Abstract
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor that mediates many cellular responses to tissue hypoxia, a common feature of acute kidney injury (AKI). Here we studied 241 patients with AKI and determined the relationship to adverse outcome of a non-synonymous polymorphism in the coding region of the HIF-1α gene where a C to T substitution occurs at position þ85 in exon 12, a change known to enhance transactivation. The baseline characteristics of the patients were not different among genotype groups except for a significantly higher prevalence of shock and number of failed organs in T-allele carriers. A significant genotype–phenotype association was found for plasma levels of vascular endothelial growth factor-A but not angiopoietin-2, two downstream targets of HIF-1α. Compared to the CC genotype, T-allele carriers had significantly higher adjusted odds for dialysis requirement or in-hospital death; assisted mechanical ventilation or dialysis requirement; and the composite of assisted mechanical ventilation, dialysis requirement or in-hospital death. The trend for higher plasma angiopoietin-2 levels was associated with significantly higher adjusted odds for in-hospital death; dialysis requirement or in-hospital death; and the composite outcome of assisted mechanical ventilation, dialysis, or in-hospital death. Despite the limited cohort size, our study found this particular HIF-1α genetic variant to be associated with disease severity and adverse outcomes in AKI. Larger studies are needed to confirm these relationships.
Keywords: acute kidney injury, gene polymorphism, HIF-1α, VEGF-A, angiopoietin-2
INTRODUCTION
Acute kidney injury (AKI), as defined by a wide range of serum creatinine increments, is a consistent and powerful predictor of in-hospital mortality, and is associated with an increase in length of stay at hospital, hospital costs, and posthospitalization resource utilization.1,2 The limited oxygen supply to renal tissue renders the kidney susceptible to hypoxia, particularly the medulla, and has been long recognized as an important factor in the pathogenesis of AKI.3 Hypoxia-inducible factor-1a (HIF-1α) is the major transcription factor that mediates cellular responses to tissue hypoxia. Similar to other organs, ischemia-reperfusion injury in the kidney evokes activation of several hypoxia-inducible genes by HIF-1α,3 and may play an important role in the development, propagation, and resolution of AKI.
In recent years, polymorphisms of host-response candidate genes have emerged as potentially important determinants of disease severity and adverse outcomes in AKI.4,5 A nonsynonymous polymorphism in the coding region of the HIF-1α gene (exon 12, position þ85, C to T single-nucleotide substitution; RefSNP no. rs11549465) resulting in proline to serine substitution (codon 1, position 582) has recently been described and shown to increase the transactivation potential of the transcription factor.6 In this study, using a candidate-gene approach, we examined the association of this HIF-1α genetic variant with measures of disease severity and adverse outcomes in a cohort of hospitalized patients with established AKI, focusing mainly on dialysis requirement, assisted mechanical ventilation, and in-hospital mortality. We also explored the association of this polymorphism with two intermediate phenotypes, plasma levels of vascular endothelial growth factor-A (VEGF-A) and angiopoietin-2 (Ang-2), pro- and antiangiogenic factors, respectively, under the control of HIF-1α. Because these factors have previously been linked to adverse outcomes in critically ill patients,7,8 we probed their potential impact in AKI.
RESULTS
Characteristics of the cohort stratified by HIF-1α genotypes
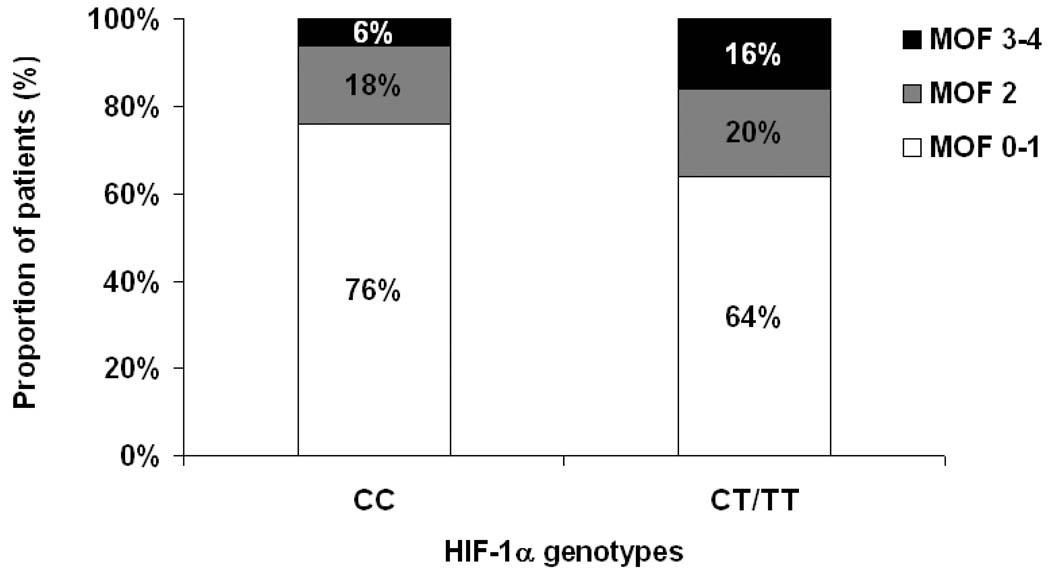
Hypoxia-inducible factor-1a genotyping was performed on 241 consecutive study participants. The minor allele frequency of the HIF-1α exonic þ85 T-allele was 14%. Testing for Hardy–Weinberg equilibrium did not detect deviation from expected frequencies (P=0.88). Table 1 displays the baseline characteristics of the cohort according to the different HIF-1α genotypes. In brief, no significant age, sex, and race differences were observed among the different genotype groups. The remaining characteristics did not differ significantly among the genotype groups, except for a higher prevalence of shock (Table 1) and a higher number of failed organs, as defined by the multiple organ failure (MOF) score, in carriers of the CT and TT genotypes (Figure 1, P=0.04). The HIF-1α T-allele was associated with a higher MOF score category (odds ratio 1.94; 95% confidence interval (CI) 1.05, 3.57; P=0.03).
