The respiratory tract forms a major mucosal interface with the external environment. Consequently, the respiratory tract is exposed constantly to inhaled foreign antigens, commensal microorganisms, and potential pathogens. The immune system has evolved a complex regulatory network to prevent unnecessary inflammation to inert antigens or commensal microorganisms that could result in excessive tissue damage without compromising host defense (reviewed in ref. [1]). Although not completely understood, the complex regulatory network in the lung includes specialized macrophage and DC populations, which in concert with signals provided by the respiratory epithelium, establish an anti-inflammatory environment. For example, alveolar macrophages express lower levels of MHC class II and costimulatory molecules, making them less efficient at inducing T cell responses [2]. Surfactant protein A is able to inhibit alveolar macrophage activation by inhibiting downstream signaling through multiple TLRs [3]. CD200, which is expressed on a wide variety of cell types, transmits a negative signal to macrophages and DCs that are expressing the CD200 receptor, which is up-regulated by TGF-β and IL-10 [4]. Finally, alveolar macrophages and DCs contribute to maintaining the suppressive environment of the lung airways by secreting inhibitory cytokines such as IL-10 and TGF-β [2]. Upon exposure to a respiratory pathogen, this barrier to innate immune activation must first be overcome to initiate an adaptive immune response. Although it is clear that the lungs and other mucosal sites exhibit an increased threshold to initiate a response, it is currently unclear to what extent the local pulmonary environment exerts regulation over an ongoing adaptive immune response. It is likely that newly activated T cells entering the lung from the local draining lymph nodes will be influenced subsequently by the specialized environment within the lung. Thus, it is critical to understand how the lung environment influences the effector activity of T cells to better understand the regulation of pulmonary adaptive immune responses.
In 2001, Chang et al. [5], and in 2002, Chang and Braciale [6] published a pair of companion studies providing the initial evidence suggesting that the lung environment influences adaptive immunity. In these studies, they reported the loss of effector functions by RSV-specific CD8 T cells residing in the lung parenchyma [5, 6]. A significant portion of RSV-specific CD8 T cells, as identified by MHC class I tetramers, failed to produce IFN-γ upon ex vivo peptide stimulation and exhibited decreased ex vivo cytolytic activity that correlated with reduced levels of perforin. The apparent loss of effector functions was found to occur only in the lung and not in secondary lymphoid tissues such as the lymph nodes or spleen. Importantly, impairment of RSV-specific CD8 T cells did not abate following virus clearance from the lung but continued to increase into memory time-points. As a similar loss of cytokine production was not seen in lung parenchymal CD8 T cells during influenza virus infection, these findings suggested that RSV might have the unique ability to limit the virus-specific CD8 T cell response that is crucial in mediating virus clearance from the lungs.
However, since these initial reports, it has become clear that impairment of virus-specific T cells in the lung is perhaps the rule rather than the exception. Additional studies have shown that CD8 T cells isolated from the lungs exhibit diminished effector functions following infection of mice with SV5 [7], pneumonia virus of mice [8], lymphocytic choriomeningitis virus [9, 10], and vaccinia virus [9]. CD8 T cells that enter into the lung parenchyma and the airways display decreased effector functions; however, CD8 T cells in the airways do not appear to become severely impaired until after the resolution of acute infection [9, 10]. Of particular note, several recent publications have demonstrated that virus-specific CD8 T cells that traffic into the lungs in the absence of infection exhibit a reduced ability to produce proinflammatory cytokines [9, 11]. Thus, the normal lung environment appears to suppress T cell effector functions independent of virus infection. This is consistent with the notion that the basal lung environment is immunosuppressive to limit unnecessary collateral damage to the lung epithelium.
