Abstract
Following activation through the TCR, CD4+ T cells can differentiate into three major subsets: Th1, Th2, and Th17 cells. IL-17-secreting Th17 cells play an important role in the pathogenesis of several autoimmune diseases and in immune responses to pathogens, but little is known about the regulation of apoptosis in Th17 cells. In this study, the sensitivity of in vitro-polarized Th1, Th2, and Th17 cells to Fas-mediated apoptosis was compared directly by different methods. The order of sensitivity of T cell subsets to Fas-mediated apoptosis is: Th1 > Th17 > Th2. The greater sensitivity of Th17 cells to Fas-mediated apoptosis compared with Th2 cells correlated with their higher expression of FasL and comparable expression of the antiapoptotic molecule FLIP. The decreased sensitivity of Th17 compared with Th1 cells correlated with the higher expression of FLIP by Th17 cells. Transgenic overexpression of FLIP in T cells protected all three subsets from Fas-mediated apoptosis. These findings provide new knowledge for understanding how survival of different subsets of T cells is regulated.
Keywords: FLIP, cell death, autoimmune
Introduction
In response to TCR cross-linking, CD4+ T cells can differentiate into several distinct subsets that can be distinguished on the basis of particular cytokines they secrete [1]. The Th1 subset secretes proinflammatory cytokines, such as IFN-γ, IL-2, and lymphotoxin. Th1 cells are essential for clearance of intracellular pathogens and are traditionally involved in cell-mediated immune responses. Th2 cells secrete cytokines such as IL-4, IL-13, IL-10, and IL-5, which are important for humoral immune responses. Th2 cells also play an important role in allergies and in responses to some parasites. Th17 cells secrete IL-17 and IL-22, proinflammatory cytokines that have been linked to the pathogenesis of autoimmune diseases and to immune responses to bacterial and fungal infections [1,2,3].
The balance between pro- and anti-inflammatory cytokines is important for development and resolution of inflammation [3]. As IL-17, produced by Th17 cells, is now recognized as an important proinflammatory cytokine that is increased in autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and psoriasis in humans [4,5,6], as well as in animal models of autoimmunity [2, 7,8,9,10], much attention has been focused on defining the role of Th17 cells in autoimmunity and tissue inflammation. The importance of Th17 cells in the pathogenesis of organ-specific autoimmune inflammation has been demonstrated in many different animal models [2, 7,8,9,10], but relatively little is known yet about the regulation of apoptosis of Th17 cells.
Apoptosis is an active process of cell death involving the sequential activation of a series of caspases after appropriate stimulation [11]. It can be induced by receptor-mediated or mitochondrial-mediated pathways, and the two pathways intersect each other [12]. Fas and FasL, DR5, and its ligand TRAIL all belong to the TNFR family [13]. They play important roles in many human and murine autoimmune diseases [11,12,13,14,15,16]. FLIP inhibits death receptor-mediated apoptosis by blocking activation of caspase-8 [17]. Bcl-xL belongs to the mitochondrial-mediated pathway and inhibits apoptosis by regulating mitochondrial membrane potential and cytochrome c release [12, 17, 18]. Fas, FasL, DR5, and TRAIL are important proapoptotic proteins, and FLIP and Bcl-xL are important antiapoptotic proteins [11,12,13,14,15,16,17,18].
The Fas/FasL pathway plays an important role in T cell apoptosis [19,20,21]. Although it is known that T cell subsets can differ in their relative sensitivity to apoptosis [19, 20, 22, 23], few studies have compared the sensitivity of Th1, Th2, and Th17 cells directly to Fas-mediated apoptosis. The goal of this study was to determine directly and compare the sensitivity of in vitro-polarized Th1, Th2, and Th17 cells with Fas-mediated apoptosis and to determine if the antiapoptotic molecule FLIP can inhibit apoptosis of each of the three subsets.
MATERIALS AND METHODS
Mice
Wild-type DBA/1 mice were generated in our breeding colony at the University of Missouri (Columbia, MO, USA) [24]. To generate CD2 FLIP-transgenic mice, a plasmid containing the recombinant CD2 FLIP construct (provided by Dr. Ralph Budd, University of Vermont, Burlington, VT, USA) was amplified in Escherichia coli and digested using KpnI and NotI (Invitrogen, Carlsbad, CA, USA). The 8.55-kb recombined construct fragment containing the FLAG-tagged mouse cFLIPL cDNA, β-globin, and CD2 enhancer was microinjected into fertilized oocytes from FVB female mice (Transgenic Core Facility, University of Missouri). Transgenic founders were screened by PCR of tail DNA using the following primers: sense, 5′-GGAGCCAGGGCTGGGCATAAAA-3′; antisense, 5′-GACTCACCCTGAAGTTCTCAGGATCC-3′. Two FVB-transgenic founders (one male and one female) were obtained. The transgenic female FVB founder was crossed with a DBA/1 male, and Tg+ F1 offspring were selected by PCR analysis of tail DNA. Tg+ F1 mice were backcrossed six times to DBA/1 mice by selecting for expression of the transgene and the DBA/1 coat color at each generation. Mice used for the indicated experiments were from the sixth backcross generations. FLIP Tg+ mice and Tg− littermates, 8–10 weeks old, were used. Mice were bred and maintained in accordance with the University of Missouri Institutional Guidelines for Animal Care.
CD4+ T cell enrichment and differentiation
CD4+ T cells were enriched by depletion of CD8+ and B220+ cells from splenocytes of naive DBA/1 mice using PE-labeled anti-CD8 and anti-B220 (eBioscience, San Diego, CA, USA) and an EasySep PE selection kit (StemCell Technologies, Canada), according to the manufacturers’ instructions. As determined by flow cytometry, >80% of the enriched cells were CD4+T cells, and >85% of CD8+ and B220+ cells were depleted. Enriched CD4+ T cells were cultured with anti-CD3 (2 μg/ml) and IL-2 (25 U/ml) for 90 h in 24-well plates at a concentration of 1 × 106 cells/well in complete RPMI medium [25]. These were considered to be nonpolarized cells (Th0) in the experiments described below. The culture medium was supplemented further with recombinant cytokines and/or anti-cytokine mAb as follows: for Th1 differentiation, rIL-12 (5 ng/ml) plus anti-IL-4 (2 μg/ml, clone 11B11); for Th2 differentiation, rIL-4 (500 U/ml) plus anti-IFN-γ (10 μg/ml, clone XMG1.2); and for Th17 differentiation, TGF-β (3 ng/ml), rIL-6 (20 ng/ml), anti-IL-4 (2 μg/ml, clone 11B11), and anti-IFN-γ (10 μg/ml, clone XMG1.2) [19, 25,26,27].