Table 1.
Characteristics of patients with AKI stratified by HIF-1α exon 12 (+85) genotypes
| Characteristic | HIF-1α exon 12 (+85) genotypes | P value | ||
|---|---|---|---|---|
| CC (N = 180) | CT (N = 56) | TT (N = 5) | ||
| Age (years) | 64 (16) | 67 (16) | 74 (14) | 0.15 |
| Male sex (%) | 54 | 48 | 40 | 0.61 |
| Race (%) | 0.62 | |||
| White | 90 | 91 | 100 | |
| Black | 7 | 4 | 0 | |
| Other | 3 | 5 | 0 | |
| Contributing cause of AKI (%) | 0.54 | |||
| Ischemic | 28 | 20 | 60 | |
| Nephrotoxic | 16 | 21 | 0 | |
| Septic | 9 | 11 | 0 | |
| Multifactorial/other | 47 | 48 | 40 | |
| Coexisting conditions (%) | ||||
| Diabetes mellitus | 42 | 48 | 60 | 0.52 |
| Heart failure | 14 | 18 | 0 | 0.53 |
| Cirrhosis | 8 | 7 | 0 | 0.93 |
| Chronic lung disease | 19 | 16 | 20 | 0.88 |
| Chronic kidney disease | 32 | 38 | 20 | 0.61 |
| ICU setting (%) | 74 | 75 | 80 | 0.96 |
| APACHE II score | 20 (6) | 20 (7) | 21 (8) | 0.78 |
| Sepsis (%) | 43 | 48 | 60 | 0.60 |
| Shock (%) | 24 | 41 | 60 | 0.01 |
| Serum creatinine (mg/dl) | ||||
| Baseline value | 1.6 (0.6) | 1.5 (0.6) | 1.4 (0.7) | 0.75 |
| Enrollment value | 3.7 (2.0) | 3.6 (1.9) | 2.4 (0.5) | 0.33 |
| Oliguria (%) | 23 | 16 | 20 | 0.61 |
The values are expressed as means (and standard deviations) or percentages.
Figure 1.
Multiple organ failure (MOF) score stratified by HIF-1α̣ genotypes. P = 0.04 by x2-test.
Association of HIF-1α genotypes with plasma VEGF-A and Ang-2 levels
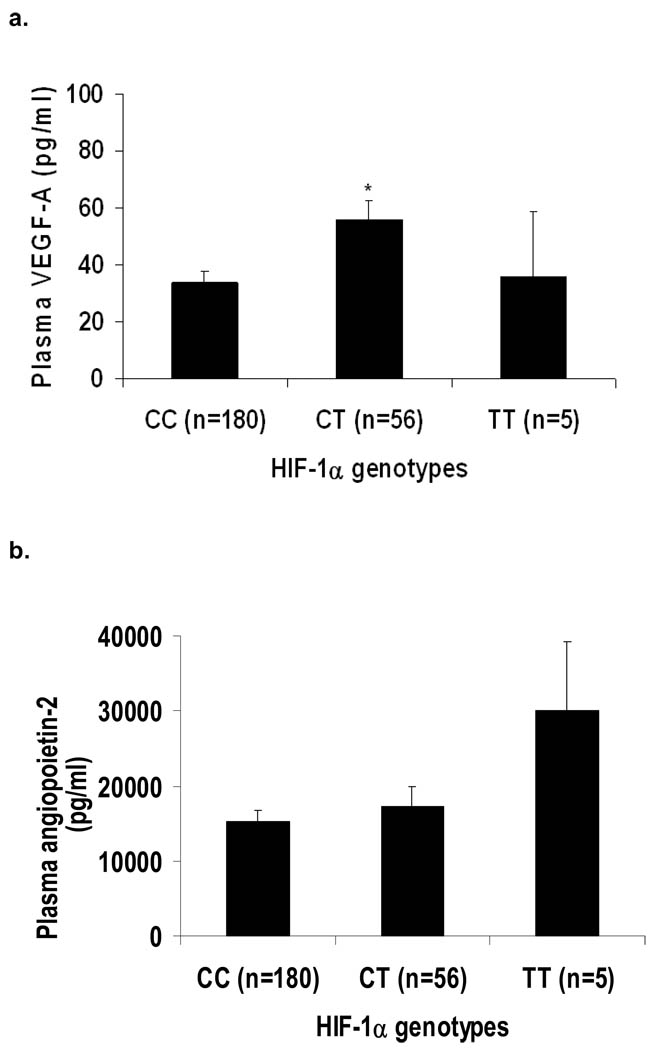
A genotype–phenotype association was demonstrable between the HIF-1α genotypes and plasma VEGF-A levels (P=0.02 for trend). Patients with the HIF-1α CT genotype had higher plasma VEGF-A levels as compared with those with the CC genotype (Figure 2a; P=0.006). When the CT and TT genotypes were combined (there were only five carriers of the TT genotype in our cohort), the T-allele carriers had markedly higher plasma VEGF-A levels as compared with carriers of the CC genotype (54±7 vs 34±4 pg/ml; P=0.008). There was no significant association between plasma Ang-2 levels and HIF-1α genotypes (P=0.24), although a trend toward higher values appeared among the five carriers of the TT genotype (Figure 2b). Similarly, when we combined the CT and TT genotypes, the T-allele carriers had higher plasma Ang-2 levels as compared to those with the CC genotype, but this did not reach statistical significance (18 335±2610 vs 15 305±1517 pg/ml; P=0.32).