In this issue of JLB, Arimilli et al. [12] further explore the molecular mechanisms used by the lungs to regulate the effector functions of T cells. They show that SV5-specific CD8 T cells in the lung tissue that are unable to produce IFN-γ following ex vivo peptide stimulation exhibit decreased calcium flux. This in turn leads to reduced activation and nuclear localization of the calcium-sensitive transcription factor NFAT1, which is responsible for regulating genes such as the cytokine IFN-γ (Fig. 1). By increasing extracellular levels of calcium during peptide stimulation, intracellular calcium levels and nuclear localization of activated NFAT1 are increased, and ex vivo production of IFN-γ is restored in previously nonfunctional CD8 T cells. Further investigation revealed that nonfunctional CD8 T cells had decreased numbers of CRAC channels, which regulate uptake of extracellular calcium. In particular, nonfunctional CD8 T cells had reduced mRNA and protein levels of ORAI1, a pore subunit that makes up the CRAC channel [13]. This work suggests that inhibition of calcium uptake by modulating CRAC channels is an important mechanism used to negatively regulate cytokine production by effector CD8 T cells in the lung.
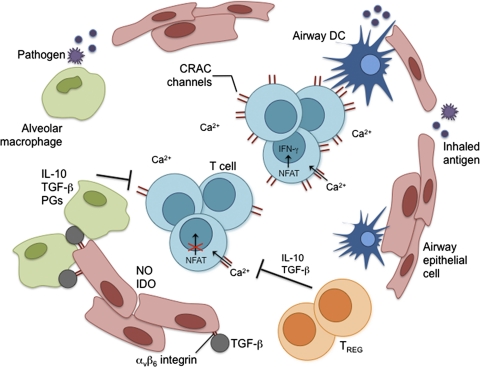
Figure 1.
Negative regulatory mechanisms in the lung. DCs are the primary sentinels of the immune response. Myeloid DCs constantly sample inhaled antigens and are responsible for immune surveillance. Plasmacytoid DCs may have an important role in inducing tolerance to inert antigens. Airway epithelial cells (AEC) produce molecules such as mucins and surfactants, which are important in regulating the innate immune response. AEC also produce inhibitory molecules such as NO, indoleamine 2,3-dioxygenase (IDO), and a variety of other cytokines. AEC and alveolar macrophages have developed a unique homeostatic cycle to ensure their proper activation. TGF-β linked to the αvβ6 integrin, which is expressed on AEC, suppresses macrophages when in close contact. However, upon receiving signals through TLRs, macrophages can suppress expression of αvβ6 and break TGF-β-mediated inhibition. Activated macrophages re-establish inhibition by producing matrix metalloproteinases that promote the expression of TGF-β and αvβ6 integrin. This relationship between AEC and alveolar macrophages is crucial for maintaining an anti-inflammatory environment. Alveolar macrophages are capable of producing anti-inflammatory molecules such as IL-10, TGF-β, and prostaglandins (PGs), which can act on DC and T cells. Regulatory CD4 T cells (TREG) are essential in maintaining homeostasis of the immune system and are important in establishing tolerance to inhaled antigens. As a method to limit collateral damage by effector CD8 T cells responding to a pathogen, intracellular calcium levels can be regulated in CD8 T cells by decreasing the expression levels of CRAC channels. Decreased uptake of extracellular calcium reduces the activation of transcription factors such as NFAT that are required for effector functions such as cytokine production.
This report opens up a variety of important questions about the regulation of effector T cells in the lungs. This study was completed in the context of a single pathogenic infection. Thus, it will be important to establish if the reduction in CRAC channels is a common negative regulatory mechanism that occurs following other respiratory infections. As cytokine production by effector CD8 T cells in the lung is inhibited independent of virus infection, it will also be important to determine if CRAC channels are reduced in this context. Additionally, several studies have suggested that the regulation of cytokine production by T cells in the lung airways differs from that of the lung tissue, which was not examined in the studies performed by Arimilli et al. [12]. Answering these questions will extend the scope of the current work and help determine if the regulation of calcium flux by immune cells is a general property of the lung and perhaps other mucosal sites such as the gastrointestinal tract.
Although this study reveals a novel, negative regulatory mechanism for dampening CD8 T cell function in the lungs, it stops short of determining the upstream signals causing the reduction in CRAC channels. Furthermore, a portion of the effector CD8 T cells in the lung examined by Arimilli et al. [12] retained their effector functions. There are a number of potential reasons for this observation, including: the functional CD8 T cells may represent cells that have entered the lung recently and therefore may not yet have modulated their CRAC channels; the functional CD8 T cells are located in distinct areas of the lung from those CD8 T cells that exhibit diminished effector activity, indicating that the regulation requires direct cell-to-cell contact or close proximity; or there may be additional positive signals that serve to counteract the immunosuppressive signals in the lung that preserve normal effector functions in a subset of the T cells.
