Abstract
During the development of immune responses to pathogens, self-antigens, or environmental allergens, naive CD4+ T cells differentiate into subsets of effector cells including Th1, Th2, and Th17 cells. The differentiation into these subsets is controlled by specific transcription factors. The activity of these effector cells is limited by nTregs and iTregs, whose differentiation and maintenance are dependent on the transcription factor Foxp3. The regulation of autoimmune diseases mediated by Th1 and Th17 cells by Tregs has been studied and reviewed extensively. However, much less has been presented about the interplay between Tregs and Th2 cells and their contribution to allergic disease. In this perspective, we discuss the regulation of Th2 cells by Tregs and vice versa, focusing on the interplay between the IL-4-activated STAT6/GATA3 pathway and Foxp3.
Keywords: allergy, lymphoid cell-mediated immunity, T cell responses, signal transduction, Foxp3
ROLE OF IL-4 AND STAT6 IN DRIVING Th2 DIFFERENTIATION
CD4+ Th2 cells are a subset of T cells that secrete the characteristic cytokines IL-4, IL-5, and IL-13 [1]. It has been well established that Th2 cells are required for providing host defense against extracellular parasitic (especially helminthic) infections, but at the same time, aberrant Th2 responses can lead to allergic inflammation and asthma. IL-4 signaling through the IL-4R in naïve CD4+ T cells leads to the phosphorylation and activation of a key transcription factor, STAT6, which then translocates into the nucleus and drives expression of IL-4-responsive genes [2]. The crucial role played by this transcription factor in driving differentiation has been elucidated by a number of groups, using in vitro and in vivo experimental systems. It is known that STAT6 deficiency abrogates the ability of IL-4 to induce the expression of many genes [3]. In addition, IL-4-mediated differentiation of T lymphocytes into Th2 effectors and proliferation of these cells were completely dependent on STAT6 in vitro [3, 4]. STAT6−/− T cells also failed to proliferate and produce Th2 cytokines in response to nematode infections. Subsequently, several groups have shown that STAT6 is not absolutely required for Th2 differentiation using in vivo models. Specifically, Jankovic et al. found large numbers of cells producing cytokines such as IL-4 and IL-5 in helminth-infected mice lacking STAT6 or IL-4Rα, although populations of Th1 cells were also present [5]. However, they found that IL-4R/STAT6 signaling was important for stabilization of the Th2 phenotype, as STAT6−/− or IL-4Rα−/− T cells could revert to Th1 cells in the presence of rIL-12. Finkelman et al. [6] made similar observations; IL-4 and STAT6 responses were not absolutely required for Th2 differentiation in vivo but were necessary for generating memory IL-4 responses in mice.
STAT6 participates in Th2 differentiation, in part, by enhancing the expression of the master regulator of Th2 differentiation, GATA3 [7]. STAT6 and GATA3 together with IL-2-mediated STAT5 activation induce the secretion of copious amounts of IL-4, IL-5, and IL-13 by activated Th2 cells [8]. Expression of these transcription factors is also necessary for Th2 lineage commitment. For example, IL-4-induced activation of STAT6 mediated the expression of Gfi-1, a transcriptional repressor that leads to the selection of GATA-3hi cell growth [9]. Thus, in spite of the somewhat varying results, it is clear that STAT6 plays an important role in Th2 cell differentiation and effector function.
nTregs AND iTregs
Regulation of effector T lymphocyte function in the periphery is mediated largely by CD4+ CD25hi FoxP3+ Tregs [10,11,12,13,14,15,16,17,18,19,20,21,22], which act to suppress effector lymphocyte functions to maintain immune tolerance to self-antigens and to prevent overly vigorous responses to innocuous foreign antigens. There are two main classes of Tregs: nTregs are generated in the thymus and released into peripheral tissues after thymic-positive selection [23]. The TCRs of nTregs recognize self-antigens and are designed to suppress effector function of pathogenic autoreactive T lymphocytes that have escaped negative selection and entered peripheral tissue [23,24,25,26]. iTregs are generated via peripheral conversion (after antigen-specific stimulation) from mature, naïve CD4+T cells or from “rescued” autoreactive effector T lymphocytes [21]. Regulatory cells can be subdivided into FoxP3-expressing and nonexpressing subsets. FoxP3+ iTregs undergo differentiation from CD4+CD25− Foxp3− T cells after stimulation by TGF-β [27]. The FoxP3–cohort has been studied mainly in vitro and consists of: Tr1 cells, which express large amounts of IL-10, and Th3 cells, which produce high levels of TGF-β [28].
Tregs constitutively express CD25, the IL-2Rα chain of the high-affinity IL-2R complex, even during the resting state [10, 19]. Signaling by the IL-2R is important for Treg survival and maintenance. In addition, Tregs express several inhibitory molecules on their surface, such as the glucocorticoid-induced TNFR family-related gene and CTLA4, and they secrete high amounts of IL-10 and TGF-β [19].
The mechanisms by which Tregs suppress the functional activity of effector cells have been explored in vitro [19, 21]. Several mechanisms for suppression of T effectors have been proposed. The first suppressive mechanism requires cell contact and is mediated by membrane-bound molecules, such as galectin 1 or TGF-β [19]. The second mechanism is through the production of secreted cytokines that inhibit effector function, such as IL-10, TGF-β, and IL-35 [22]. The third mechanism is through the consumption of limiting growth factors, such as IL-2 [29]. It was shown recently that the ability of Tregs to suppress Th2 effectors depended specifically on the expression of the transcription factor IRF4, although the mechanism of this specific suppression was not evaluated [30]. In addition to suppressing effector T cell function, Tregs suppress DC function by a cell contact-dependent mechanism [19].