Figure 2.
(2A) Plasma vascular endothelial growth factor-A (VEGF-A) and (2B) plasma Ang-2 level stratified by HIF-1α̣genotypes. The data are presented as mean (SE) values that are adjusted for the white blood cell and platelet counts. * P = 0.006 vs. CC genotype.
Association of HIF-1α genotypes with adverse clinical outcomes
As shown in Table 2, carriers of the HIF-1α CT/TT genotype had a higher requirement for dialysis (P=0.03) or assisted mechanical ventilation (P=0.05), as well as higher composite end points of dialysis requirement or in-hospital death (P=0.001), assisted mechanical ventilation or dialysis requirement (P=0.005), and assisted mechanical ventilation, dialysis requirement, or in-hospital death (P=0.001).
Table 2.
Outcomes of patients with AKI stratified by HIF-1α exon 12 (+85) genotypes
| Characteristic | HIF-1α exon 12 (+85) genotypes | P value | ||
|---|---|---|---|---|
| CC (N = 180) | CT (N = 56) | TT (N = 5) | ||
| Requirement for assisted mechanical ventilation (%) | 22 | 32 | 60 | 0.05 |
| Persistent mechanical ventilation at hospital discharge (%) |
14 | 23 | 40 | 0.09 |
| Dialysis requirement (%) | 37 | 55 | 60 | 0.03 |
| In-hospital death (%) | 22 | 32 | 20 | 0.27 |
| Dialysis requirement or in-hospital death (%) | 43 | 70 | 60 | 0.002 |
| Assisted mechanical ventilation or dialysis requirement (%) |
46 | 68 | 80 | 0.008 |
| Assisted mechanical ventilation, dialysis requirement, or in-hospital death (%) |
48 | 75 | 80 | 0.001 |
The values are expressed as means (and standard deviations) or percentages.
Results of the logistic regression analyses are displayed in Table 3. In the unadjusted analyses, compared with the CC genotype, the HIF-1α T-allele carrier state was associated with 2.89-fold higher odds for dialysis requirement or in-hospital death, 2.58-fold higher odds for assisted mechanical ventilation or dialysis requirement, and 3.28-fold higher odds for assisted mechanical ventilation, dialysis requirement, or in-hospital death. These associations persisted with 3.61-, 3.39-, and 4.74-fold higher odds for these respective composite end points after adjustment for sex, race, and APACHE II score. Similar associations were observed after adjustment for sex, race, APACHE II score, and shock (Table 3), as well as after adjustment for sex, race, and age, and either the MOF score or sepsis (data not shown).
Table 3.
Association of HIF-1α exon 12 (+85) genotypes with adverse clinical outcomes in patients with AKI
| HIF-1α CT/TT genotypes (vs. CC genotype) | OR | 95% CI | P value |
|---|---|---|---|
| In-hospital death | |||
| Unadjusted | 1.64 | 0.86, 3.13 | 0.14 |
| Adjusted for race, sex, and APACHE II score | 1.53 | 0.72, 3.29 | 0.27 |
| Adjusted for race, sex, APACHE II score, and shock | 1.49 | 0.69, 3.24 | 0.31 |
| Dialysis requirement or in-hospital death | |||
| Unadjusted | 2.89 | 1.56, 5.36 | < 0.001 |
| Adjusted for race, sex, and APACHE II score | 3.61 | 1.77, 7.39 | < 0.001 |
| Adjusted for race, sex, APACHE II score, and shock | 3.87 | 1.87, 8.01 | < 0.001 |
| Assisted mechanical ventilation or dialysis requirement | |||
| Unadjusted | 2.58 | 1.39, 4.78 | 0.003 |
| Adjusted for race, gender, and APACHE II score | 3.39 | 1.60, 7.19 | 0.001 |
| Adjusted for race, sex, APACHE II score, and shock | 3.67 | 1.70, 7.89 | 0.001 |
| Assisted mechanical ventilation, dialysis requirement, or in-hospital death | |||
| Unadjusted | 3.28 | 1.71, 6.29 | 0.0004 |
| Adjusted for race, gender, and APACHE II score | 4.74 | 2.16, 10.43 | 0.0001 |
| Adjusted for race, sex, APACHE II score, and shock | 4.73 | 2.14, 10.47 | 0.0001 |
95% CI denotes the 95% confidence interval for the odds ratio (OR)
Using the additive models, each copy of the HIF-1α T-allele was independently associated with 2.90-fold higher odds for dialysis requirement or in-hospital death (95% CI 1.52, 5.56), 3.08-fold higher odds for assisted mechanical ventilation or dialysis requirement (95% CI 1.55, 6.16), and 4.05-fold higher odds for assisted mechanical ventilation, dialysis requirement or in-hospital death (95% CI 1.96, 8.41), after adjustment for sex, race, and APACHE II score. The addition of shock to these models did not affect any of these point estimates (data not shown).
