now the Parke-Davis Professor of Pharmaceutical Sciences and the Director of the Center for Drug Delivery and Nanomedicine.
Alexander Kabanov obtained his PhD in the chemical kinetics and catalysis at the M.V. Lomonosov State University, Moscow USSR in 1987 and DSC in biochemistry from the same university in 1990. Working in Moscow he further initiated studies using polymeric micelles, DNA/polycation complexes and other nanoscale polymeric systems for the delivery of small drugs and biomacromolecules. In 1994 he moved to the Unites States and took an appointment with University of Nebraska Medical Center (UNMC) College of Pharmacy where he is now the Parke-Davis Professor of Pharmaceutical Sciences and the Director of the Center for Drug Delivery and Nanomedicine.
 Serguei Vinogradov obtained his PhD in the chemistry of biologically active & natural compounds at the M.M. Shemyakin Institute of Bioorganic Chemistry of the Russian Academy of Science at 1979. After decline of the USSR he continued his research with C. Helene, CNRS Laboratory of Biophysics in Paris, N. Thuong, Center of Molecular Biophysics in Orleans (France), and with A. Kabanov, University of Nebraska Medical Center in Omaha (USA). He is now Research Professor at the UNMC College of Pharmacy and Center for Drug Delivery and Nanomedicine. His research areas are nanogel drug-delivery systems for nucleotide therapeutics, bioconjugate chemistry, targeting tumors and viral diseases, development of nanointerventions for CNS and non-viral gene therapy.
Serguei Vinogradov obtained his PhD in the chemistry of biologically active & natural compounds at the M.M. Shemyakin Institute of Bioorganic Chemistry of the Russian Academy of Science at 1979. After decline of the USSR he continued his research with C. Helene, CNRS Laboratory of Biophysics in Paris, N. Thuong, Center of Molecular Biophysics in Orleans (France), and with A. Kabanov, University of Nebraska Medical Center in Omaha (USA). He is now Research Professor at the UNMC College of Pharmacy and Center for Drug Delivery and Nanomedicine. His research areas are nanogel drug-delivery systems for nucleotide therapeutics, bioconjugate chemistry, targeting tumors and viral diseases, development of nanointerventions for CNS and non-viral gene therapy.
Contributor Information
Alexander V. Kabanov, Center for Drug Delivery and Nanomedicine and Department of Pharmaceutical Sciences, College of Pharmacy, University of Nebraska Medical Center, 986025 Nebraska Medical Center, Omaha, NE 68198-5830 (United States); Faculty of Chemistry, M.V. Lomonosov Moscow State University, 119899 Moscow, Russian Federation.
Serguei V. Vinogradov, Center for Drug Delivery and Nanomedicine and Department of Pharmaceutical Sciences, College of Pharmacy, University of Nebraska Medical Center, 986025 Nebraska Medical Center, Omaha, NE 68198-5830 (United States) vinograd@unmc.edu
References
- [1].Vinogradov SV, Batrakova EV, Kabanov AV. Coll. Surf. B: Biointerfaces. 1999;16:291–304. [Google Scholar]
- [2].Lemieux P, Vinogradov SV, Gebhart CL, Guerin N, Paradis G, Nguyen HK, Ochietti B, Suzdaltseva YG, Bartakova EV, Bronich TK, St-Pierre Y, Alakhov VY, Kabanov AV. J. Drug Target. 2000;8:91–105. doi: 10.3109/10611860008996855. [DOI] [PubMed] [Google Scholar]
- [3].Sunamoto J, Akiyoshi K. Macromolecules. 1993;26:3062–3068. [Google Scholar]
- [4].Morimoto N, Hasegawa U, Sugawara A, Yamane S, Akiyoshi K. In: Nanotechnology in Carbohydrate Chemistry. Yuassa H, editor. Transworld Research Network; Trivandrum, India: 2006. pp. 67–85. [Google Scholar]