Flow cytometry
Polarized cells were washed and stained with FITC-conjugated anti-CD4 and PE-conjugated anti-Fas (Jo2) or PE-conjugated anti-FasL (Kay10) at 4°C for 30 min. For intracellular staining, polarized cells were washed, placed in fresh medium, and cultured with 50 ng/ml PMA and 500 ng/ml ionomycin at 37°C for 5 h. Brefeldin A (10 ug/ml) was added for the last 3 h of incubation. After staining with FITC-conjugated anti-CD4, cells were treated with freshly prepared fixation/permeabilization buffer (eBioscience) and incubated at 4°C for 45 min. After washing with 1× permeabilization buffer (eBioscience), intracellular staining was performed at 4°C for 45 min using APC-conjugated anti-IFN-γ together with PE-conjugated anti-IL-5 or PE-conjugated anti-IL-17. All antibodies were from eBioscience. CellQuest software and FACSCalibur were used for data collection, and FlowJo software was used for data analysis.
Expression of Annexin V (a marker for apoptosis) and staining with PI (a vital dye) on polarized T cells stimulated with and without anti-Fas were also analyzed by flow cytometry (FACScan) using an Annexin V apoptosis detection kit (eBioscience). AnnexinV+PI− cells are early apoptotic cells, and Annexin V+PI+ cells are late apoptotic cells [28, 29].
Induction of apoptosis
Following 72 h culture with anti-CD3 and cytokines as described above, cells were washed and placed in fresh medium without cytokines or anti-CD3 and cultured overnight (16 h) in the presence of 1 μg/ml agonist anti-Fas (Jo-2, BD PharMingen, San Diego, CA, USA) or 1 ug/ml anti-CD3. The amount of anti-Fas used in these experiments was found to be optimal in earlier studies with cultured thyrocytes [17] and splenocytes (our upublished data).
TUNEL staining
Apoptosis of T cell subsets was determined by TUNEL assay using an Apoptag kit (Chemicon, El Segundo, CA, USA) as described previously [16, 30]. To quantify the number of apoptotic cells, all cells in five to six randomly selected high-power fields (magnification, ×400) were counted manually using image analysis software MetaMorph, Version 6.3r6 (MDS Analytical Technologies, Sunnyvale, CA, USA). Positive cells were expressed as a percentage of total cells. Δ Apoptosis was expressed as the percent TUNEL+ cells with anti-Fas minus the percent TUNEL+ cells without anti-Fas or anti-CD3.
Measurement of caspase-3 activity
Cellular caspase-3 activity recognizing the sequence DEVD was measured using a caspase-3/CPP32 colorimetric assay kit (BioVision, Mountain View, CA, USA). After overnight treatment with anti-Fas, polarized T cells were lysed in lysis buffer and centrifuged at 10,000 g for 10 min. The supernatant was harvested, 50 μg protein was diluted to 50 μl with lysis buffer, and the lysate was mixed with 50 μl 2× reaction buffer (containing 10 mM DTT) and 200 μM DEVD-pNA. The reaction was performed at 37°C for 2 h. The cleaved pNA, with a light emission at 400 nm, was quantified using a spectrophotometer (BioRad, Hercules, CA, USA). The fold increase in caspase-3 activity (relative caspase-3 activity) was determined by comparing the absorbance of pNA from samples treated with anti-Fas with that from samples treated with isotype IgG. Assays were done in triplicate.
RT-PCR
Cultured T cells were washed with PBS, centrifuged, and homogenized in TRIzol (Invitrogen). RNA was extracted and reverse-transcribed as described previously [31, 32]. β-Actin was used as a housekeeping gene to verify that the same amount of RNA was amplified. Primers were described previously [30,31,32].
Confocal laser-scanning immunofluorescence microscopy
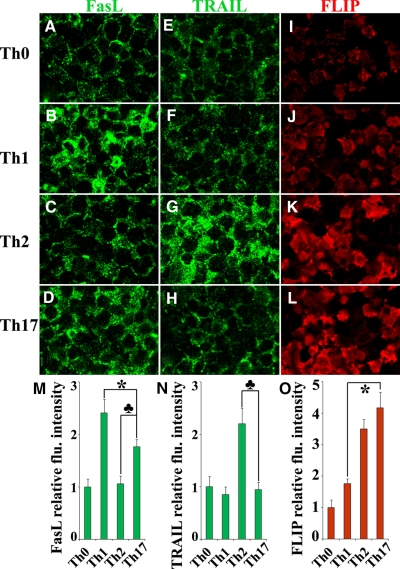
To detect differential expression of FasL, TRAIL, and FLIP by polarized Th1, Th2, and Th17 cells, immunofluorescence and confocal laser-scanning microscopy were performed as described previously [30] using rabbit anti-FasL, anti-TRAIL (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-FLIP (Abcam, Cambridge, MA, USA). FasL and TRAIL were visualized by Alexa 488 (green; Molecular Probes, Eugene, OR, USA), and FLIP was visualized by Alexa 568 (red; Molecular Probes). Slides were observed with a BioRad Radiance 2000 confocal system coupled to an Olympus IX70 inverted microscope. As a negative control, primary antibody was replaced with an equal amount of normal rabbit IgG, and these controls were always negative. The staining intensity in five to six randomly selected high-power fields of three slides from each group was analyzed by MetaMorph software. Images were also arranged into panels, and the red or green channel was saved as a grayscale Tagged Image File Format file using Photoshop CS2 (Adobe, San Jose, CA, USA). Average integrated intensity within the area covered by cells was measured for each panel in the grayscale images using MetaMorph image analysis software. Results are expressed as the average integrated fluorescence intensity of three slides ± sem relative to that of Th0 cells.
ELISA assay
Supernatants of polarized T cells were collected and frozen at –20°C. IFN-γ, IL-5, and IL-17 concentrations in supernatants were determined using ELISA kit (mouse ELISA Ready-Set-Go) from eBioscience as instructed.
Statistics
All experiments were repeated two or three times. Statistical analysis of data was performed using an unpaired two-tailed Student’s t-test. A P value <0.05 was considered significant.