Treg differentiation and suppressive function is dependent on expression of the transcription factor Foxp3 [17, 20], which is a member of the forkhead-winged helix family of transcription factors; all members have a forkhead domain that is needed to bind DNA [31]. Foxp3 can act as a transcriptional activator or repressor; it regulates cytokine gene transcription in effector and regulatory T lymphocytes. Several studies have shown that Tregs can decrease effector T lymphocyte IL-2 expression but have yet to show if this is a direct effect by Tregs on T cells or an indirect effect caused by Treg inhibition of T cell proliferation [32]. Foxp3 suppresses IL-2 production by Tregs via its interaction with AP-1 [33]. This renders Tregs dependent on exogenous sources of IL-2 or other γc cytokines for survival and protection from cell death [33]. Furthermore, in vitro-cultured Tregs rapidly lose expression of Foxp3 in the absence of exogenous γc cytokines [33].
Naturally occurring mutations in the FOXP3 gene in humans and mice cause immunopathology that has a major Th2 component [13, 34, 35]. Humans develop the IPEX syndrome as a result of accumulation of certain mutations in FOXP3. Clinical manifestations of IPEX (also called X-linked autoimmunity-allergic disregulation syndrome or XLAAD) include autoimmunity together with severe atopy, eczema, food allergies, and eosinophilic inflammation [34]. Humans with IPEX have a mutated Foxp3 gene whose product is unable to dimerize to form the necessary heterotetrameric complex [31]. Scurfy mice also lack Foxp3 expression and are used commonly to study IPEX in an animal model [13, 14]. However, original Scurfy mutants possess another closely linked sparse-fur mutation that can confound studies about the loss of Foxp3 expression [36]. In studies using Foxp3-deficient mice to study the role of Foxp3 in allergic dysregulation, a specific knock-in mutation of the Foxp3 locus was generated using targeted mutagenesis [36]. These FoxP3−/− mice from various genetic backgrounds developed autoimmune disease and allergic airway inflammation.
RELATIONSHIP BETWEEN Th2 CELLS AND Tregs
Recent research in this field suggests that although Th2 cells and Tregs belong to different subsets of the CD4+ T cell lineage, they may be related more closely than earlier thought. Th2 differentiation requires strong STAT5 activation through IL-2 signaling [8]; the same is important for development of Tregs [37]. In addition to IL-2, nTregs and iTregs need TGF-β for their survival to induce and maintain Foxp3 expression and for mediating their suppressive function [38]. TGF-β, in turn, suppresses differentiation of Th2 cells by inhibiting the expression of transcription factors GATA3 and Gfi-1 [39].
Several years ago, two groups independently found that diminished expression of Foxp3 caused Tregs to revert back to effector T cells (especially Th2-like cells), which produce cytokines such as IL-2 and IL-4 [40, 41]. A close relationship between Tregs and Th2 cells is illustrated further by the observation that the presence of a Th2 bias in the scurfy mouse is not a result of the complete absence of Tregs but a result of the lack of suppressive function of nTregs and iTregs in these mice [42]. They also report that these Tregs display a Th2-like phenotype, as they express GATA3 in addition to producing elevated levels of Th2 cytokines. These results suggest that there may be some plasticity between Th2 cells and iTregs.
IRF4 is a transcription factor that is critical for Th2 differentiation [43, 44]. High levels of IRF4 were found to enhance production of IL-10 by Th2 cells [45]. Interestingly, IRF4 was identified as a Foxp3-induced gene in Tregs, and Tregs constitutively expressed high levels of IRF4 [30]. As mentioned above, Zheng et al. [30] showed that IRF4 expression in Tregs was necessary to suppress Th2-mediated responses specifically. They proposed that IRF4 interacts with Foxp3 and modifies gene expression to initiate a Th2-specific, suppressive mechanism. The nature of this Th2-specific, suppressive mechanism is unclear. This strategy of co-opting effector T cell transcriptional machinery by Tregs, leading to specific suppression, appears to be used for Th1 and Th17 suppression as well [46, 47]. Although the mechanism is not known, it has been proposed that sharing transcriptional regulation allows Tregs to adapt to the local environment and mediate suppression of the specific type of inflammation [47].
IL-4 has been reported to have stimulatory and inhibitory effects on Treg development and maintenance, making it difficult to develop a clear understanding of how IL-4 regulates Tregs in vivo. Recent studies showed that IL-4 signaling through STAT6 was required for Foxp3 mRNA expression and maintenance of Foxp3 protein in nTregs [48, 49]. This pathway was also important for Treg proliferation and survival. It was also shown that IL-4 could induce the formation of iTregs from naïve CD4+ T cells [50]. On the other hand, Th2 cells have been reported to suppress Tregs. Most of the reports focus on iTregs, although nTregs can also be affected. The inhibitory effect of IL-4 on iTregs is brought about by the differentiation of Th2 cells (and production of cytokines such as IL-4), which can potently block TGF-β-mediated differentiation of Foxp3+ Tregs from naïve CD4+ T cells [51]. Furthermore, it has been shown that the production of IL-4 makes Th2 cells refractory to Treg suppression in a STAT6- and GATA3-dependent manner [49]. It is possible that the effect of IL-4 on Tregs, either positive or negative, depends on the timing of exposure to IL-4, relative to the Treg-inducing stimulus and the context of the inflammatory environment.
