Abstract
Administration of DNA vaccines via gene gun has emerged as an important form of antigen-specific immunotherapy. The MHC class II transactivator (CIITA) is a master regulator of MHC II expression and also induces expression of class I molecules. We reasoned that the gene gun administration of CIITA DNA with DNA vaccines employing different strategies to improve MHC I and II processing could enhance DNA vaccine potency. We observed that DC-1 cells transfected with CIITA DNA lead to higher expression of MHC I and II molecules, leading to enhanced antigen presentation through the MHC class I/II pathways. Furthermore, our data suggested that co-administration of DNA encoding calreticulin (CRT) linked to HPV-16 E6 antigen (CRT/E6) with CIITA DNA leads to enhanced E6-specific CD8+ T cell immune responses in vaccinated mice. In addition, co-administration of the combination of CRT/E6 DNA with CIITA DNA and DNA encoding the invariant chain (Ii) linked to the pan HLA-DR reactive epitope (Ii-PADRE) further enhanced E6-specific CD8+ T cell immune responses in vaccinated mice. Treatment with the combination vaccine was also shown to enhance the antitumor effects and prolong survival in TC-1 tumor-bearing mice. Vaccination with the combination vaccine also led to enhanced E6-specific CD8+ memory T cells and led to long-term protection against TC-1 tumors and prolonged survival in vaccinated mice. Thus, our findings suggest that the combination of CIITA DNA with CRT/E6 and Ii-PADRE DNA vaccines represents a potentially effective means to combat tumors in the clinical setting.
Keywords: CIITA, DNA vaccine, CRT-E6, Ii-PADRE, MHC molecules
Introduction
DNA vaccines have emerged as an interesting approach for antigen-specific immunotherapy because they are safe, stable and easy to produce. Gene gun administration of DNA vaccines represents an effective means of directly delivering antigenic DNA into dendritic cells (DCs), the most potent of the professional antigen-presenting cells. The antigen-expressing DCs mature and migrate to the draining lymph nodes, where they activate naive T lymphocytes in vivo to differentiate into activated, antigen-specific T cells (1, 2). Gene gun administration enables us to test the strategies that require direct delivery of the DNA vaccines into DCs to improve the potency of these DNA vaccines. We have previously used the gene gun delivery system for the development of several innovative strategies to enhance DNA vaccine potency (for review, see (3, 4)).
One of these strategies involves intracellular targeting of the encoded antigen to subcellular compartments to enhance MHC class I and class II antigen presentation in DCs. For example, DNA vaccines encoding antigen linked to calreticulin (CRT) are able to target the linked antigen to the endoplasmic reticulum, resulting in enhanced MHC class I presentation of the linked antigen (5). CRT is an abundant Ca2+-binding protein that resides in the endoplasmic reticulum (for review see (6)) and has been shown to aid in antigen presentation by associating with peptides delivered to the ER by transporter associated with antigen processing (TAP) molecules (7) and with MHC class I molecules (8). We have demonstrated that DNA vaccines encoding CRT linked to model antigen HPV-16 E6 and E7 generated increased HPV antigen-specific CD8+ T cell responses and antitumor effects (5, 9). Therefore, DNA vaccines encoding CRT linked to a tumor-specific antigen present the opportunity to enhance vaccine potency via enhancing MHC class I processing and presentation.
Another strategy to enhance DNA vaccine potency involves the induction of CD4+ T cell help. The activation of CD8+ T cells can be significantly enhanced by CD4+ T helper cells (for review see (10)). Thus, strategies to induce CD4+ T helper cells at sites of CD8+ T cell priming can potentially enhance CTL immune responses. In previous studies, it has been shown that DNA vaccines encoding invariant (Ii) chain in which the CLIP region is replaced with a high-affinity and “promiscuous” CD4+ T-cell epitope such as the Pan HLA-DR reactive epitope (PADRE) (11) leads to the stable, accelerated presentation of PADRE through MHC class II molecules. More recently, we have shown that immunization with DNA vaccines encoding Ii-PADRE DNA leads to the generation of high numbers of PADRE-specific CD4+ T-cell immune responses in mice (12). Furthermore, co-administration of E7 DNA vaccines with Ii-PADRE DNA has led to enhanced E7-specific CD8+ T cell immune responses and antitumor effects in vaccinated mice (12, 13). Therefore, DNA vaccination encoding antigen of interest with Ii-PADRE DNA serves as a potentially useful means to improve DNA vaccine potency through the induction of CD4+ T cells.