RESULTS
Polarization of enriched splenic CD4+ T cells
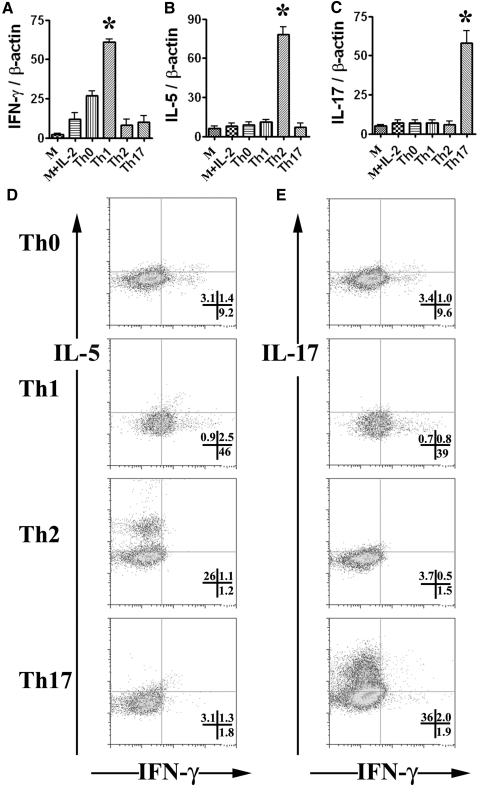
CD4+ T cells proliferate and differentiate to Th1, Th2, or Th17 cell subsets in the presence of specific cytokines [1, 2]. To compare the sensitivity of Th1, Th2, and Th17 cells with Fas-mediated apoptosis, CD4+ T cells enriched from spleens of DBA/1 mice were cultured under different conditions, as described in Materials and Methods. RT-PCR analysis showed that IFN-γ, IL-5, and IL-17 mRNA increased significantly in enriched CD4+ T cells cultured under Th1, Th2, and Th17 polarization conditions, respectively (Fig. 1, A–C). IFN-γ but not IL-5 or IL-17 mRNA also increased in enriched CD4+ T cells cultured under Th0 culture conditions (Fig. 1, A–C). Consistent with the mRNA levels, IHC showed that enriched CD4+ T cells cultured under Th1, Th2, and Th17 polarization conditions expressed higher IFN-γ, IL-5, and IL-17 protein, respectively (data not shown).
Figure 1.
Polarization of enriched CD4+ T cells from mouse spleens. Splenic CD4+ T cells from DBA/1 mice were enriched using magnetic beads and cultured for 72 h in medium alone (M), medium + IL-2 (M+IL-2), anti-CD3 and IL-2 (Th0), or anti- CD3 and IL-2 under Th1, Th2, and Th17 polarization conditions, and mRNA was extracted and amplified by RT-PCR as described in Materials and Methods. IFN-γ, IL-5, and IL-17 mRNA levels relative to β-actin are shown (A–C). Results are expressed as the mean ratio of cytokine densitometric units/β-actin ± sem (×100) of five wells/group and are representative of three independent experiments. A significant difference among Th1, Th2, or Th17 cells with others is indicated (*, P<0.05). (D and E) Polarized cells were stimulated with PMA and ionomycin for 5 h, with addition of brefeldin A during the final 3 h. After staining with FITC anti-CD4, fixed and permeabilized cells were stained with APC-conjugated anti-IFN-γ together with PE-conjugated anti-IL-5 or PE-conjugated anti-IL-17. Representative data are shown (D and E), and numbers in quadrants indicate the frequency of cells staining positive for the indicated cytokines.
Intracellular cytokine staining was used to confirm and quantitate the extent of polarization to the desired T cell subsets by measuring the signature cytokines IFN-γ, IL-5, and IL-17. As shown in Figure 1, D and E, each T cell subset was enriched for production of the appropriate cytokine, e.g., Th1 cells highly expressed IFN-γ but expressed little IL-5 or IL-17, whereas Th17 cells expressed IL-17 but little IFN-γ or IL-5, and Th2 cells expressed IL-5 but little IL-17 or IFN-γ. IFN-γ, IL-5, and IL-17 concentrations in supernatants of polarized T cells were also determined by ELISA. Although IL-5 protein was not detected in culture supernatants, enriched CD4+ T cells cultured under Th1 conditions produced high amounts of IFN-γ (>3000 pg/ml) and little IL-17 (3–8 pg/ml), and cells cultured under Th17 polarization conditions produced high amounts of IL-17 (7000–12,000 pg/ml) and little IFN-γ (<6 pg/ml; data not shown). These results indicate that the culture conditions used here effectively polarized enriched CD4+ T cells to Th1, Th2, and Th17 cells.
Sensitivity of CD4+ T cell subsets to Fas-mediated apoptosis
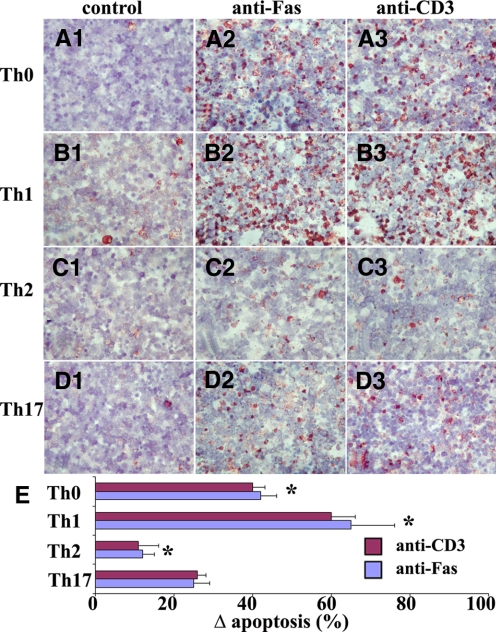
After stimulation with anti-CD3 and cytokines as described in Materials and Methods, T cells were stimulated overnight with isotype control IgG, agonist anti-Fas (1 μg/ml), or anti-CD3 (1 μg/ml), and apoptotic cells were determined by TUNEL staining. Few or no TUNEL+cells (red) were detected in any T cell subsets cultured with isotype IgG (Fig. 2, A1–D1). Apoptosis was increased in cell subsets cultured with agonist anti-Fas or anti-CD3 (Fig. 2, A2–D2 and A3–D3), but Th0, Th1, Th2, and Th17 cells differed greatly in their sensitivity to apoptosis induced by anti-Fas or by anti-CD3. TUNEL+ cells (red) in five to six randomly selected high-power fields of three slides from each group were counted, and the results are summarized in Figure 2E. Th1 cells were more sensitive than the other T cell subsets to apoptosis induced by restimulation through the TCR (anti-CD3) or by cross-linking Fas, and Th2 cells were the most resistant to apoptosis (Fig. 2E). These differences were also evident by staining for active caspase-3 (another important marker for apoptosis) using IHC (percentage of caspase-3+ cells: Th1, 80%; Th0, 39%; Th17, 28%; and Th2, 16%; data not shown). When caspase-3 activity was assayed semi-quantitatively using the colorometric detection method described in Materials and Methods, the relative caspase-3 activity of each T cell subset was consistent with the differences observed by IHC, and Th1 cells had more caspase activity than the other T cell subsets (Fig. 3A).
Figure 2.