INTERPLAY OF TRANSCRIPTION FACTORS
Many factors induced by the antigen TCR, costimulatory molecules, and cytokine receptors [20] modulate the transcriptional regulation of Foxp3 expression. Model systems have been developed to identify the cytokines and transcription factors that regulate its expression. One factor critical for induction of Foxp3 is TGF-β, which is necessary for the conversion of CD4+Foxp3− T cells into CD4+Foxp3+Tregs [52,53,54,55,56]. TGF-β-deficient mice showed a defect in peripheral Foxp3+ Treg homeostasis with decreased levels of Foxp3 in the low numbers of CD25+CD4+ T cells found in these mice [57]. In addition, TGF-β-deficient mice develop a lethal lymphoproliferative disorder characterized by enhanced Th1 and Th2 effector responses, suggesting that TGF-β is required for suppressive function [57]. In contrast, other investigators using different models of in vivo TGF-β deficiency (Smad3−/−, neonatal TGFβ1−/−, and dominant-negative TGF-β-type RII-transgenic mice) have shown that Tregs isolated from these mice were effectively immunosuppressive in vitro and in vivo [58]. Nevertheless, our current state of knowledge, based on many published observations, establishes TGF-β as a crucial factor regulating Treg development and function.
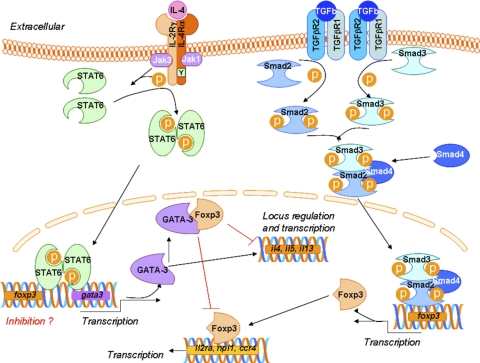
The observation that polarized Th2 cells inhibited Foxp3+ Treg development [51] suggested that there could be a direct interaction between transcription factors critical for Th2 responses (IL-4-induced STAT6 and GATA3) and transcription factors critical for Tregs (TGF-β-induced Foxp3). Indeed, initial reports suggested that Foxp3 can bind to the Th2 lineage-specific transcription factor GATA3, but the consequences of this interaction were not well understood [51, 59]. One outcome of this interaction was that Foxp3 could inhibit the differentiation of Th2 cells directly (Fig. 1). Foxp3 not only interacted with GATA3 but also inhibited the ability of GATA3 to transactivate the promoter of a Th2 target gene, Il5.
Figure 1.
Transcription factor interplay in the Th2/iTreg balance. Naive CD4+ T cells receive signals through the antigen TCR and costimulatory molecules to begin the differentiation process. IL-4 binds to the type I IL-4R, present on the surface of T cells, and induces the activation of STAT6, which drives transcription of GATA3 and suppresses transcription of Foxp3. GATA3 controls the Th2 cytokine locus and regulates IL-5 transcription. TGF-β binds to the TGF-βR, leading to activation of the Smad family of transcription factors. In cooperation with signals from the TCR and IL-2R, the Smads drive transcription of Foxp3, which then regulates the expression of genes important for the Treg phenotype. GATA3 can bind to Foxp3 directly and prevent its ability to induce the Treg genes. On the other hand, Foxp3 can bind to GATA3 and prevent its ability to regulate Th2 genes. Il2ra, IL-2R antagonist; npl1, nonphototropic hypocotyl 1-like protein.
The relationship among Foxp3, GATA3, and STAT6 was more fully addressed in a recent study by Dardalhon and colleagues [60]. They showed that IL-4 inhibited Foxp3 induction in iTregs and that this inhibition was critically dependent on STAT6. Naïve T cells from IL-4-transgenic mice were cultured in vitro in the presence of increasing doses of TGF-β. Under such conditions, TGF-β did not induce the generation of Foxp3+ Tregs. These results suggest that IL-4 inhibition of Foxp3+Tregs is dominant over the TGF-β effect on Foxp3 expression. As STAT6 is a canonical IL-4Rα signaling pathway critical for Th2 cell differentiation, the authors assessed the involvement of STAT6 in IL-4-mediated Foxp3 inhibition by using STAT6−/−/Foxp3-GFP mice. Over 80% of T cells from these mice showed high Foxp3 expression, even in the presence of IL-4, in combination with TGF-β. In addition, T cells from STAT6−/− mice were unable to give rise to the Foxp3−IL-9+IL-10+ T cell population in response to TGF-β plus IL-4. Similarly, this T cell population could not be detected in TGF-β plus IL-4 T cell cultures from GATA3−/− mice. Therefore, in the absence of STAT6 or GATA3, IL-4 did not antagonize the TGF-β-mediated induction of Foxp3 expression. This work clearly shows that IL-4 has a critical, down-regulatory effect on iTregs. The authors also demonstrated that Foxp3 and GATA3 physically associated with each other by coimmunoprecipitation assays in transiently transfected human embryo kidney 293 cells. This direct association of GATA3 with Foxp3 and cross-inhibition creates a balancing act between transcription factor dominance and T cell differentiation (Fig. 2), reminiscent of the interaction between Foxp3 and retinoic acid receptor-related orphan receptor-γt, which reciprocally regulate the induction of Tregs and Th17 cells [52, 61,62,63].
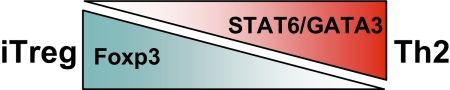
Figure 2.
Levels of transcription factors can tip the balance to Th2 or to iTreg differentiation. As GATA3 and Foxp3 interact with each other and suppress functional activity, the relative amounts of GATA3 and Foxp3 can determine the differentiation fate of the CD4+ T cell to Th2 or iTreg, respectively.