All of the related studies so far have only demonstrated that virus-specific CD8 T cells in the lungs lose effector functions following ex vivo assays. Thus, perhaps the most important next step is to examine the function of effector T cells in situ during respiratory infections. Compared with effector T cells in secondary lymphoid tissues, how well do effector T cells in the lungs produce proinflammatory cytokines such as IFN-γ and TNF-α? Do they exhibit diminished cytolytic activity? Determining the answers to these critical questions will be important to verify the current in vitro-based observations. If it is shown that the activity of effector T cells is inhibited in vivo, then it will be important to determine if any intervention attempted to alleviate the inhibition of effector T cell activity results in increased immunopathology.
As this story has evolved, the lung has emerged as a unique microenvironment. Since respiratory pathogens represent a major cause of morbidity and mortality worldwide, continued research into how the immune response is regulated in the lung is vital in learning how to develop new pulmonary-based vaccines or therapeutic interventions.
Acknowledgments
R. B. F. is supported by the Training in Mechanisms of Parasitism training grant T32 AI007511. S. M. V. is supported by The National Institutes of Health grant R01 AI063520.
Footnotes
SEE CORRESPONDING ARTICLE ON PAGE 977
Abbreviations: AEC=airway epithelial cell(s), CRAC=calcium release-activated calcium, DC=dendritic cell, RSV=respiratory syncytial virus
References
- Holt P G, Strickland D H, Wikstrom M E, Jahnsen F L. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- Wissinger E, Goulding J, Hussell T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin Immunol. 2009;21:147–155. doi: 10.1016/j.smim.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Henning L N, Azad A K, Parsa K V, Crowther J E, Tridandapani S, Schlesinger L S. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol. 2008;180:7847–7858. doi: 10.4049/jimmunol.180.12.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelgrove R J, Goulding J, Didierlaurent A M, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick J D, Barclay A N, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- Chang J, Srikiatkhachorn A, Braciale T J. Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection. J Immunol. 2001;167:4254–4260. doi: 10.4049/jimmunol.167.8.4254. [DOI] [PubMed] [Google Scholar]
- Chang J, Braciale T J. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- Gray P M, Arimilli S, Palmer E M, Parks G D, Alexander-Miller M A. Altered function in CD8+ T cells following paramyxovirus infection of the respiratory tract. J Virol. 2005;79:3339–3349. doi: 10.1128/JVI.79.6.3339-3349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen E A, van der Kant P A, Rychnavska Z S, van Bleek G M, Easton A J, van der Most R G. Activation and inactivation of antiviral CD8 T cell responses during murine pneumovirus infection. J Immunol. 2005;175:6597–6604. doi: 10.4049/jimmunol.175.10.6597. [DOI] [PubMed] [Google Scholar]
- Fulton R B, Olson M R, Varga S M. Regulation of cytokine production by virus-specific CD8 T cells in the lungs. J Virol. 2008;82:7799–7811. doi: 10.1128/JVI.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbracht S, Unsold H, Ehl S. Functional impairment of cytotoxic T cells in the lung airways following respiratory virus infections. Eur J Immunol. 2006;36:1434–1442. doi: 10.1002/eji.200535642. [DOI] [PubMed] [Google Scholar]
- Arimilli S, Palmer E M, Alexander-Miller M A. Loss of function in virus-specific lung effector T cells is independent of infection. J Leukoc Biol. 2008;83:564–574. doi: 10.1189/jlb.0407215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimilli S, Sharma S K, Yammani R, Reid S D, Parks G D, Alexander-Miller M A. Pivotal Advance: nonfunctional lung effectors exhibit decreased calcium mobilization associated with reduced expression of ORAI1. J Leukoc Biol. 2010;87:979–990. doi: 10.1189/jlb.0809575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan P G. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]