Comparison of sensitivity of T cell subsets with Fas-mediated apoptosis by TUNEL staining. T cell subsets from enriched CD4+ T cells were generated as described in Materials and Methods. After overnight culture with isotype IgG (A1–D1), agonist anti-Fas (A2–D2) or anti-CD3 (A3–D3) apoptotic cells were determined by TUNEL staining. TUNEL+ cells (red) in five to six randomly selected high-power fields of three slides/group were counted using MetaMorph, and TUNEL+ cells were expressed as a percentage of total cells. Δ Apoptosis represents the percentage of TUNEL+ cells in the presense of anti-Fas or anti-CD3 minus the percentage of TUNEL+ cells with isotype IgG. Results are expressed as the mean Δ apoptosis ± sem (E). Results are representative of three independent experiments. A significant difference in the sensitivity to Fas-mediated or anti-CD3-induced apoptosis between Th17 cells and other groups is indicated (*, P<0.05). Original magnification, A1–D3, ×400.
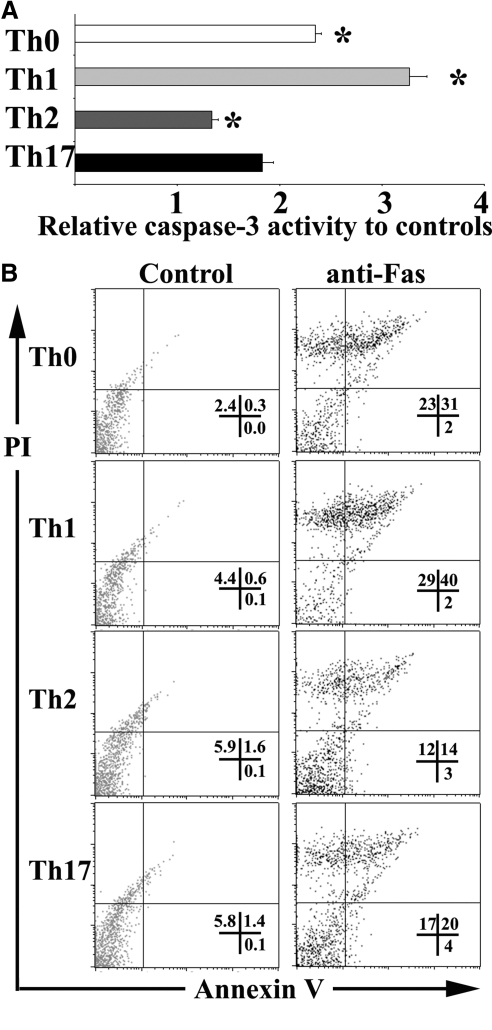
Figure 3.
Analysis of sensitivity of T cell subsets to Fas-mediated apoptosis by measurement of capase-3 activity and Annexin V staining. Polarized T cells from enriched CD4+ T cells were generated as described in Materials and Methods. (A) Cellular caspase-3 activity, recognizing the sequence DEVD, was measured using a caspase-3/CPP32 colorimetric assay kit as described in Materials and Methods. The fold increase in caspase-3 activity was determined by comparing the absorbance of pNA from samples treated with anti-Fas with that from samples treated with isotype IgG. Results are expressed as mean caspase-3 activity relative to controls ± sem. Assays were done in triplicate. A significant difference between Th17 cells and other groups is indicated (*, P<0.05). (B) After overnight stimulation with anti-Fas, apoptotic CD4+ T cells were determined by Annexin V and PI staining by gating on CD4+ cells. Numbers are the percentage of CD4+ cells in the corresponding quadrants. Results are representative of two independent experiments.
Similar differences in sensitivity to Fas-mediated apoptosis were obtained using flow cytometry for detection of Annexin V, another important marker for apoptosis (Fig. 3B). In the absence of anti-Fas, few cells (<2%) were Annexin V+, and the percentages of Annexin V+ cells in each subset were comparable. In the presence of anti-Fas, the percentages of Annexin V+ cells increased as follows: Th0, 33%; Th1, 42%; Th2, 17%; and Th17, 24% (Fig. 3). Thus, the order of sensitivity of polarized Th cells to Fas-mediated apoptosis is: Th1 > Th0 > Th17 > Th2, as indicated by several different apoptosis detection methods (Table 1).
TABLE 1.
Summary of the Characteristics of Sensitivity of Th0, Th1, Th2, and Th17 Cells to Fas-Mediated Apoptosisa
| Method | Th0 | Th1 | Th2 | Th17 |
|---|---|---|---|---|
| TUNEL stainingb | 42% | 65% | 12% | 25% |
| Caspase-3 activityc | 2.4 | 3.3 | 1.3 | 1.8 |
| Annexin V stainingd | 32% | 42% | 17% | 24% |
TUNEL+ cells were expressed as a percentage of total cells. Δ Apoptosis represents the percentage of TUNEL+ cells in the presence of anti-Fas minus the percentage of TUNEL+ cells with isotype IgG. Results are the mean Δ apoptosis and are representative of three independent experiments.
Results are mean fold increase in caspase-3 activity of T cell subsets treated with anti-Fas relative to controls by caspase-3/CPP32 colorimetric assay.
Results are representative data of Annexin V+ staining of T cell subsets treated with anti-Fas.
Expression of pro- and antiapoptotic molecules in polarized T cells
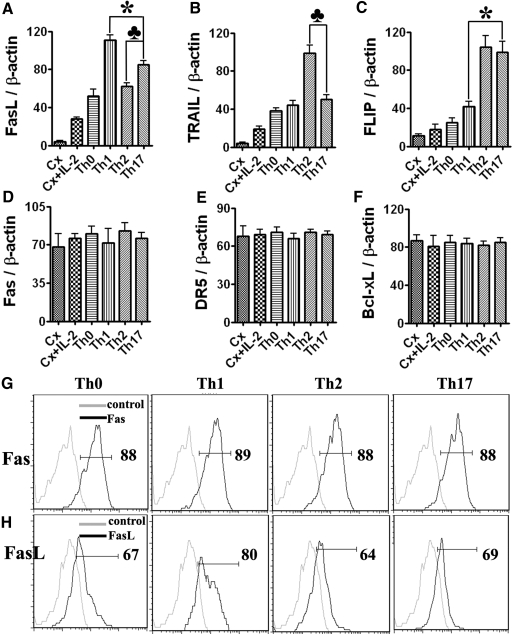
The balance between pro- and antiapoptotic molecules plays an important role in apoptosis [11,12,13,14,15,16,17,18]. To gain insight into the basis for the differential sensitivity of Th1, Th2, and Th17 cells to Fas-mediated apoptosis, mRNA expression of several pro- and antiapoptotic molecules in polarized CD4+ T cells was determined by RT-PCR (Fig. 4). There was minimal expression of the proapoptotic molecules FasL and TRAIL and the antiapoptotic molecule FLIP in cells cultured in the absence of anti-CD3, in the presence and absence of IL-2, and all three molecules were up-regulated following anti-CD3 stimulation. FasL mRNA expression in Th17 cells was lower than in Th1 cells but higher than in Th2 or Th0 cells (Fig. 4A), and TRAIL mRNA expression was higher in Th2 cells than in the other three subsets (Fig. 4B). Expression of the antiapoptotic molecule FLIP was higher in Th2 and Th17 cells compared with Th1 or Th0 cells (Fig. 4C), and mRNA expression of Fas, DR5, and Bcl-xL was similar in Th0, Th1, Th2, and Th17 cells (Fig. 4, D–F). Protein expression of Fas and FasL by CD4+ T cells of each subset was also quantitated by flow cytometry (Fig. 4, G and H). Consistent with the mRNA expression, the percentage of Fas-positive cells was similar for each T cell subset, indicating that the greater sensitivity of Th1 cells to apoptosis was not a result of higher expression of Fas by Th1 cells, which had the highest percentage of FasL-positive cells (Fig. 4, G and H).