This observation of IL-4-induced, STAT6-dependent Foxp3 inhibition is in accord with our recent finding that there is a higher number of Foxp3+ nTregs and iTregs in naïve and immunized STAT6−/− mice as compared with STAT6+/+ mice [64]. Moreover, a recent study by Takaki and colleagues [65] provides an additional mechanism for regulation of Tregs by IL-4/STAT6. Using in vitro cultures of CD25−CD4+ cells with TGF-β1, the authors showed that Foxp3 levels were only transiently induced in these cells; however, the Foxp3 expression could be maintained by the addition of anti-IL-4 antibody to the cultures. Similarly, TGFβ-1-induced Foxp3 expression was sustained longer in STAT6−/− CD25−CD4+ T cells, as compared with that observed in STAT6+/+ cells. During this time period, GATA3 was not induced, and IL-4 did not inhibit activation of Smad transcription factors by TGF-β. Therefore, the authors proposed that STAT6 inhibits Foxp3 mRNA expression directly in these developing iTregs. Using chromatin immunoprecipitation and in vitro-binding assays, they identified a silencer region in the Foxp3 transcript with a specific STAT6-binding site and showed that binding of STAT6 to this site reduced TGF-β1-induced transcriptional activation and chromatin modification. This IL-4-induced suppression was reversed by retinoic acid. The authors suggest that the use of anti-IL-4 could be efficient in the induction of tolerance in Th2-mediated diseases such as allergy. However, as mentioned above, several reports showed that IL-4 had a positive effect on Foxp3 expression and Treg number and/or function [48,49,50, 66]. This discrepancy in observations may be a result of differences between in vitro and in vivo studies, the use of mouse and human cells, the timing of IL-4 exposure, the presence of other factors such as retinoic acid, and by different mechanisms regulating Foxp3 transcription or message stability. It was shown that IL-4-mediated enhancement of Foxp3+ Treg function persisted in STAT6−/− cells [48], suggesting that there is an IL-4-dependent, STAT6-independent enhancement of Treg function by IL-4. These controversial findings illustrate the need for further studies aimed at clarifying the inter-relationship between Th2 and Tregs.
INTERPLAY BETWEEN Th2 CELLS AND Tregs IN ALLERGY AND ASTHMA
Allergic asthma is an increasing worldwide health problem; ∼300 million people in the world currently have asthma [67]. It is a chronic inflammatory disease of the airway associated with eosinophilic inflammation, airway obstruction, and AHR. This reaction can be suppressed by Tregs, which maintain airway tolerance [68,69,70]. Thus, allergic asthma is essentially a breakdown of immune tolerance to an environmental allergen. Environmental allergens stimulate airway epithelial cells and lung DCs, resulting in the priming of CD4+ T cells to become Th2 cells [71]. Repeated exposure to this allergen causes massive expansion of the allergen-specific CD4+ Th2 cells and overproduction of Th2 cytokines (Fig. 3). The skewed Th2-immune response underlies the development of allergic asthma. Taking into consideration the antagonistic relationship between Th2 cytokine gene expression and Foxp3 expression, it is not surprising that Foxp3 expression in Tregs helps to mediate regulation of Th2-mediated allergic airway inflammation and tolerance at mucosal surfaces [72].
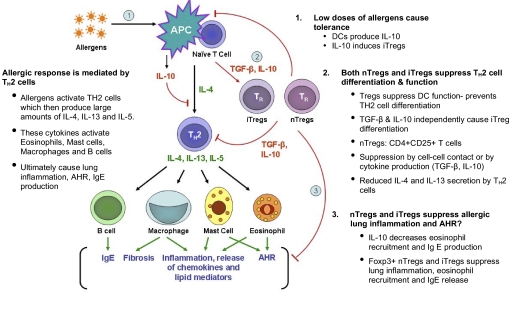
Figure 3.
nTregs and iTregs can influence allergic lung inflammation by multiple mechanisms. Exposure to low doses of allergen leads to immune tolerance as a result of the production of IL-10 by DCs. IL-10 produced by DCs also stimulates differentiation of IL-10-producing iTregs. nTregs and iTregs can suppress allergic lung inflammation at three different stages. 1. Tregs can suppress DC function, thus blocking priming of naïve CD4 T cells and their subsequent differentiation into Th2 effectors. 2. They can inhibit Th2 activity by direct cell–cell contact or by producing cytokines such as TGF-β and/or IL-10. This results in lower Th2 cytokine production. 3. Finally, both classes of Tregs can dampen recruitment of eosinophils and other cells into the lung, leading to lower inflammation, and can reduce IgE secretion by B cells. However, the role of Tregs in blocking AHR is somewhat controversial.
Many experimental studies in mice have shown that airway exposure to low doses of allergen without adjuvant causes a state of unresponsiveness to subsequent stimulatory allergen challenge [72,73,74,75,76,77,78,79]. Gereke and associates [72] have shown that such allergen-induced tolerance was associated with the induction of suppressive Foxp3+ Tregs. Akbari and collegues [75] associated it with IL-10-producing pulmonary DCs, which stimulated the development of IL-10-producing Tregs in an ICOS-ICOS ligand-dependent costimulation pathway. Pulmonary DC and alveolar epithelial cells present allergen in a tolerizing mode under such exposure [72, 75]. IL-10-producing alveolar macrophages (AM) can also play an important role in suppression of the Th2 response to an allergen in the lung, although currently, there is no evidence associating AM with Tregs [77]. Moreover, several experimental studies have demonstrated that a prolonged exposure of airways to low-dose antigen has a suppressive effect on an established Th2 pulmonary inflammation [76,77,78]. These effects were largely Treg-dependent; however, TGF-β1-producing macrophages also can play a prominent, suppressive role [76, 77].