- [5].Vinogradov SV, Bronich TK, Kabanov AV. Adv Drug Deliv Rev. 2002;54:135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- [6].Nayak S, Lyon LA. Angew Chem Int Ed Engl. 2005;44:7686–7708. doi: 10.1002/anie.200501321. [DOI] [PubMed] [Google Scholar]
- [7].Oha JK, Drumrighta R, Siegwart DJ, Matyjaszewskib K. Prog. Polym. Sci. 2008;33:448–477. [Google Scholar]
- [8].Akiyoshi K, Kobayashi S, Shichibe S, Mix D, Baudys M, Kim SW, Sunamoto J. J Control Release. 1998;54:313–320. doi: 10.1016/s0168-3659(98)00017-0. [DOI] [PubMed] [Google Scholar]
- [9].Yu S, Yao P, Jiang M, Zhang G. Biopolymers. 2006;83:148–158. doi: 10.1002/bip.20539. [DOI] [PubMed] [Google Scholar]
- [10].Yu S, Hu J, Pan X, Yao P, Jiang M. Langmuir. 2006;22:2754–2759. doi: 10.1021/la053158b. [DOI] [PubMed] [Google Scholar]
- [11].Daoud-Mahammed S, Couvreur P, Gref R. Int J Pharm. 2007;332:185–191. doi: 10.1016/j.ijpharm.2006.09.052. [DOI] [PubMed] [Google Scholar]
- [12].Speiser P. In: Reverse Micelles. Luisi PL, Straub BE, editors. Plenum Press; New York: 1984. pp. 339–346. [Google Scholar]
- [13].Khmelnitsky YL, Neverova IN, Gedrovich AV, Polyakov VA, Levashov AV, Martinek K. Eur J Biochem. 1992;210:751–757. doi: 10.1111/j.1432-1033.1992.tb17477.x. [DOI] [PubMed] [Google Scholar]
- [14].McAllister K, Sazani P, Adam M, Cho MJ, Rubinstein M, Samulski RJ, DeSimone JM. J Am Chem Soc. 2002;124:15198–15207. doi: 10.1021/ja027759q. [DOI] [PubMed] [Google Scholar]
- [15].Sahiner N, Godbey WT, McPherson GL, John VT. Colloid and Polymer Science. 2006;284:1121–1129. [Google Scholar]
- [16].Kaneda I, Sogabe A, Nakajima H. J Colloid Interface Sci. 2004;275:450–457. doi: 10.1016/j.jcis.2004.02.086. [DOI] [PubMed] [Google Scholar]
- [17].Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Frechet JM. Proc Natl Acad Sci U S A. 2003;100:4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goh SL, Murthy N, Xu M, Frechet JM. Bioconjug Chem. 2004;15:467–474. doi: 10.1021/bc034159n. [DOI] [PubMed] [Google Scholar]
- [19].Kwon YJ, Standley SM, Goh SL, Frechet JM. J Control Release. 2005;105:199–212. doi: 10.1016/j.jconrel.2005.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Oh JK, Tang C, Gao H, Tsarevsky NV, Matyjaszewski K. J Am Chem Soc. 2006;128:5578–5584. doi: 10.1021/ja060586a. [DOI] [PubMed] [Google Scholar]
- [21].Oh JK, Siegwart DJ, Lee HI, Sherwood G, Peteanu L, Hollinger JO, Kataoka K, Matyjaszewski K. J Am Chem Soc. 2007;129:5939–5945. doi: 10.1021/ja069150l. [DOI] [PubMed] [Google Scholar]
- [22].Donini C, Robinson DN, Colombo P, Giordano F, Peppas NA. Int J Pharm. 2002;245:83–91. doi: 10.1016/s0378-5173(02)00335-6. [DOI] [PubMed] [Google Scholar]
- [23].Hennink WE, van Nostrum CF. Adv Drug Deliv Rev. 2002;54:13–36. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- [24].Xu DM, Yu JH, Liu YB, Sun HW, Xu JY, Sheng KL, Yao SD, Xu YH, Lu HL. Intern J Nanosci. 2006;5:753–756. [Google Scholar]
- [25].Xu DM, Yao SD, Liu YB, Sheng KL, Hong J, Gong PJ, Dong L. Int J Pharm. 2007;338:291–296. doi: 10.1016/j.ijpharm.2007.01.050. [DOI] [PubMed] [Google Scholar]
- [26].Gupta M, Gupta AK. J Pharm Pharm Sci. 2004;7:38–46. [PubMed] [Google Scholar]
- [27].Gupta M, Gupta AK. J Control Release. 2004;99:157–166. doi: 10.1016/j.jconrel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- [28].Vinogradov SV, Kohli E, Zeman A, Kabanov AV. Polymer Prepr. 2006;47:27–28. [PMC free article] [PubMed] [Google Scholar]