Figure 4.
mRNA expression of pro- and antiapoptotic molecules in T cell subsets. mRNA was extracted from polarized T cells and amplified by RT-PCR as described in Materials and Methods. mRNA expression level of FasL, TRAIL, FLIP, Fas, DR5, and Bcl-xL is shown (A–F). Results are expressed as the mean ratio of cytokine densitometric units/β-actin ± sem (×100) of five wells/group and are representative of three independent experiments. A significant difference between Th17 and Th1 cells is indicated (*, P<0.05). A significant difference between Th17 and Th2 cells is indicated (♣, P<0.05). Cx, enriched CD4+ T cells without cytokine treatment. To evaluate Fas and FasL expression in CD4+ T cells, polarized cells were washed and stained with FITC-conjugated anti-CD4 and PE-conjugated anti-Fas or PE-conjugated anti-FasL. The percentage of Fas+ (G) or FasL+ cells (H) in CD4+ T cells in different T cell subsets is indicated. Results are representative of two independent experiments.
Confocal microscopy was also used to determine the relative fluoresence intensity of apoptotic and antiapoptotic molecules in the various T cell subsets (Fig. 5). The intensity of FasL protein expression in Th17 cells was weaker than in Th1 cells but stronger than in Th2 cells, and the fluorescence intensity of the TRAIL protein was higher in Th2 cells than in other subsets, and the intensity of FLIP protein in Th17 and Th2 cells was stronger than in Th1 or Th0 cells (Fig. 5). Consistent with the results obtained by mRNA and flow cytometry, the intensity of Fas protein expression in different T cell subsets was similar (data not shown). These results indicate that down-regulation of the proapoptotic molecule FasL and up-regulation of the antiapoptotic molecule FLIP in Th17 cells at mRNA and protein levels correlated with their reduced sensitivity to Fas-mediated apoptosis compared with Th1 cells. On the other hand, increased expression of FasL mRNA and protein in Th17 cells correlated with their higher sensitivity to Fas-mediated apoptosis compared with Th2 cells, although Th2 and Th17 cells expressed comparable levels of FLIP.
Figure 5.
Protein expression of pro- and antiapoptotic molecules in T cell subsets. FasL (A–D), TRAIL (E–H), and FLIP (I–L) protein expression in polarized T cells from enriched CD4+ T cells was detected by confocal microscopy. Proapoptotic molecules FasL and TRAIL were visualized by Alexa 488 (green) and antiapoptotic molecule FLIP by Alexa 568 (red). The staining intensity in five to six randomly selected high-power fields of three slides from each group was analyzed by MetaMorph software. Results are expressed as the average integrated fluorescence intensity of three slides ± sem relative to that of Th0 cells (M–O). A significant difference in the fluorenscence intensity between Th17 and Th1 cells is indicated (*, P<0.05). A significant difference in the fluorenscence intensity between Th17 and Th2 cells is indicated (♣, P<0.05). Shown are representative areas on slides from two independent experiments. Original magnification, A–L, ×800.
Transgenic overexpression of FLIP protects Th0, Th1, Th2, and Th17 cells from Fas-mediated apoptosis
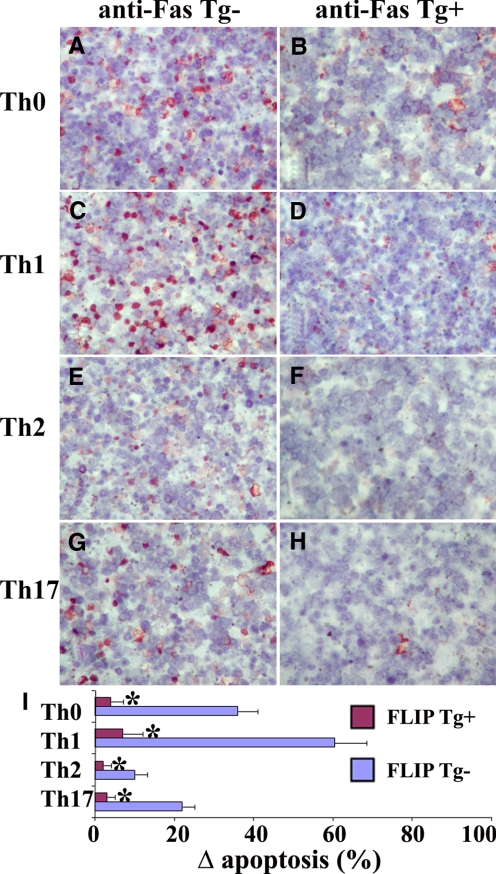
As shown in Figure 4, higher expression of FLIP in Th17 compared with Th1 cells correlated with the reduced sensitivity of Th17 cells to Fas-mediated apoptosis. To determine directly if transgenic overexpression of FLIP in T cells could protect polarized T cells from Fas-mediated apoptosis, transgenic mice were generated that expressed cFLIPL under control of the CD2 promoter. Western blot indicated that FLIP-transgenic mice expressed the transgene at levels over fivefold higher than endogenous FLIP in CD4+and CD8+T cells as well as in B cells (our unpublished data). CD4+ T cells isolated from CD2 FLIP Tg+ DBA/1 mice and their Tg− littermates were polarized as in previous experiments. After stimulation with anti-Fas as described in Materials and Methods, many apoptotic TUNEL+cells (red) were detected in polarized Th0 and Th1 cells of FLIP Tg− mice, and few TUNEL+ cells were detected in their Th2 and Th17 cells (Fig. 6, A, C, E, and G). Many fewer TUNEL+ cells of any subset were detected in T cells from FLIP Tg+ mice (Fig. 6, B, D, F, and H). When TUNEL+cells (red) in five to six randomly selected high-power fields of three slides/group were manually counted and summarized (Fig. 6I), the results indicated that transgenic overexpression of FLIP inhibited apoptosis in all T cell subsets (Th0, Th1, Th2, and Th17 cells).