Tregs have been shown to suppress allergic airway responses by multiple mechanisms (Fig. 3). It was shown that adoptive transfer of unfractionated, Th2-polarized CD4+ T cells caused less allergic response to allergen challenge as compared with Treg-depleted CD4+ T cells [80] and that allergen-specific Tregs transferred to sensitized mice suppressed inflammation [81]. However, these two transfer studies identified different mechanisms of suppression. In the first case, the suppression was induced by Foxp3+ Tregs expressing TGF-β through a cell–cell contact-dependent mechanism without IL-10 involvement and was inhibited by anti-TGF-β-neutralizing antibodies [80]. In the second case, suppression was mediated by Tregs through an IL-10-dependent mechanism and was abrogated by neutralizing anti-IL-10 antibodies [81]. Interestingly, this latter suppression was not dependent on IL-10 production by Tregs but on their ability to enhance IL-10 production by CD4+ T cells. However, recent studies using mice engineered, such that only Foxp3+ Tregs lacked IL-10, clearly established that IL-10 produced by Foxp3+ Tregs was important for suppressing allergic lung inflammation in response to allergen challenge [82].
Further studies used anti-CD25 mAb to deplete nTreg. Treg depletion resulted in increased IgE synthesis and Th2 cytokine production in response to the house dust mite [83]. Another interesting consequence of Treg depletion was an increase in pulmonary mDCs. These cells had an increased ability to stimulate effector T cells and also had elevated expression levels of costimulatory molecules, such as MHC II and CD80 [83]. Thus, one possible mechanism used by Tregs to suppress allergic airway inflammation could be the reduction of mDC stimulation to prevent excess antigen presentation and activation of CD4+ Th2 cells. Another study about Treg depletion reported an increase in DCs following Treg depletion and indicated a complex feedback system between Tregs and DCs, in which DC levels regulated Treg homeostasis [84]. It is important to note that the anti-CD25 mAb does not necessarily deplete Tregs but may induce shedding of CD25 from the surface of Tregs and render them functionaly inactive, as they are unable to bind IL-2 [85]. In contrast to Treg depletion, adoptive transfer of Tregs has been shown to have protective effects against allergic respiratory symptoms. Work by Hadeiba and Locksley [86] illustrated Treg ability to suppress Th2-immune responses by decreasing IL-4 production, serum IgE levels, and lung eosinophillic infiltration.
A major feature of allergic asthma is AHR to challenge with methacholine. Many studies using mouse models of experimental asthma have demonstrated a dissociation between AHR and inflammation in the allergic lung response [87,88,89]. Recent studies have shown that depending on the experimental setting, Tregs can efficiently block the inflammatory component of an allergic response, but not AHR, [83, 86] or inhibit AHR without having a substantial effect on Th2 cytokine production [90].
These mouse models have allowed the characterization of the essential role of Tregs in suppressing allergic diseases by multiple mechanisms. Recent clinical studies have shown that Tregs function in humans in ways similar to that observed in mice, including human Treg expression of Foxp3 [91], suppression of effector T cell proliferation and cytokine production [92], decreased allergen-specific proliferation [93], and enhanced Th2 cytokine production by PBMC after Treg depletion [92, 94]. Pediatric patients with allergic respiratory symptoms have, on average, a lower number of CD4+ CD25+ Tregs than compared with healthy control patients [95]. Furthermore, the iTregs isolated from these patients were functional and able to suppress effector T lymphocytes in vitro [95]. Corticosteroid treatment of asthma results in the increase in Treg numbers in the peripheral blood and bronchoalveolar lavage of asthmatic children and restores their suppressive function, suggesting a potent immunomodulatory, Treg-mediated mechanism(s) of the disease by this drug [96]. Recent advances in understanding the immunological mechanisms of allergen SIT and sublingual immunotherapy further illustrate the important role of Tregs in allergen-specific peripheral tolerance (reviewed in refs. [59, 97]). Therefore, Tregs could be useful cells for SIT of allergic diseases including asthma.
The relative contribution of nTregs versus iTregs in allergic asthma is not clear. It is reasonable to predict that iTregs may be dominant in the control of asthma, as allergic phenomena result from a skewed Th2 effector response to a harmless environmental allergen rather than a self-antigen [98]. nTregs have a TCR repertoire oriented toward recognizing self-antigens, but the iTreg TCR repertoire has been reported to have similar diversity to the TCR repertoire found on mature, peripheral, naïve CD4+ cells [23,24,25,26, 98]. A model system designed to test the role of iTregs in the absence of nTregs revealed that iTregs can mediate mucosal tolerance and moderate the inflammatory response during chronic allergic lung inflammation [99]. nTregs and iTregs have been implicated in the control of lung inflammation, and it may be that they work synergistically to combat allergic asthma (Fig. 3).
To complicate things further, there is a body of research strongly implicating a major role for Foxp3−, IL-10-producing Tr1 cells in the control of asthma [69, 97, 100]. Research by Akdis and colleagues [91] has shown that the dominant allergen-specific T cell population in healthy individuals consists of Tr1 cells. However, the relationship between Foxp3–Tr1 cells and Foxp3+ iTregs producing IL-10 is not clear. As noted above, production of IL-10 by Foxp3+ cells was essential for suppression of inflammation and AHR after allergen challenge [82]. Recent evidence suggests that Tregs in the periphery can become unstable and lose Foxp3 expression, adopting some effector functions [101]. Perhaps in the inflammed Th2 lung environment, Foxp3+ cells can become Foxp3–cells, producing high amounts of IL-10. Other findings suggest a necessary role for TGF-β in immunosupression by showing a requirement for effector T cells to express TGF-βRs to prevent constitutive T cell activation [102]. Thus, a number of distinct but possibly related regulatory cell populations can dampen allergic inflammation.