Association of plasma VEGF-A or Ang-2 levels with adverse clinical outcomes
We constructed a restrictive cubic spline to ascertain the crude relationship between plasma VEGF-A or Ang-2 and dialysis requirement or in-hospital death (Figure 3). Increasing plasma levels of VEGF-A were not associated with this composite outcome (P=0.39; Figure 3a). By contrast, increasing plasma levels of Ang-2 were associated with a progressively higher probability of dialysis requirement or in hospital death (P<0.0001; Figure 3b).
Figure 3.
Restrictive cubic spline depicting the unadjusted relationship (with 95% confidence intervals) of (3A) plasma vascular endothelial growth factor-A (VEGF-A) (P = 0.39) and (3B) plasma angiopoietin-2 (P < 0.0001) with predicted probability of dialysis requirement or in-hospital death.
Plasma VEGF-A (odds ratio 0.95; 95% CI 0.91, 0.99; P=0.03) and plasma Ang-2 (odds ratio 3.83; 95% CI 2.55, 5.75; P<0.0001) were also associated with the MOF score, but these associations were in opposite directions.
Results of the logistic regression analyses are displayed in Table 4. In the unadjusted analyses, plasma VEGF-A level was associated with 0.90-fold lower odds for in-hospital death, which persisted after adjustment for sex, race, APACHE II score, and HIF-1α genotypes. However, there was no association between this proangiogenic factor and the composite end points (Table 4). In the unadjusted analyses, plasma Ang-2 level was associated with 3.28-fold higher odds for in-hospital death, 2.34-fold higher odds for dialysis requirement or in-hospital death, 2.12-fold higher odds for assisted mechanical ventilation or dialysis requirement, and 2.27-fold higher odds for the composite of assisted mechanical ventilation, dialysis requirement, or in-hospital death. These associations persisted for in-hospital death, and for all three composite end points after adjustment for sex, race, APACHE II score, and HIF-1α genotypes (Table 4). Of note, the HIF-1α T-allele was independently associated with 3.57-fold higher odds for dialysis requirement or in-hospital death (95% CI 1.73, 7.35), 3.37-fold higher odds for assisted mechanical ventilation or dialysis requirement (95% CI 1.59, 7.15), and 4.69-fold higher odds for assisted mechanical ventilation, dialysis requirement or in-hospital death (95% CI 2.13, 10.33), after adjustment for sex, race, APACHE II score, and Ang-2 level. There was no interaction between HIF-1α genotypes and either plasma VEGF-A or Ang-2 for the various end points (data not shown).
Table 4.
Association of plasma VEGF-A and Ang-2 levels with adverse clinical outcomes in patients with AKI
| Outcome variable | Plasma VEGF-A level (per 5-unit increase) |
P value | C statistic |
Plasma Ang-2 level (per 1-log- unit increase) |
P value | C statistic |
|---|---|---|---|---|---|---|
| In-hospital death | ||||||
| Unadjusted | 0.90 (0.84, 0.96) | 0.003 | 0.649 | 3.28 (2.14, 5.04) | < 0.0001 | 0.768 |
| Adjusted for sex, race and APACHE II score | 0.92 (0.86, 0.99) | 0.02 | 0.843 | 2.37 (1.48, 3.78) | 0.0003 | 0.844 |
| Adjusted for sex, race, APACHE II score, and HIF-1α̣ genotypes | 0.92 (0.86, 0.98) | 0.01 | 0.851 | 2.35 (1.46, 3.78) | 0.0004 | 0.847 |
| Dialysis requirement or in-hospital death | ||||||
| Unadjusted | 1.00 (0.98, 1.02) | 0.94 | 0.500 | 2.34 (1.65, 3.33) | < 0.0001 | 0.696 |
| Adjusted for sex, race, and APACHE II score | 1.02 (0.99, 1.04) | 0.19 | 0.776 | 1.68 (1.14, 2.48) | 0.009 | 0.785 |
| Adjusted for sex, race, APACHE II score, and HIF-1α genotypes | 1.01 (0.98, 1.03) | 0.49 | 0.806 | 1.61 (1.08, 2.39) | 0.02 | 0.814 |
| Assisted mechanical ventilation or dialysis requirement | ||||||
| Unadjusted | 1.00 (0.98, 1.02) | 0.99 | 0.504 | 2.12 (1.51, 2.80) | < 0.0001 | 0.678 |
| Adjusted for sex, race, and APACHE II score | 1.02 (0.99, 1.04) | 0.24 | 0.810 | 1.36 (0.92, 2.02) | 0.13 | 0.806 |
| Adjusted for sex, race, APACHE II score, and HIF-1α genotypes | 1.01 (0.98, 1.03) | 0.57 | 0.833 | 1.29 (0.87, 1.93) | 0.21 | 0.833 |
| Assisted mechanical ventilation, dialysis requirement or in-hospital death | ||||||
| Unadjusted | 1.00 (0.98, 1.02) | 0.72 | 0.553 | 2.27 (1.60, 3.23) | < 0.0001 | 0.691 |
| Adjusted for sex, race, and APACHE II score | 1.01 (0.99, 1.03) | 0.43 | 0.803 | 1.50 (1.01, 2.24) | 0.04 | 0.806 |
| Adjusted for sex, race, APACHE II score, and HIF-1α genotypes | 1.00 (0.98, 1.03) | 0.98 | 0.839 | 1.42 (0.94, 2.13) | 0.09 | 0.842 |
95% CI denotes the 95% confidence interval for the odds ratio (OR); VEGF-A denotes vascular endothelial growth factor A; and Ang-2, angiopoietin-2.