Figure 6.
Transgenic overexpression of FLIP in T cells protects T cell subsets from Fas-mediated apoptosis. CD4+ T cells enriched from CD2 FLIP Tg+ DBA/1 mice and their Tg− littermates were polarized and stimulated with anti-Fas as described in Materials and Methods. Apoptotic cells were determined by TUNEL staining (A–H). TUNEL+ cells (red) in five to six randomly selected high-power fields of three slides/group were counted using MetaMorph. TUNEL+ cells were expressed as a percentage of total cells. Δ Apoptosis represents the percentage of TUNEL+ cells in the presence of anti-Fas minus the percentage of TUNEL+ cells with isotype IgG. Results are expressed as the mean Δ apoptosis ± sem and summarized. Shown are representative areas of slides from three independent experiments. A significant difference in the percentage of TUNEL+ cells of polarized T cells from FLIP Tg+ mice compared with Tg− littermates is indicated (*, P<0.05). Original magnification, A–H, ×800.
DISCUSSION
The balance between various T cell subsets and the cytokines they produce dictates the nature of an inflammatory response [1,2,3, 33,34,35]. The rate of elimination of particular subsets of effector cells when an immune response is terminated plays an important role in determining whether an inflammatory response will be chronic or will resolve. The Fas pathway is important for apoptosis of T cells activated during an immune response, although other pathways are also involved, as in the case of Th2 cells, where the granzyme B pathway has been shown to be important [36]. Th17 cells have been shown to be important effector cells for some autoimmune diseases such as experimental autoimmune encephalomyelitis, arthritis, and colitis [1, 7,8,9,10, 37, 38] and are important for clearing some infections [1, 2]. However, little is known yet about the sensitivity to Fas-mediated apoptosis of Th17 cells compared with nonpolarized Th0, Th1, or Th2 subsets. The goal of this study was to compare directly the sensitivity of short-term in vitro-polarized subsets of CD4+ T cells with Fas-mediated apoptosis and to determine if differences in expression of the antiapoptotic molecule FLIP might influence their sensitivity to Fas-mediated apoptosis. By multiple approaches, Th17 cells were shown to be more sensitive than Th2 cells but less sensitive than Th1 or Th0 cells to Fas-mediated or anti-CD3-induced apoptosis. The greater sensitivity to Fas-mediated apoptosis of Th17 compared with Th2 cells is consistent with their higher expression of FasL, and the decreased sensitivity to Fas-mediated apoptosis of Th17 compared with Th1 cells is consistent with their higher expression of FLIP (Fig. 4). Furthermore, transgenic overexpression of FLIP in T cells protected polarized Th1, Th2, and Th17 cells, as well as nonpolarized Th0 cells, from Fas-mediated apoptosis (Fig. 6). Thus, the difference in sensitivity among Th1, Th2, and Th17 cells to Fas-mediated apoptosis is clear-cut and provides a means to distinguish these T cell subsets in addition to their known differences in cytokine production.
Recent studies using a model of eye inflammation showed that donor Th1 cells effectively recruited recipient CD4+ cells and rapidly became a minority among the infiltrating cells, whereas donor Th17 cells remained in the majority for up to 1 week [27]. These studies suggested that Th17 and Th1 cells might differ in their sensitivity to apoptosis during the development of autoimmune inflammation, and subsequent studies by this group established that Th17 cells were in fact more resistant than Th1 cells to apoptosis induced after restimulation by antigen [39]. Consistent with those in vivo studies, this in vitro study demonstrates directly that Th17 cells are less sensitive than Th1 cells to Fas-mediated apoptosis, and recent studies by others [40] indicate that Th17 cells are less sensitive to activation-induced cell death compared with Th1 cells. Thus, the superiority of Th17 cells in inducing inflammation and the rapid decline of donor Th1 cells, but not Th17 cells, in recipient eyes might be attributed, at least in part, to the lesser sensitivity of Th17 cells to Fas-mediated apoptosis. Differences in the sensitivity of Th1 and Th17 subsets to Fas-mediated and anti-CD3-induced apoptosis could have a major impact on the development or/and resolution of inflammation. The results of this study identify a major functional difference between Th1 and Th17 cells, which could result in the preferential survival of Th17 cells and might be relevant in some in vivo disease states where Th17 dominance is observed.
Although the T cell subsets used in this study were short-term in vitro-polarized cells, 46% of enriched CD4+ T cells were effectively polarized to Th1 cells, 36% to Th17 cells, and 26% to Th2 cells (Fig. 1). However, many CD4+ T cells had not completely differentiated to Th1, Th17, or Th2 cells. Therefore, the T cell subsets studied here include differentiated Th1, Th17, and Th2 cells as well as Th0 CD4+ T cells, which may still be in the process of differentiating to more polarized Th1, Th17, or Th2 cells.
In a previous study, Th17 cells exhibited significant, spontaneous cell death in the absence of IL-2 [20]. In this study, Th0, Th1, Th2, and Th17 cells were generated in the presence of IL-2 using conditions comparable with those used previously by J. S. Ellis and others [19, 20, 22, 25,26,27]. Although spontaneous cell death was greater in all T cell subsets when cells were polarized in the absence of IL-2, the presence or absence of IL-2 did not influence the relative differences in Fas-mediated apoptosis of polarized T cells shown in this study (data not shown). Splenocytes from IFN-γ−/− mice were also used in this study, as IFN-γ has been shown to increase Fas-mediated apoptosis of some T cell subsets [19, 20]. The results were consistent with results of others [19, 20], indicating that Fas-mediated apoptosis of T cells activated under Th1 polarization-inducing conditions was dependent on IFN-γ, and Fas-mediated apoptosis of Th17 and Th2 cells was IFN-γ-independent (data not shown).