- [29].Kohli E, Han HY, Zeman AD, Vinogradov SV. J Control Release. 2007;121:19–27. doi: 10.1016/j.jconrel.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee H, Mok H, Lee S, Oh YK, Park TG. J Control Release. 2007;119:245–252. doi: 10.1016/j.jconrel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- [31].Mok H, Park TG. Bioconjug Chem. 2006;17:1369–1372. doi: 10.1021/bc060119i. [DOI] [PubMed] [Google Scholar]
- [32].Ma Q, Remsen EE, Kowalewski T, Wooley KL. J Am Chem Soc. 2001;123:4627–4628. doi: 10.1021/ja0156542. [DOI] [PubMed] [Google Scholar]
- [33].Ma Q, Remsen EE, Clark CG, Jr., Kowalewski T, Wooley KL. Proc Natl Acad Sci U S A. 2002;99:5058–5063. doi: 10.1073/pnas.052653099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pochan DJ, Chen Z, Cui H, Hales K, Qi K, Wooley KL. Science. 2004;306:94–97. doi: 10.1126/science.1102866. [DOI] [PubMed] [Google Scholar]
- [35].Chen Z, Cui H, Hales K, Li Z, Qi K, Pochan DJ, Wooley KL. J Am Chem Soc. 2005;127:8592–8593. doi: 10.1021/ja050290p. [DOI] [PubMed] [Google Scholar]
- [36].Harrisson S, Wooley KL. Chem Commun (Camb) 2005:3259–3261. doi: 10.1039/b504313a. [DOI] [PubMed] [Google Scholar]
- [37].Joralemon MJ, O’Reilly RK, Hawker CJ, Wooley KL. J Am Chem Soc. 2005;127:16892–16899. doi: 10.1021/ja053919x. [DOI] [PubMed] [Google Scholar]
- [38].Vinogradov SV, Kohli E, Zeman AD. Pharm Res. 2006;23:920–930. doi: 10.1007/s11095-006-9788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee WC, Li YC, Chu IM. Macromol Biosci. 2006;6:846–854. doi: 10.1002/mabi.200600101. [DOI] [PubMed] [Google Scholar]
- [40].Bronich TK, Keifer PA, Shlyakhtenko LS, Kabanov AV. J Am Chem Soc. 2005;127:8236–8237. doi: 10.1021/ja043042m. [DOI] [PubMed] [Google Scholar]
- [41].Bontha S, Kabanov AV, Bronich TK. J Control Release. 2006;114:163–174. doi: 10.1016/j.jconrel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- [42].Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. J Am Chem Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- [43].Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, DeSimone JM. J Control Release. 2007;121:10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shin Y, Chang JH, Liu J, Williford R, Shin Y, Exarhos GJ. J Control Release. 2001;73:1–6. doi: 10.1016/s0168-3659(01)00247-4. [DOI] [PubMed] [Google Scholar]
- [45].Shin Y, Liu J, Chang JH, Exarhos GJ. Chem Commun (Camb) 2002:1718–1719. doi: 10.1039/b204703a. [DOI] [PubMed] [Google Scholar]
- [46].Wong JE, Muller CB, Laschewsky A, Richtering W. J Phys Chem B. 2007;111:8527–8531. doi: 10.1021/jp0687145. [DOI] [PubMed] [Google Scholar]
- [47].Wong JE, Díez-Pascual ML, Richtering W. Macromolecules. 2008 000, 00. [Google Scholar]
- [48].Wong JE, Gaharwar AK, Muller-Schulte D, Bahadur D, Richtering W. J Colloid Interface Sci. 2008;324:47–54. doi: 10.1016/j.jcis.2008.05.024. [DOI] [PubMed] [Google Scholar]
- [49].Kazakov S, Levon K. Curr Pharm Des. 2006;12:4713–4728. doi: 10.2174/138161206779026281. [DOI] [PubMed] [Google Scholar]
- [50].Schillemans JP, Flesch FM, Hennink WE, van Nostrum CF. Macromolecules. 2006;39:5885–5890. [Google Scholar]
- [51].Yan M, Ge J, Liu Z, Ouyang P. J Am Chem Soc. 2006;128:11008–11009. doi: 10.1021/ja064126t. [DOI] [PubMed] [Google Scholar]
- [52].Yan M, Liu Z, Lu D, Liu Z. Biomacromolecules. 2007;8:560–565. doi: 10.1021/bm060746a. [DOI] [PubMed] [Google Scholar]
- [53].Oishi M, Myagawa N, Sakura T, Nagasaki Y. Reactive & Functional Polymers. 2007;67:662–668. [Google Scholar]