DISCUSSION
This study evaluates the relationship of a functional genetic variant in the coding region of the HIF-1α gene to adverse clinical outcomes in a large cohort of hospitalized patients with established AKI of mixed etiology and severity. The observed and expected genotype frequencies were not significantly different, thereby fulfilling the Hardy–Weinberg equilibrium, and there were no race, gender, or age differences within genotype groups. The HIF-1α genotypes influenced plasma levels of VEGF-A but not Ang-2, two HIF-1α-regulated gene products. After adjustment for sex, race, and APACHE II score, the HIF-1α T-allele carriers (CT and TT genotypes) had a higher prevalence of shock and multiple organ failure, and higher odds for the composite end points of dialysis requirement or in-hospital death, assisted mechanical ventilation or dialysis requirement, and assisted mechanical ventilation, dialysis requirement, or in-hospital death. Although plasma VEGF-A levels were significantly higher among carriers of the HIF-1α T-allele, higher levels of this proangiogenic factor were associated with lower odds for in-hospital death. By contrast, although there was a nonsignificant trend toward higher plasma Ang-2 levels among the HIF-1α T-allele carriers, higher levels of this antiangiogenic factor were associated with higher odds for in-hospital death and the three afore-mentioned composite end points.
The limitation in oxygen supply to renal tissue renders the kidney susceptible to hypoxia and has been long recognized as an important factor in the pathogenesis of AKI.3 HIF-1α is a transcription factor that mediates cellular responses to tissue hypoxia.9 By forming a heterodimeric complex with the b-unit (HIF-1b) on hypoxic responsive elements, HIF-1α activates transcription of a wide array of genes as a part of the cellular response to hypoxia. HIF-1b is ubiquitously expressed and maintained at constant cellular levels, whereas HIF-1α protein levels and transcriptional activity are tightly regulated in response to oxygen levels. Under normoxic conditions, HIF-1α is hydroxylated on proline residues by oxygen-dependent prolyl hydroxylases, which mediate ubiquitination and degradation of HIF-1α. The critical proline residues that mediate binding when hydroxylated are at position P402 and P564, both located in the oxygen dependent domain.
Given its importance in mediating cellular repsonses to hypoxia, one might anticipate that polymorphisms disrupting the function of HIF-1α would alter tissue responses in conditions such as AKI. Several polymorphisms in the gene encoding HIF-1α have been described. Among them, the non-synonymous polymorphism in the coding region of the HIF-1α gene (exon 12, position þ85, C to T singlenucleotide substitution) results in proline to threonine substitution (codon 1, position 582).6,10 This mutation at P582 renders HIF-1α less sensitive to hydroxylation-dependent degradation, thereby increasing the transactivation potential of this transcription factor.11 Such stabilization leads to higher abundance of HIF-1α12 and subsequent upregulation of HIF-1α-dependent genes. The P582 HIF-1α T-allele carrier has been associated with more aggressive forms of cancer, inlcuding renal cell carcinoma,13 breast cancer,14 prostate cancer,11 and non-small cell lung cancer.15 However, nononcological clinical studies examining the influence of this polymorphism have yielded conflicting results with the HIF-1α T-allele being associated with a higher prevalence of type-2 diabetes mellitus,12 and less collateral vessel formation among patients with coronary artery disease.16
In this study, the potential mechanisms underlying the association of the HIF-1α T-allele with higher requirement for organ support are unknown. Our data seemingly contradict experimental observations that hypoxic preconditioning and subsequent HIF-1α activation protect against ischemic and nephrotoxic renal injury,17,18 and that the extent of HIF-1α induction inversely correlates with tissue injury.19 However, targeted deletion of HIF-1α has also been shown to be protective against sepsis,20 which would support the potential aggravating effect of the HIF-1α stabilizing genetic variant observed in our study. In this regard, a recently described new activator of prolyl-hydroxylase 2 that is capable of rapidly degrading HIF-1α,21 might be a potential therapeutic agent in states of excessive HIF-1α activation and might be of clinical interest in AKI. It is also difficult to translate experimental results to human AKI. We propose that the poor outcomes observed among carriers of the stabilizing HIF-1α T-allele might be due, in part, to the slower degradation of the mutated HIF-1α after reversal of tissue hypoxia, that is, when oxygen supply is restored, thereby creating an effective state of ‘hypoxia extension.’ This hypothesized maladaptive response of HIF-1α might be conducive in part to an aberrant cytokine activation pattern that has been described earlier in sepsis, and linked to HIF-1α activation.22,23
With more than 60 HIF-1α target genes identified to date, and their involvement in a multitude of biological processes relevant to kidney function, including glucose and energy metabolism, angiogenesis, cell migration, cell–cell and cell–matrix interaction, proliferation, and apoptosis,3,24 there are numerous potential downstream effectors of HIF-1α that might contribute to the poor outcomes observed in our study. We chose to examine two intermediate HIF-1α phenotypes, VEGF-A and Ang-2. VEGF-A represents one of the well-described HIF-1α target genes.25 Although we were able to demonstrate a genotype–phenotype association between HIF-1α and plasma VEGF-A, higher plasma VEGF-A levels were associated with improved survival. The need for dialysis and assisted mechanical ventilation are clinical end points that are potentially associated with vascular leakage phenomena. Although VEGF-A is a wellestablished vascular permeability factor,26 we failed to establish an association between this intermediate HIF-1α phenotype and organ support. We can only speculate as to whether circulating VEGF-A levels among patients with AKI are not sufficiently high to cause vascular leakage as observed in vitro,27 or whether a balance exists between different effectors with opposing effects on vascular leakage.