SUMMARY
The studies highlighted in this perspective demonstrate a need for further detailed investigations of the relationship between Th2 cells and Tregs and their balance in allergic disease. The possible contributions of the different Treg populations (nTregs and iTregs, Tr1, Th3) to the maintenance of mucosal tolerance in the pulmonary system are complex. The cooperation between different populations of resident lung cells and immune cells is critically important, not only for maintaining normal pulmonary homeostasis but also for limiting harmful inflammatory and/or hyper-reactivity reactions induced by allergen exposure in sensitive individuals. The control of the Th2/Treg balance by the key transcription factors STAT6, GATA3, and Foxp3 suggests therapeutic targets to control allergic disease. However, such an approach would need to be carefully considered and fine-tuned to avoid immunosuppression and susceptiblity to infectious pathogens and to cancer.
AUTHORSHIP
S. Chapoval, P. Dasgupta, N. J. Dorsey, and A. D. Keegan worked together to research the literature, prepare the perspective, and develop the figures.
ACKNOWLEDGMENTS
This perspective was supported by Public Health Service grant AI038985 from the National Institutes of Health (A. D. K.). We thank Dr. Jonathan Skupsky and Dr. David Scott for discussions about Treg function in the context of Th2 responses. We apologize to the many authors of important studies whom we did not cite as a result of space limitations and the focused nature of this perspective.
Footnotes
Abbreviations: AHR=airway hyper-responsiveness, AM=alveolar macrophage(s), DC=dendritic cell, Foxp3=forkhead box p3, γc=cytokine receptor common γ chain, Gfi-1=growth factor independence 1, ICOS=inducible costimulatory, IPEX=immunodysregulation, polyendocrinopathy, and enteropathy, X-linked, IRF4=IFN regulatory factor 4, iTreg=inducible or adaptive regulatory T cell, mDC=myeloid dendritic cell, nTreg=natural regulatory T cell, SIT=specific immunotherapy, Tr1=T regulatory type 1 cell, Treg= regulatory T cell
References
- Zhu J, Paul W E. CD4 T cells: fates functions and faults. Blood. 2008;112:1557–1568. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Welch A E, Hanson E M, Boothby M R, Keegan A D. Interleukin-4 (IL-4) and IL-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- Kaplan M H, Schindler U, Smiley S T, Grusby M J. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Shimoda K, van Deursen J, Sangster M Y, Sarawar S R, Carson R T, Tripp R A, Chu C, Quelle F W, Nosaka T, Vignali D A, Doherty P C, Grosveld G, Paul W E, Ihle J N. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Jankovic D, Kullberg M C, Noben-Trauth N, Caspar P, Paul W E, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- Finkelman F D, Morris S C, Orekhova T, Mori M, Donaldson D, Reiner S L, Reilly N L, Schopf L, Urban J F., Jr Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- Zheng W, Flavell R A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Zhu J, Cote-Sierra J, Guo L, Paul W E. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- Zhu J, Jankovic D, Grinberg A, Guo L, Paul W E. Gfi-1 plays an important role in IL-2-mediated Th2 cell expansion. Proc Natl Acad Sci USA. 2006;103:18214–18219. doi: 10.1073/pnas.0608981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T-cells expressing IL-2 receptor α chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Fontenot J D, Gavin M A, Rudensky A Y. Foxp3 programs the development and functions of CD4+CD25+ regulatory T-cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko S A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kasprowicz D J, Smallwood P S, Tyznik A J, Ziegler S F. Scurfin (FoxP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol. 2003;171:1216–1223. doi: 10.4049/jimmunol.171.3.1216. [DOI] [PubMed] [Google Scholar]
- Bluestone J A, Abbas A K. Natural versus adaptive regulatory T-cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Ziegler S F. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Rudensky A Y. Foxp3 in control of the regulatory T-cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T-cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Shevach E M. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Josefowicz S Z, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman C J, Szymczak-Workman A L, Collison L W, Pillai M R, Vignali D A A. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini M, Vignali D A A. Regulatory T-cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima M, Hirokawa M, Fujishima N, Sawada K. TCRαβ repertoire diversity of human naturally occurring CD4+CD25+ regulatory T cells. Immunol Lett. 2005;99:193–197. doi: 10.1016/j.imlet.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hsieh C S, Zheng Y, Liang Y, Fontenot J D, Rudensky A Y. An intersection between the self-reactive regulatory and non-regulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- Lathrop S K, Santacruz N A, Pham D, Luo J, Hsieh C S. Antigen specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Sakaguchi S. Foxp3 and Aire in thymus-generated Treg cells: a link in self-tolerance. Nat Immunol. 2007;8:333–334. doi: 10.1038/ni0407-333. [DOI] [PubMed] [Google Scholar]
- Yamagiwa S, Gray J D, Hashimoto S, Horwitz D A. A role for TGF-β in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- Umetsu D T, Akbari O, Dekruyff R H. Regulatory T cells control the development of allergic disease and asthma. J Allergy Clin Immunol. 2003;112:480–487. [PubMed] [Google Scholar]