- [54].Oishi M, Hayashi H, Uno T, Ishii T, Iijima M, Nagasaki Y. Macromol Chemystry and Physics. 2007;208:1172–1182. [Google Scholar]
- [55].Sun H, Yu J, Gong P, Xu D, Hong J, Zhang C, Yao S. Intern J Nanosci. 2006;5:253–258. [Google Scholar]
- [56].Hong J, Gong P, Xu D, Dong L, Yao S. J Biotechnol. 2007;128:597–605. doi: 10.1016/j.jbiotec.2006.11.016. [DOI] [PubMed] [Google Scholar]
- [57].Jordan A, Scholz R, Maier-Hauff K, van Landeghem FK, Waldoefner N, Teichgraeber U, Pinkernelle J, Bruhn H, Neumann F, Thiesen B, von Deimling A, Felix R. J Neurooncol. 2006;78:7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- [58].Xu S, Nie Z, Seo M, Lewis P, Kumacheva E, Stone HA, Garstecki P, Weibel DB, Gitlin I, Whitesides GM. Angew Chem Int Ed Engl. 2005;44:724–728. doi: 10.1002/anie.200462226. [DOI] [PubMed] [Google Scholar]
- [59].Zhang H, Tumarkin E, Peerani R, Nie Z, Sullan RM, Walker GC, Kumacheva E. J Am Chem Soc. 2006;128:12205–12210. doi: 10.1021/ja0635682. [DOI] [PubMed] [Google Scholar]
- [60].Francis GE, Delgado C, Fisher D, Malik F, Agrawal AK. J Drug Target. 1996;3:321–340. doi: 10.3109/10611869608996824. [DOI] [PubMed] [Google Scholar]
- [61].Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. J Control Release. 2005;107:143–157. doi: 10.1016/j.jconrel.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hayashi H, Iijima M, Kataoka K, Nagasaki Y. Macromolecules. 2004;37:5389–5396. [Google Scholar]
- [63].Immordino ML, Dosio F, Cattel L. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- [64].Vinogradov SV, Batrakova EV, Kabanov AV. Bioconjug Chem. 2004;15:50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nayak S, Lee H, Chmielewski J, Lyon LA. J Am Chem Soc. 2004;126:10258–10259. doi: 10.1021/ja0474143. [DOI] [PubMed] [Google Scholar]
- [66].Shiokawa T, Hattori Y, Kawano K, Ohguchi Y, Kawakami H, Toma K, Maitani Y. Clin Cancer Res. 2005;11:2018–2025. doi: 10.1158/1078-0432.CCR-04-1129. [DOI] [PubMed] [Google Scholar]
- [67].Stella B, Marsaud V, Arpicco S, Geraud G, Cattel L, Couvreur P, Renoir JM. J Drug Target. 2007;15:146–153. doi: 10.1080/10611860600935826. [DOI] [PubMed] [Google Scholar]
- [68].Vinogradov SV, Kabanov AV. Polymer Prepr. 2004;228:296. [PMC free article] [PubMed] [Google Scholar]
- [69].Vinogradov SV. Curr Pharm Des. 2006;12:4703–4712. doi: 10.2174/138161206779026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ricka J, Tanaka T. Macromolecules. 1984;17:2916–2921. [Google Scholar]
- [71].Bronich T, Vinogradov S, Kabanov AV. Nano Letters. 2001;1:535–540. [Google Scholar]
- [72].Lee SH, Choi SH, Kim SH, Park TG. J Control Release. 2007 [Google Scholar]
- [73].Varga I, Szalai I, Meszaros R, Gilanyi T. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys. 2006;110:20297–20301. doi: 10.1021/jp064282m. [DOI] [PubMed] [Google Scholar]
- [74].Zhang Y, Guan Y, Zhou S. Biomacromolecules. 2007;8:3842–3847. doi: 10.1021/bm700802p. [DOI] [PubMed] [Google Scholar]
- [75].Eichenbaum GM, Kiser PF, Simon SA, Needham D. Macromolecules. 1998;31:5084–5093. doi: 10.1021/ma970897t. [DOI] [PubMed] [Google Scholar]
- [76].Nagahama K, Mori Y, Ohya Y, Ouchi T. Biomacromolecules. 2007;8:2135–2141. doi: 10.1021/bm070206t. [DOI] [PubMed] [Google Scholar]
- [77].Kato N, Hasegawa U, Morimoto N, Saita Y, Nakashima K, Ezura Y, Kurosawa H, Akiyoshi K, Noda M. J Cell Biochem. 2007 doi: 10.1002/jcb.21160. [DOI] [PubMed] [Google Scholar]
- [78].Missirlis D, Tirelli N, Hubbell JA. Langmuir. 2005;21:2605–2613. doi: 10.1021/la047367s. [DOI] [PubMed] [Google Scholar]