As stated in Materials and Methods, Annexin V+PI− cells are early-stage apoptotic cells, Annexin V+PI+ cells are late-stage apoptotic cells, and Annexin V−PI+ cells are dead cells. These three stages represent a continuous process of apoptosis, and early-stage apoptosis progresses to late-stage apoptosis and finally to death. As shown in Figure 3B, few early-stage apoptotic cells were detected when T cells were treated with anti-Fas for 16 h, and most apoptotic cells were found in late-stage apoptosis or as dead cells. There was greater apoptosis in Th1 cells compared with Th17 and in Th17 cells when compared with Th2 cells. To address the possibility that the paucity of early-stage apoptotic cells was a result of the overnight (16-h) treatment with anti-Fas, we performed other experiments in which the polarized T cells were treated with anti-Fas for 8 h or 12 h. There were many early apoptotic cells when cells were exposed to anti-Fas for 8 h or 12 h, but there was little difference in the percentages of apoptotic cells among Th1, Th2, and Th17 cells (data not shown). Therefore, we believe that 16 h of treatment of polarized T cells with anti-Fas is the optimal time to detect differences in sensitivity of T cell subsets to Fas-mediated or anti-CD3-induced apoptosis.
Unequal death of Th17 and Th1 cells could be explained by several intrinsic differences between the two subsets, as pro- and antiapoptotic molecules play a role in apoptosis [11,12,13,14,15,16,17,18]. This study focused on several major pro-and antiapoptotic molecules studied in our laboratory [16, 17, 41, 42]. Although the proapoptotic molecule FasL was up-regulated in Th1 and Th17 cells compared with Th0, Th2, or nonactivated T cells, increased expression of the antiapoptotic molecule FLIP in Th17 compared with Th1 cells (Fig. 4) is likely to be critical for the greater resistance of Th17 cells to apoptosis. The importance of FLIP was confirmed further by results obtained using transgenic mice overexpressing FLIP on lymphocytes, as all subsets of T cells were protected from Fas-mediated apoptosis by transgenic overexpression of FLIP (Fig. 6). Consistent with our results, a critical role for differences in FLIP expression by Th1 versus Th17 cells was shown recently by others [40] to be important in determining differences in the sensitivity of these two subsets to activation-induced cell death. Our studies confirm those results and extend them by demonstrating that the importance of FLIP expression levels in determining the differential sensitivity of Th1 and Th17 cells to apoptosis also applies to Fas-mediated apoptosis. The results presented here suggest further that expression levels of FasL and FLIP may contribute to the unequal death of Th17 and Th1 cells.
T cells from FLIP-transgenic mice are not immortal, as T cells from FLIP-transgenic and nontransgenic mice are equally sensitive to irradiation-mediated apoptosis (our unpublished data). On the other hand, the proapoptotic molecules Fas and DR5 and the antiapoptotic molecule Bcl-xL are comparably expressed in Th0, Th1, Th2, and Th17 cells (Fig. 4). As expression of Fas was similar in all T cell subsets (Fig. 4), differences in Fas expression do not explain the differential sensitivity of T cell subsets to Fas-mediated apoptosis. Our results are consistent with those of others showing that Bcl-xL expression was similar in Th1 and Th2 cells [23]. As Bcl-xL is an important apoptotic molecule in the mitochondrial signal pathway, it may not contribute to apoptosis induced by death receptor-mediated pathways such as the Fas/FasL pathway in mice as suggested by others [43,44,45]. Other molecules that negatively regulate apoptosis by the death receptor or mitochondrial pathways might play another role in protecting Th17 cells.
In this study, Th2 cells were shown to be less sensitive than Th17 cells to Fas-mediated apoptosis. As Th17 and Th2 cells expressed similar levels of FLIP, FLIP is unlikely to contribute significantly to the difference in sensitivity of Th17 and Th2 cells to Fas-mediated apoptosis. Th2 cells expressed less FasL, and this may be the most important basis for their relative resistance to Fas-mediated apoptosis compared with the other T cell subsets. Although TRAIL was higher in Th2 cells compared with Th17 or Th1 cells (Fig. 4), the lesser sensitivity of Th2 cells to Fas-mediated apoptosis suggests that neither the TRAIL nor the Fas pathway was the predominant factor determining apoptosis of Th2 cells. Similar results were also reported by others [19] when comparing the sensitivity of Th1 and Th2 cells with death receptor-mediated apoptosis. In our studies, Th2 cells expressed a much higher granzyme B message compared with the other subsets (data not shown). This is consistent with the fact that apoptosis in Th2 cells may be controlled primarily by molecules such as granzyme B [36].
In conclusion, Th17 cells are less sensitive than Th1 cells to Fas-mediated apoptosis as a result of their higher expression of FLIP, and Th17 cells are more sensitive to Fas-mediated apoptosis than Th2 cells as a result of their higher expression of FasL. These findings provide new knowledge for distinguishing T cell subsets in addition to their known differences in cytokine production and functions in immune response and autoimmune diseases. This information may be relevant for devising new strategies for controlling some infectious diseases and for controlling inflammation and tissue damage in autoimmune diseases.
AUTHORSHIP
Helen Braley-Mullen and Yujiang Fang designed the study. Yujiang Fang performed all of the experiments. Helen Braley-Mullen and Yujiang Fang analyzed and interpreted the data. Yujiang Fang wrote the draft, and Helen Braley-Mullen revised the manuscript. Shiguang Yu, Jason S. Ellis, and Tumenjargal Sharav helped with the flow cytometry and analysis.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant DK35527 and the Arthritis Foundation Eastern Missouri Chapter. We thank Dr. Gerardo Fernandez (Molecular Cytology Core) for assistance with image analysis.
DISCLOSURE
The authors have no conflicts of interest to disclose.