- Scheffold A, Murphy K M, Hofer T. Competition for cytokines: T(reg) cells take all. Nat Immunol. 2007;8:1285–1287. doi: 10.1038/ni1207-1285. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim J M, Chu T-T, Corcoran L, Treuting P, Klein U, Rudensky A Y. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control Th2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner J H, Ziegler S F. Functional analysis of FOXP3. Ann N Y Acad Sci. 2008;1143:151–169. doi: 10.1196/annals.1443.014. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Pandiyan P, Lenardo M J. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol Direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatila T A, Blaeser F, Ho N, Lederman H M, Voulgaropoulos C, Helms C, Bowcock A M. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C L, Christie J, Ramsdell F, Bunkow M E, Ferguson P J, Whitesell L, Kelly T E, Saulsbury F T, Chance F T, Ochs H D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Lin W, Truong N, Grossman W J, Haribhai D, Williams C B, Wang J, Martín M G, Chatila T A. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Davidson T S, DiPaolo R J, Andersson J, Shevach E M. Cutting edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- Marie J C, Letterio J J, Gavin M, Rudensky A Y. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Davidson T S, Wei G, Jankovic D, Cui K, Schones D E, Guo L, Zhao K, Shevach E M, Paul W E. Down-regulation of Gfi-1 expression by TGF-β is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J Exp Med. 2009;206:329–341. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L M, Rudensky A Y. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- Wan Y Y, Flavell R A. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- Lahl K, Mayer C T, Bopp T, Huehn J, Loddenkemper C, Eberl G, Wirnsberger G, Dornmair K, Geffers R, Schmitt E, Buer J, Sparwasser T. Nonfunctional regulatory T cells and defective control of Th2 cytokine production in natural Scurfy mutant mice. J Immunol. 2009;183:5662–5672. doi: 10.4049/jimmunol.0803762. [DOI] [PubMed] [Google Scholar]
- Lohoff M, Mittrücker H W, Prechtl S, Bischof S, Sommer F, Kock S, Ferrick D A, Duncan G S, Gessner A, Mak T W. Dysregulated T helper cell differentiation in the absence of IRF4. Proc Natl Acad Sci USA. 2002;99:11808–11812. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J, Mowen K A, McBride K D, Smith E D, Singh H, Glimcher L H. IRF4 interacts with NFATc2 to modulate IL-4 gene expression. J Exp Med. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahyi A N, Chang H C, Dent A L, Nutt S L, Kaplan M H. IRF4 regulates the expression of a subset of Th2 cytokines. J Immunol. 2009;183:1598–1606. doi: 10.4049/jimmunol.0803302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M A, Tucker-Heard G, Perdue N R, Killebrew J R, Urdahl K B, Campbell D J. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein R M, Liang Y, Kas A, Rudensky A Y. CD4+ regulatory T cells control Th17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerten P, Shen C, Bullens D M, Van Assche G, Van Gool S, Geboes K, Rutgeerts P, Ceuppens J L. Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. J Autoimmun. 2005;25:112–120. doi: 10.1016/j.jaut.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Pillemer B B, Qi Z, Melgert B, Oriss T B, Ray P, Ray A. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. J Immunol. 2009;183:155–163. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapenko A, Kalden J R, Lipsky P E, Schulze-Koops H. The IL-4 receptor α chain binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25–CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- Wei J, Duramad O, Perng O A, Reiner S L, Liu Y J, Qin F X. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom T B, Oukka M, Weiner H L, Kuchroo V K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Cobbold S P, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei K J, Li L, Marinos N, McGrady G, Wahl S M. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H B, Paik D J, Jang E, Hong S, Youn J. Acquisition of anergic and suppressive activities in transforming growth factor-β-costimulated CD4+CD25– T cells. Int Immunol. 2004;16:1203–1213. doi: 10.1093/intimm/dxh123. [DOI] [PubMed] [Google Scholar]
- Fantini M C, Becker C, Tubbe I, Nikolaev A, Lehr H A, Galle P, Neurath M F. Transforming growth factor β induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J C, Letterio J J, Gavin M, Rudensky A Y. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo C A, Letterio J J, Thornton A M, McHugh R S, Mamura M, Mizuhara H, Shevach E M. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel P Y, Kuipers H, Boyman O, Rhyner C, Ouaked N, Rückert B, Karagiannidis C, Lambrecht B N, Hendriks R W, Crameri R, Akdis C A, Blaser K, Schmidt-Weber C B. GATA3-driven Th2 responses inhibit TGF-β1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel R A, Mitsdoerffer M, Strom T B, Elyaman W, Ho I C, Khoury S, Oukka M, Kuchroo V K. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3(–) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lopes J E, Chong M M, Ivanov I I, Min R, Victora G D, Shen Y, Du J, Rubtsov Y P, Rudensky A Y, Ziegler S F, Littman D R. TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S F, Buckner J H. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009;11:594–598. doi: 10.1016/j.micinf.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORγ t-mediated IL-17A mRNA transcription through direct interaction with RORγ t. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- Chapoval S P, Kelly-Welch A, Smith E, Keegan A D. Antagonistic role of STAT6 for regulatory T cells. Cytokine. 2008;43:243. [Google Scholar]
- Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. STAT6 inhibits TGF-β1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J Biol Chem. 2008;283:14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J, Rovis F, Mitchell P, Afzali B, Tsang J Y, Garin M, Lechler R I, Lombardi G, Garden O A. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007;19:785–799. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]
- Bahadori K, Doyle-Waters M M, Marra C, Lynd L, Alasaly K, Swiston J, FitzGerald J M. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. doi: 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D S, Larche M, Durham S R. Tregs and allergic disease. J Clin Invest. 2004;114:1389–1397. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdis M, Akdis C A. Therapeutic manipulation of immune tolerance in allergic disease. Nat Rev Drug Discov. 2009;8:645–660. doi: 10.1038/nrd2653. [DOI] [PubMed] [Google Scholar]