Although there was a lack of association between HIF-1α genotypes and plasma Ang-2 levels, this intermediate marker was associated with the same adverse outcomes predicted by the HIF-1α T-allele. The mechanism of Ang-2 regulation by HIF-1α is not as well established as that of VEGF-A,28,29 and its induction tends to be cell-type specific.28,30 Ang-2 levels are elevated in critically ill patients with sepsis7,31 and severe trauma,32 and correlate with disease severity.31 Furthermore, among septic patients with acute lung injury, Ang-2 levels correlate with impaired pulmonary gas exchange7 and predict acute lung injury.33 In our study, higher plasma Ang-2 levels were associated with higher probability of mechanical ventilation or dialysis requirement, and dialysis requirement or in-hospital death. Thus, Ang-2 appeared to be a suitable downstream effector of HIF-1α, and the inability to demonstrate a genotype–phenotype association might be due to our limited sample size. Nonetheless, further studies to identify candidate downstream mediators of HIF-1α are required. The fact that HIF-1α T-allele carrier state remained independently associated with the composite end point of dialysis requirement or in-hospital death even after adjustment for Ang-2 level is in support of other more important downstream HIF-1α gene effectors.
To our knowledge, this is the first study testing the hypothesis of whether a functionally relevant polymorphism of the HIF-1α gene is associated with adverse clinical outcomes in patients with AKI. The heterogeneity of our cohort was offset by the selective inclusion of patients with more severe AKI requiring formal consultation of the nephrology service. Although sizeable for a hospital-based study, our cohort was relatively small for genetic epidemiological studies. Our population was 90% white, reducing the potential impact of race and ethnicity on genotype prevalence. Of note, we found no ethnic differences among genotype groups, but when the analyses were confined to white subjects, the point estimates were not significantly altered (data not shown). The use of baseline covariates such as the APACHE II or MOF score, which incorporate several demographic, physiological, and laboratory variables that are individually associated with the composite end points, strengthens our results. Our composite end points were also chosen to account for survival bias when dialysis requirement and assisted mechanical ventilation are being assessed as outcome measures. The demonstrable genotype–phenotype associations lend some additional credibility to the observed association between the HIF-1α T-allele and the various composite end points. Finally, there is a possibility that the HIF-1α candidate gene polymorphism we studied might be in linkage disequilibrium with other unidentified pathogenic genetic variants.
In summary, this study supports the hypothesis that HIF-1α gene polymorphism predicts adverse outcomes in hospitalized patients with AKI. Larger studies are needed to confirm these genetic associations and identify the candidate downstream effectors of HIF-1α. These observations bolster previous knowledge that cytokine and oxidative-stress gene polymorphisms play an important role in the regulation of host inflammatory responses, and contribute to altered morbidity and mortality among patients with AKI.4,5
MATERIALS AND METHODS
Study design and participants
This was a prospective cohort study of hospitalized patients with AKI, which was conducted between November 2003 and January 2008 at two tertiary care hospitals located in Boston, Massachusetts, USA. All consecutive hospitalized adult patients with AKI, in whom nephrology consultation was requested, were eligible for enrollment. AKI was defined as a rise in serum creatinine of 0.5, 1.0, or 1.5 mg per 100 ml from a baseline level of ≤1.9, 2.0–4.9, or ≥5.0 mg per 100 ml, respectively.34 The adopted definition preceded the AKI network’s recently developed criteria.35
Exclusion criteria were as follows: age <18 years, pregnancy, long-term dialysis, organ transplantation within the prior year, and presence of acute obstructive uropathy. Written informed consent was obtained from all participants or next of kin. The institutional review board of each participating center approved the study protocol.
Data collection
Medical records were reviewed prospectively to retrieve hospitalization data, including baseline demographic characteristics, coexisting conditions, and renal variables. At enrollment, oliguria was defined by 24-h urine output <400 ml. Advanced chronic kidney disease was assessed on the basis of a pre-morbid estimated glomerular filtration rate <30 ml/min per 1.73m2, as calculated by the Modification of Diet in Renal Disease (MDRD) study equation.36 At enrollment, the presence of sepsis was ascertained using the systemic inflammatory response syndrome (SIRS) criteria,37 and two severity-of-illness scores were calculated, the Acute Physiology and Chronic Health Evaluation (APACHE) II score38 and the MOF score.39
Blood sampling and DNA extraction
At enrollment, EDTA-anticoagulated whole blood was collected. Plasma was separated, and the remaining blood was aliquoted and stored at −80°C for subsequent DNA extraction. Genomic DNA was extracted using a spin column method according to the manufacturer’s instructions (Qiagen Inc., Valencia, CA, USA). Final DNA concentration was set at 50–200 ng/ml, and was determined by minigel electrophoresis.