Footnotes
Abbreviations: APC=allophycocyanin, Bcl-xL=B cell leukemia/lymphoma extra long, cFLIPL=long form of cellular Fas-associated death domain-like inhibitory protein, DEVD=Asp-Glu-Val-Asp, DR5=death receptor 5, FasL= Fas ligand, FLIP=Fas-associated death domain-like IL-1β-converting enzyme inhibitory protein, IHC=immunohistochemistry, PI=propidium iodide, pNA=para-nitroanilide, Tg+/Tg−=transgenic positive/negative strains
References
- Bettelli E, Korn T, Oukka M, Kuchroo V K. Induction and effector functions of Th17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Betteli E, Oukka M, Kuchroo V K. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- O'Shea J J, Murray P J. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giulian F, Arbour N, Becher B, Prat A. Human Th17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh M L, Miossec P. The role of T cells in rheumatoid arthritis: new subsets and new targets. Curr Opin Rheumatol. 2007;19:284–288. doi: 10.1097/BOR.0b013e32805e87e0. [DOI] [PubMed] [Google Scholar]
- Teunissen M B, Koomen C W, de Waal Malefyt R, Wierenga E A, Bos J D. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Cua D J, Sherlock J, Chen Y, Murphy C A, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira S A, Gorman D, Kastelein R A, Sedgwich J D. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sude K, Iwakura Y. Suppression of immue induction of collagen-induced arthritis in IL-17 deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek M A, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua D J, Kastelein R A, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xu Q, Krajewski S, Krajewska M, Xie Z, Fuess S, Kitada S, Paulowski K, Godzik A, Reed J C. BAR: an apoptosis regulator at the intersection of caspases and Bcl-2 family proteins. Proc Natl Acad Sci USA. 2000;97:2597–2602. doi: 10.1073/pnas.97.6.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson F A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Stassi G, De Maria R. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat Rev Immunol. 2002;2:195–204. doi: 10.1038/nri750. [DOI] [PubMed] [Google Scholar]
- Suvannavejh G C, Dal Canto M C, Matis L A, Miller S D. Fas-mediated apoptosis in clinical remissions of relapsing experimental autoimmune encephalomyelitis. J Clin Invest. 2000;105:223–231. doi: 10.1172/JCI8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Sharp G C, Yagita H, Braley-Mullen H. A critical role for TRAIL in resolution of granulomatous experimental autoimmune thyroiditis. J Pathol. 2008;216:505–513. doi: 10.1002/path.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Braley-Mullen H. Cultured murine thyroid epithelial cells expressing transgenic Fas-associated death domain-like interleukin-1β converting enzyme inhibitory protein are protected from Fas-mediated apoptosis. Endocrinology. 2008;149:1121–1129. doi: 10.1210/en.2008-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Zhang X R, Zhang L Y, Devadas S, Li L, Keegan A D, Shi Y F. Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: role of apoptosis in T helper subset differentiation. Cell Death Differ. 2003;10:203–210. doi: 10.1038/sj.cdd.4401138. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu G, Zhang L, Roberts A I, Shi Y. Th17 cells undergo Fas-mediated activation-induced cell death independent of IFN-γ. J Immunol. 2008;181:190–196. doi: 10.4049/jimmunol.181.1.190. [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen K, Sharp G C, Yagita H, Braley-Mullen H. Expression and regulation of Fas and Fas ligand on thyrocytes and infiltrating cells during induction and resolution of granulomatous experimental autoimmune thyroiditis. J Immunol. 2001;167:6678–6686. doi: 10.4049/jimmunol.167.11.6678. [DOI] [PubMed] [Google Scholar]
- Toscano M A, Branco G A, Ilarregui J M, Croci D O, Correale J, Hernandez J D, Zwirner N W, Poirier F, Riley E M, Baum L, Rabinovich G A. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- Zhang X, Brunner T, Carter L, Dutton R W, Rogers P, Bradley L, Sato T, Reed J C, Green D, Swain S L. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Wei Y, Sharp G C, Braley-Mullen H. Decreasing TNF-α results in less fibrosis and earlier resolution of granulomatous experimental autoimmune thyroiditis. J Leukoc Biol. 2007;81:306–314. doi: 10.1189/jlb.0606402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Tartar D M, Gregg R K, Divekar R D, Bell J J, Lee H H, Yu P, Ellis J S, Hoeman C M, Franklin C L, Zaghouani H. Innocuous IFNγ induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–218. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummvoll G H, DiPaolo R J, Huter E N, Davidson T S, Glass D, Ward J M, Shevach E M. Th1, Th2 and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C A, Shi G, Yin H, Vistica B P, Wawrousek E F, Chan C C, Gery I. Both Th1 and Th17 are immunopathogenic but differ in other key biological activities. J Immunol. 2008;180:7414–7422. doi: 10.4049/jimmunol.180.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidyl serine expression on early apoptotic cells using fluorescein labeled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Kurita-Ochiai T, Amano S, Fukushima K, Ochiai K. Cellular events involved in butyric acid-induced T cell apoptosis. J Immunol. 2003;171:3576–3584. doi: 10.4049/jimmunol.171.7.3576. [DOI] [PubMed] [Google Scholar]
- Fang Y, Sharp G C, Braley-Mullen H. IL-10 promotes resolution of granulomatous experimental autoimmune thyroiditis. Am J Pathol. 2008;172:1591–1602. doi: 10.2353/ajpath.2008.071067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, DeMarco V G, Sharp G C, Braley-Mullen H. Expression of transgenic FLIP on thyroid epithelial cells inhibits induction and promotes resolution of granulomatous experimental autoimmune thyroiditis in CBA/J mice. Endocrinology. 2007;148:5734–5745. doi: 10.1210/en.2007-0939. [DOI] [PubMed] [Google Scholar]
- Fang Y, Wei Y, DeMarco V, Chen K, Sharp G C, Braley-Mullen H. Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis in DBA/1 mice. Am J Pathol. 2007;170:875–887. doi: 10.2353/ajpath.2007.060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R L. Origins of the TH1-TH2 model: a personal perspective. Nat Immunol. 2006;7:539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo V K. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Steinman L. A brief history of TH-17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Devadas S, Das J, Liu C, Zhang L, Roberts A I, Pan Z, Moore P A, Das G, Shi Y. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25:237–247. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Langrish C L, Chen Y, Blumenschein W M, Mattson J, Basham B, Sedgwick J D, McClanahan T, Kastelein R A, Cua D J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C O, Cong Y, Weaver C T, Schoeb T R, McClanahan T K, Fick R B, Kastelein R A. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- Shi G, Ramaswamy M, Vistica B P, Cox C A, Tan C, Wawrousek E F, Siegel R M, Gery I. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. J Immunol. 2009;183:7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Iclozan C, Yamazak T, Yang X, Anasetti C, Dong C, Yu X Z. Abundant c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood. 2009;114:1026–1028. doi: 10.1182/blood-2009-03-210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Chen K, Wei Y, Sharp G C, McKee L, Braley-Mullen H. Apoptosis of thyrocytes and effector cells during induction and resolution of granulomatous experimental autoimmune thyroiditis. Int Immunol. 2000;12:1629–1639. doi: 10.1093/intimm/12.12.1629. [DOI] [PubMed] [Google Scholar]
- Chen K, Wei Y, Sharp G C, Braley-Mullen H. Mechanisms of spontaneous resolution versus fibrosis in granulomatous experimental autoimmune thyroiditis. J Immunol. 2003;171:6236–6243. doi: 10.4049/jimmunol.171.11.6236. [DOI] [PubMed] [Google Scholar]
- Steemans M, Goossens V, Van de Craen M, Van Herreweghe F, Vancompernolle K, De Vos K, Vandenabeele P, Grooten J. A caspase-activated factor (CAF) induces mitochondrial membrane depolarization and cytochrome c release by a nonproteolytic mechanism. J Exp Med. 1998;188:2193–2198. doi: 10.1084/jem.188.11.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin S A, Zamzami N, Castedo M, Daugas E, Wang H G, Geley S, Fassy F, Reed J C, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]