- [79].Bronich TK, Bontha S, Shlyakhtenko LS, Bromberg L, Hatton TA, Kabanov AV. J Drug Target. 2006;14:357–366. doi: 10.1080/10611860600833781. [DOI] [PubMed] [Google Scholar]
- [80].Jin W, Xu P, Zhan Y, Shen Y, Van Kirk EA, Alexander B, Murdoch WJ, Liu L, Isaak DD. Drug Deliv. 2007;14:279–286. doi: 10.1080/10717540601036856. [DOI] [PubMed] [Google Scholar]
- [81].Ogawa K, Sato S, Kokufuta E. Langmuir. 2005;21:4830–4836. doi: 10.1021/la047071m. [DOI] [PubMed] [Google Scholar]
- [82].Ogawa K, Sato S, Kokufuta E. Langmuir. 2007;23:2095–2102. doi: 10.1021/la061767t. [DOI] [PubMed] [Google Scholar]
- [83].Vinogradov SV. Expert Opin Drug Deliv. 2007;4:5–17. doi: 10.1517/17425247.4.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kabanov VA, Skobeleva VB, Rogacheva VB, Zezin AB. Journal of Physical Chemistry B. 2004;108:1485–1490. [Google Scholar]
- [85].Oh KT, Bronich TK, Kabanov VA, Kabanov AV. Biomacromolecules. 2007;8:490–497. doi: 10.1021/bm060599g. [DOI] [PubMed] [Google Scholar]
- [86].Vinogradov SV, Bronich TK, Kabanov AV. Bioconjug. Chem. 1998;9:805–812. doi: 10.1021/bc980048q. [DOI] [PubMed] [Google Scholar]
- [87].Missirlis D, Kawamura R, Tirelli N, Hubbell JA. Eur J Pharm Sci. 2006;29:120–129. doi: 10.1016/j.ejps.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [88].Kabanov AV, Alakhov VY. Crit. Rev. Ther. Drug Carrier Syst. 2002;19:1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- [89].Chang C, Wang ZC, Quan CY, Cheng H, Cheng SX, Zhang XZ, Zhuo RX. J Biomater Sci Polym Ed. 2007;18:1591–1599. [PubMed] [Google Scholar]
- [90].Oishi M, Sumitani S, Nagasaki Y. Bioconjug Chem. 2007;18:1379–1382. doi: 10.1021/bc7002154. [DOI] [PubMed] [Google Scholar]
- [91].Vinogradov SV, Kohli E, Zeman AD. Mol Pharm. 2005;2:449–461. doi: 10.1021/mp0500364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kabanov AV, Nazarova IR, Astafieva IV, Batrakova EV, Alakhov VY, Yaroslavov AA, Kabanov VA. Macromolecules. 1995;28:2303–2314. [Google Scholar]
- [93].Allen C, Maysinger D, Eisenberg A. Coll. Surfaces, B: Biointerfaces. 1999;16:3–27. [Google Scholar]
- [94].Ng EY, Ng WK, Chiam SS. J Med Syst. 2008;32:85–92. doi: 10.1007/s10916-007-9110-z. [DOI] [PubMed] [Google Scholar]
- [95].Soni S, Babbar AK, Sharma RK, Maitra A. J Drug Target. 2006;14:87–95. doi: 10.1080/10611860600635608. [DOI] [PubMed] [Google Scholar]
- [96].Chen H, Gu Y, Hub Y, Qian Z. PDA J Pharm Sci Technol. 2007;61:303–313. [PubMed] [Google Scholar]
- [97].Tyagi R, Lala S, Verma AK, Nandy AK, Mahato SB, Maitra A, Basu MK. J Drug Target. 2005;13:161–171. doi: 10.1080/10611860500046732. [DOI] [PubMed] [Google Scholar]
- [98].Galmarini CM, Mackey JR, Dumontet C. Lancet Oncol. 2002;3:415–424. doi: 10.1016/s1470-2045(02)00788-x. [DOI] [PubMed] [Google Scholar]
- [99].Galmarini CM, Kohli E, Zeman AD, Warren G, Vinogradov SV. Mol Cancer Therap. 2008 doi: 10.1158/1535-7163.MCT-08-0616. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kabanov AV. Adv Drug Deliv Rev. 2006;58:1597–1621. doi: 10.1016/j.addr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kwon YJ, James E, Shastri N, Frechet JM. Proc Natl Acad Sci U S A. 2005;102:18264–18268. doi: 10.1073/pnas.0509541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kwon YJ, Standley SM, Goodwin AP, Gillies ER, Frechet JM. Mol Pharm. 2005;2:83–91. doi: 10.1021/mp0498953. [DOI] [PubMed] [Google Scholar]
- [103].Standley SM, Mende I, Goh SL, Kwon YJ, Beaudette TT, Engleman EG, Frechet JM. Bioconjug Chem. 2007;18:77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]