HIF-1α genotyping analyses
Genotyping of the HIF-1α gene in the coding region (exon 12, position þ85, C to T substitution) was performed with the allelespecific primers TTCCTTCGATCAGTTGTCAC (þ85C) and TTCCTTCGATCAGTTGTCAT (þ85T), and the consensus primer TGAGGCTGTCCGACTTTGAG, which produces a PCR product of 280 bp. Perfectly matched primer pairs resulted in amplification of target sequences, whereas mismatched primer pairs did not. In each reaction, a second set of primers for exon 3 of the HLA-DRBI gene was used as a control for PCR efficiency. The PCR amplification reactions were carried out at 95°C for 3 min, followed by 5 cycles at 95°C for 30 s, 66°C for 50 s, and 72°C for 50 s. This was followed by 16 cycles with gradual decline of the annealing temperature by 1°C per cycle from 65 to 50°C; 10 cycles at 95°C for 30 s, 55°C for 50 s, and 72°C for 50 s; and a final cycle at 72°C for 5 min.
Measurement of plasma VEGF-A and Ang-2
Plasma VEGF-A and Ang-2 were measured by sandwich ELISA using the commercially available kits (Quantikine VEGF Immunoassay, and Quantikine Angiopoietin-2 Immunoassay, R&D, Minneapolis, MN, USA). Two measurements were performed per sample, and outliers, as defined by values two standard deviations below or above the mean, were re-measured. For VEGF-A, the average inter- and intra-assay coefficient of variation was 7.3 and 5.4%, respectively, and for Ang-2, 9.0 and 5.9%, respectively. The VEGF-A and Ang-2 results are expressed in pg/ml and are adjusted for the white blood cell and platelet counts, as these molecules are expressed, stored, and secreted by these circulating blood cells.40–42
Outcome measures
The primary end point of our study was the composite of dialysis requirement or in-hospital death. Secondary outcome measures were severity of organ dysfunction, as measured by the MOF score, organ support, as defined by need for assisted mechanical ventilation or dialysis requirement, in-hospital death and the composite of dialysis requirement, assisted mechanical ventilation or in-hospital death. The composite end points of organ support or in-hospital death were chosen as they take into consideration survival bias for dialysis requirement or assisted mechanical ventilation.
Statistical analyses
The genotype frequencies were tested for Hardy–Weinberg equilibrium using a standard x2-test. Comparisons between genotype groups were made by the Kruskall–Wallis test or the two-tailed Mann–Whitney test for continuous variables, and by x2-test or Fisher’s exact test for categorical variables. Results are expressed as means (with standard deviation) or percentages.
A general linear model was used to estimate the mean plasma VEGF-A and Ang-2 levels in each genotype category, adjusting for white blood cell and platelet counts. For this analysis, the results are displayed as means ± s.e. A proportional odds model was used to examine the association of the HIF-1α T-allele carrier, plasma VEGF-A and Ang-2 level with the MOF score.
Logistic regression analyses were used to examine the association of the HIF-1α CT/TT genotypes or T-allele carrier (vs CC genotype) with the afore-mentioned outcomes, using a recessive genetic model. All models were adjusted for sex, race, and APACHE II score. We performed similar analyses using an additive genetic model (i.e., copies of the HIF-1α minor allele). In a sensitivity analysis, other covariates were used including sex, race, age, and either sepsis or the MOF score. The results of the logistic regression analyses are displayed as odds ratios with 95% CI.
The functional form of plasma VEGF-A and Ang-2 level with dialysis requirement or in-hospital death was examined and graphically displayed using restricted cubic spline functions with four knots.43 Plots of the restricted splines were constructed using the Design library of the R package.44
Logistic regression analyses were also used to examine the association of plasma VEGF-A and Ang-2 levels, our two hypothesized downstream effectors of HIF-1α, with similar clinical end points. For these analyses, the Ang-2 variable was log transformed due to a skewed distribution. Models were adjusted for sex, race, APACHE II score, and HIF-1α genotypes. We also formally tested for interactions between HIF-1α genotypes and either VEGF-A or Ang-2 for the outcomes of interest.
All statistical analyses were performed using the SAS software (SAS Institute, Cary, NC, USA) version 9.1. All P-values are twosided, and a P-value of less than 0.05 was considered significant.
Acknowledgments
We are indebted to the patients and their next of kin for participating in this study. This study was supported by grants from the National Institutes of Health (DK065102 and DK077751, to B.L.J.). O.L. was supported by a grant from the American Heart Association. We thank Robert W. MacKinnon, RN, for assistance with enrollment of study participants.