We reasoned that a strategy that is capable of enhancing the MHC class I and II expression on DCs may further enhance DNA vaccine potency. The major histocompatability complex (MHC) class II transactivator (CIITA) is known as a master control factor for the genes required for MHC class II antigen-presentation (14, 15). It has also been shown that CIITA induces the surface expression of MHC I molecules (16, 17). Thus, we reasoned that co-administration of DNA vaccines with CIITA DNA would increase the levels of MHC class I/II molecules and lead to enhanced presentation of the antigen via the MHC class I and II processing pathways, resulting in enhanced DNA vaccine potency. Therefore, the employment of CIITA DNA in DNA vaccines represents a potential strategy to improve vaccine potency through enhancing antigen presentation via the MHC class I and II processing pathways.
In the current study, we employed a combination of DNA vaccines encoding CRT/E6, Ii-PADRE DNA and CIITA DNA to further improve DNA vaccine potency. We showed that DC-1 cells transfected with CIITA DNA exhibited increased MHC I/II expression resulting in enhanced antigen presentation through the MHC class I/II pathways. Furthermore, we found that co-administration of the combination of CRT/E6 DNA with CIITA DNA and Ii-PADRE further enhanced E6-specific CD8+ T cell immune responses and improved the antitumor effects against E7-expressing tumors. Thus, the combination of CIITA DNA with CRT/E6 and Ii-PADRE DNA vaccines represents a potentially effective means to enhance the potency of DNA vaccines. The clinical implications of the study are discussed.
Materials and Methods
Antibodies, peptides, cell lines and mice
The HPV-16 E6 (YDFAFRDL) and PADRE (AKFVAAWTLKAAA) peptides were synthesized by Macromolecular Resources (Denver, CO) at a purity of ≥ 70%. Antibodies against mouse CD4 (PE-conjugated, clone L3T4), IFN-γ (FITC-conjugated, clone XMG1.2), CD8a (PE-conjugated, clone Ly-1), I-Ab (PE-conjugated, clone AF6-120.1), H-2Kb (PE-conjugated, clone KH95), and H-2Db (PE-conjugated, clone AF6-88.5) were purchased from BD Pharmingen (San Jose, CA).
The immortalized DC line was kindly provided by Dr. Kenneth Rock (University of Massachusetts, Worcester, MA) (18). With continued passage, we have generated subclones of dendritic cells (DC-1) that are easily transfected using Lipofectamine 2000 (Invitrogen) (19). The production and maintenance of TC-1 have been described previously (20).
Six- to eight-week-old female C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) and housed in the oncology animal facility of the Johns Hopkins Hospital (Baltimore, MD). All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Plasmid DNA constructs and DNA preparation
The generation of pcDNA-Ii and pcDNA-Ii-PADRE has been described previously (12). pcDNA3-CRT/E6 (9) was generated as described previously. The generation of the pcDNA3/F-CIITA and pcDNA3/F-CIITAΔ59-94 (pcDNA3-mtCIITA) expression vectors have been previously described (21). The DNA were amplified and purified as described previously (22).
Generation of PADRE-specific CD4+ T cell line and E6-specific CD8+ T cell line
Six-week-old female C57BL/6 mice were immunized with pcDNA3-Ii-PADRE by gene gun. After prime and booster vaccination at 1week interval, splenocytes were harvested 1 week after the last vaccination. For initial in vitro stimulation, 5 ×106 splenocytes were pulsed with IL-2 (10U/ml) and PADRE peptide (1μg/ml) in RPMI media containing 10% FBS for 6 days. Propagation of the PADRE-specific CD4+ T cell line was performed in 24-well plates by mixing 1 × 106 splenocytes containing PADRE-specific CD4+ T cells with 1×106 irradiated DCs that were pulsed with PADRE peptide, and then cultured in RPMI media containing IL-2 (10U/ml) for 6 days. This procedure was repeated weekly. Flow cytometry was performed to demonstrate the expression of the CD4 marker. The preparation of E6-specific CD8+ T cell line was performed similarly.