- Lloyd C M, Hawrylowicz C M. Regulatory T cells in asthma. Immunity. 2009;31:438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B N. Biology of lung dendritic cell subsets at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am J Respir Crit Care Med. 2009;179:344–355. doi: 10.1164/rccm.200804-592OC. [DOI] [PubMed] [Google Scholar]
- Holt P G, Batty J E, Turner K J. Inhibition of specific IgE responses in mice by pre-exposure to inhaled antigen. Immunology. 1981;42:409–417. [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Yokoyama A, Kohno N, Hamada H, Hiwada K. Prolonged antigen exposure ameliorates airway inflammation but not remodeling in a mouse model of bronchial asthma. Int Arch Allergy Immunol. 2001;126:126–134. doi: 10.1159/000049503. [DOI] [PubMed] [Google Scholar]
- Akbari O, Freeman G J, Meyer E H, Greenfield E A, Chang T T, Sharpe A H, Berry G, DeKruyff R H, Umetsu D T. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- Swirski F K, Sajic D, Robbins C S, Gajewska B U, Jordana M, Stämpfli M R. Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J Immunol. 2002;169:3499–3506. doi: 10.4049/jimmunol.169.7.3499. [DOI] [PubMed] [Google Scholar]
- Niu N, Le Goff M K, Li F, Rahman M, Homer R J, Cohn L. A novel pathway that regulates inflammatory disease in the respiratory tract. J Immunol. 2007;178:3846–3855. doi: 10.4049/jimmunol.178.6.3846. [DOI] [PubMed] [Google Scholar]
- Van Hove C L, Maes T, Joos G F, Tournoy K G. Prolonged inhaled allergen exposure can induce persistent tolerance. Am J Respir Cell Mol Biol. 2007;36:573–584. doi: 10.1165/rcmb.2006-0385OC. [DOI] [PubMed] [Google Scholar]
- Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol. 2004;172:3842–3849. doi: 10.4049/jimmunol.172.6.3842. [DOI] [PubMed] [Google Scholar]
- Ostroukhova M, Seguin-Devaux C, Oriss T B, Dixon-McCarthy B, Yang L, Ameredes B T, Corcoran T E, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-β and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearley J, Barker J E, Robinson D S, Lloyd C M. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov Y P, Rasmussen J P, Chi E Y, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson W R, Jr, Muller W, Rudensky A Y. Regulatory T cell-derived IL-10 limits inflammation at environmental surfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Lewkowich I P, Herman N S, Schleifer K W, Dance M P, Chen B L, Dienger K M, Sproles A A, Shah J S, Köhl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrasse-Jèze G, Deroubaix S, Mouquet H, Victora G D, Eisenreich T, Yao K H, Masilamani R F, Dustin M L, Rudensky A Y, Liu K, Nussenzweig M C. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm A P, McMahon J S, Podojil J R, Begolka W S, DeGutes M, Kasprowicz D J, Ziegler S F, Miller S D. Cutting edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- Hadeiba H, Locksley R M. Lung CD25 CD4 regulatory T cells suppress type 2 immune responses but not bronchial hyperreactivity. J Immunol. 2003;170:5502–5510. doi: 10.4049/jimmunol.170.11.5502. [DOI] [PubMed] [Google Scholar]
- Hogan S P, Matthaei K I, Young J M, Koskinen A, Young I G, Foster P S. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol. 1998;161:1501–1509. [PubMed] [Google Scholar]
- Wilder J A, Collie D D, Wilson B S, Bice D E, Lyons C R, Lipscomb M F. Dissociation of airway hyperresponsiveness from immunoglobulin E and airway eosinophilia in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1999;20:1326–1334. doi: 10.1165/ajrcmb.20.6.3561. [DOI] [PubMed] [Google Scholar]
- Makela M J, Kanehiro A, Borish L, Dakhama A, Loader J, Joetham A, Xing Z, Jordana M, Larsen G L, Gelfand E W. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci USA. 2000;97:6007–6011. doi: 10.1073/pnas.100118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell J T, Wikstrom M E, Stumbles P A, Sly P D, Turner D J. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L307–L319. doi: 10.1152/ajplung.00521.2007. [DOI] [PubMed] [Google Scholar]
- Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber C B, Blaser K, Akdis C A. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling E M, Smith T, Nguyen X D, Pridgeon C, Dallman M, Arbery J, Carr V A, Robinson D S. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- Sletten G B, Halvorsen R, Egaas E, Halstensen T S. Memory T cell proliferation in cow’s milk allergy after CD25+ regulatory T cell removal suggests a role for casein-specific cellular immunity in IgE-mediated but not in non-IgE-mediated cow’s milk allergy. Int Arch Allergy Immunol. 2007;142:190–198. doi: 10.1159/000097021. [DOI] [PubMed] [Google Scholar]
- Taams L S, Vukmanovic-Stejic M, Smith J, Dunne P J, Fletcher J M, Plunkett F J, Ebeling S B, Lombardi G, Rustin M H, Bijlsma J W. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–1630. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Lee J H, Yu H H, Wang L C, Yang Y H, Lin Y T, Chiang B L. The levels of CD4+CD25+ regulatory T cells in pediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007;148:53–63. doi: 10.1111/j.1365-2249.2007.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D, Koller B, Mehlhorn A T, Reinhardt D, Nicolai T, Schendel D J, Griese M, Krauss-Etschmann S. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Akdis C A, Barlan I B, Bahceciler N, Akdis M. Immunological mechanisms of sublingual immunotherapy. Allergy. 2006;61:11–14. doi: 10.1111/j.1398-9995.2006.01159.x. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille M A, Lafaille J J. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille M A, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille J J. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz C M, O'Garra A. Potential role of IL-10 secreting regulatory T-cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout S, Jeker L T, Bluestone J A. Plasticity of CD4+ Foxp3+ T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y Y, Flavell R A. Regulatory T cells, transforming growth factor-β, and immune suppression. Proc Am Thorac Soc. 2007;4:271–276. doi: 10.1513/pats.200701-020AW. [DOI] [PMC free article] [PubMed] [Google Scholar]