REFERENCES
- 1.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Liangos O, Wald R, O’Bell J, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 3.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaber B, Rao M, Guo D, et al. Cytokine promoter gene polymorphisms and mortality in acute renal failure. Cytokine. 2004;25:212–219. doi: 10.1016/j.cyto.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Perianayagam MC, Liangos O, Kolyada AY, et al. NADPH oxidase p22phox and catalase gene variants are associated with biomarkers of oxidative stress and adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:255–263. doi: 10.1681/ASN.2006070806. [DOI] [PubMed] [Google Scholar]
- 6.Tanimoto K, Yoshiga K, Eguchi H, et al. Hypoxia-inducible factor-1alpha polymorphisms associated with enhanced transactivation capacity, implying clinical significance. Carcinogenesis. 2003;24:1779–1783. doi: 10.1093/carcin/bgg132. [DOI] [PubMed] [Google Scholar]
- 7.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro NI, Yano K, Okada H, et al. A prospective, observational study of soluble FLT-1 and vascular endothelial growth factor in sepsis. Shock. 2008;29:452–457. doi: 10.1097/shk.0b013e31815072c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 10.Clifford SC, Astuti D, Hooper L, et al. The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx1 and HIF-1alpha in renal cell carcinoma. Oncogene. 2001;20:5067–5074. doi: 10.1038/sj.onc.1204602. [DOI] [PubMed] [Google Scholar]
- 11.Fu XS, Choi E, Bubley GJ, et al. Identification of hypoxia-inducible factor-1alpha (HIF-1alpha) polymorphism as a mutation in prostate cancer that prevents normoxia-induced degradation. Prostate. 2005;63:215–221. doi: 10.1002/pros.20190. [DOI] [PubMed] [Google Scholar]
- 12.Yamada N, Horikawa Y, Oda N, et al. Genetic variation in the hypoxiainducible factor-1alpha gene is associated with type 2 diabetes in Japanese. J Clin Endocrinol Metab. 2005;90:5841–5847. doi: 10.1210/jc.2005-0991. [DOI] [PubMed] [Google Scholar]
- 13.Ollerenshaw M, Page T, Hammonds J, et al. Polymorphisms in the hypoxia inducible factor-1alpha gene (HIF1A) are associated with the renal cell carcinoma phenotype. Cancer Genet Cytogenet. 2004;153:122–126. doi: 10.1016/j.cancergencyto.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Choi JY, Lee KM, et al. Rare variant of hypoxia-inducible factor-1alpha (HIF-1A) and breast cancer risk in Korean women. Clin Chim Acta. 2008;389:167–170. doi: 10.1016/j.cca.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Koukourakis MI, Papazoglou D, Giatromanolaki A, et al. C2028T polymorphism in exon 12 and dinucleotide repeat polymorphism in intron 13 of the HIF-1alpha gene define HIF-1alpha protein expression in non-small cell lung cancer. Lung Cancer. 2006;53:257–262. doi: 10.1016/j.lungcan.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Resar JR, Roguin A, Voner J, et al. Hypoxia-inducible factor 1alpha polymorphism and coronary collaterals in patients with ischemic heart disease. Chest. 2005;128:787–791. doi: 10.1378/chest.128.2.787. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto M, Makino Y, Tanaka T, et al. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol. 2003;14:1825–1832. doi: 10.1097/01.asn.0000074239.22357.06. [DOI] [PubMed] [Google Scholar]
- 18.Weidemann A, Bernhardt WM, Klanke B, et al. HIF activation protects from acute kidney injury. J Am Soc Nephrol. 2008;19:486–494. doi: 10.1681/ASN.2007040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberger C, Heyman SN, Rosen S, et al. Up-regulation of HIF in experimental acute renal failure: evidence for a protective transcriptional response to hypoxia. Kidney Int. 2005;67:531–542. doi: 10.1111/j.1523-1755.2005.67110.x. [DOI] [PubMed] [Google Scholar]
- 20.Peyssonnaux C, Cejudo-Martin P, Doedens A, et al. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 21.Choi HJ, Song BJ, Gong YD, et al. Rapid degradation of hypoxia-inducible factor-1alpha by KRH102053, a new activator of prolyl hydroxylase 2. Br J Pharmacol. 2008;154:114–125. doi: 10.1038/bjp.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blouin CC, Page EL, Soucy GM, et al. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 23.Frede S, Stockmann C, Freitag P, et al. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 25.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 27.Chandra A, Barillas S, Suliman A, et al. A novel fluorescence-based cellular permeability assay. J Biochem Biophys Methods. 2007;70:329–333. doi: 10.1016/j.jbbm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Kelly BD, Hackett SF, Hirota K, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 29.Fong GH. Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis. 2008;11:121–140. doi: 10.1007/s10456-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 30.Yamakawa M, Liu LX, Date T, et al. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93:664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- 31.Orfanos SE, Kotanidou A, Glynos C, et al. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35:199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 32.Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247:320–326. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 33.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008 doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 34.Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 35.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network (AKIN): report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.K/DOQI NKF. Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. Am J Kidney Dis. 2002;39 Suppl 1:S1–S266. [PubMed] [Google Scholar]
- 37.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 38.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 39.Knaus WA, Wagner DP. Multiple systems organ failure: epidemiology and prognosis. Crit Care Clin. 1989;5:221–232. [PubMed] [Google Scholar]
- 40.Ito A, Hirota S, Mizuno H, et al. Expression of vascular permeability factor (VPF/VEGF) messenger RNA by plasma cells: possible involvement in the development of edema in chronic inflammation. Pathol Int. 1995;45:715–720. doi: 10.1111/j.1440-1827.1995.tb03387.x. [DOI] [PubMed] [Google Scholar]
- 41.Maloney JP, Silliman CC, Ambruso DR, et al. In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol. 1998;275(3 Part 2):H1054–H1061. doi: 10.1152/ajpheart.1998.275.3.H1054. [DOI] [PubMed] [Google Scholar]
- 42.Frechette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84:434–439. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 43.Stone C, Koo CY. Proceedings of the Statistical Computing Section of the American Statistical Association. 1995. Additive splines in statistics; pp. 45–48. [Google Scholar]
- 44.R Foundation for Statistical Computing. Vienna, Austria: 2005. R: A language and environment for statistical computing. (Accessed at http://www.R-project.org) [Google Scholar]