Transfection and detection of MHC class molecules
DC-1 cells (1.0 × 106) were transiently transfected with pcDNA3, pcDNA3-mtCIITA or pcDNA3-CIITA plamid constructs using Lipfectamine 2000 according to the vendor's manual. Cells were grown at 37°C and 5% CO2. At 24 h after transfection, the effects of CIITA on the expression of MHC class I and II molecules were characterized by flow cytometry. Naïve DC-1 cells or DC-1 cells cotransfected with the same plasmid constructs (pcDNA3, pcDNA3-mtCIITA or pcDNA3-CIITA) and pcDNA-Ii or pcDNA-Ii-PADRE (5×104/well) were mixed with PADRE-specific CD4+ T cells (5×105/well) in 24 well plates.
Naïve DC-1 cells or DC-1 cells transiently cotransfected with the plasmid constructs pcDNA3, pcDNA3-mtCIITA or pcDNA3-CIITA and pcDNA3 or pcDNA3-CRT/E6 (5×104/well) were mixed with E6-specific CD8+ T cells (5×105/well) in 24 well plates. Cells were cultured at 37°C and 5% CO2 for 24 hrs. After mixed culture, the cells were subjected to intracellular cytokine staining with fluorescein-conjugated anti-mouse IFN-γ.
DNA vaccination using a gene gun
DNA-coated gold particles were prepared according to a previously described protocol (22). DNA-coated gold particles were delivered to the shaved abdominal region of mice, using a helium-driven gene gun (Bio-Rad, Hercules, CA) with a discharge pressure of 400 lb/in2. C57BL/6 mice were immunized with various combinations of the DNA constructs illustrated in Table 1. Each cartridge contained 1 μg of plasmid DNA mixture and mice received 2 shots/mouse of the DNA mixtures by gene gun bombardment for a total of 2 μg/mouse. Each mouse received a booster of the same regimen 1 week later.
Table 1.
Vaccinations of Mice with various DNA combinations with pcDNA3-CIITA**
| Mouse Group # | Names of the DNA Constructs used in the Mixtures* | Notes | ||
|---|---|---|---|---|
| 1 | pcDNA3-CRT/E6 | pcDNA3 | pcDNA3-Ii | |
| 2 | pcDNA3-CRT/E6 | pcDNA3-CIITA | pcDNA3-Ii | |
| 3 | pcDNA3-CRT/E6 | pcDNA3 | pcDNA3-Ii-PADRE | |
| 4 | pcDNA3-CRT/E6 | pcDNA3-mtCIITA | pcDNA3-Ii-PADRE | |
| 5 | pcDNA3-CRT/E6 | pcDNA3-CIITA | pcDNA3-Ii-PADRE | |
| 6 | pcDNA3 | pcDNA3-CIITA | pcDNA3-Ii-PADRE | |
| Amount of DNA in one bullet | 0.33 μg | 0.33 μg | 0.33 μg | Total DNA ≈ 1.0 μg/bullet |
C57BL/6 mice (5 per group) were administered 2 bullets of the DNA mixtures twice with a 1-wk interval.
pcDNA3 Vector backbone for all plasmids; CRT calreticulin E6 HPV type-16 E6 protein; CIITA MHC class II transactivator; Ii Invariant chain; PADRE Pan HLA DR-binding epitope
Intracellular cytokine staining and flow cytometry analysis
Splenocytes were harvested from mice (5 per group) 1 week or 60 days after the last vaccination. Prior to intracellular cytokine staining, 5×106/mouse of pooled splenocytes from each vaccination group were incubated for 16 h with 1 μl/ml of E6 peptide (YDFAFRDL) containing an MHC class I (H-2Kb or Db) epitope (aa 50-57) for detecting antigen-specific CD8+ T-cell precursors or MHC class II (I-Ab) PADRE peptide (AKFVAAWTLKAAA) for detecting antigen-specific CD4+ T cell precursors in the presence of GolgiPlug (BD Pharmingen, San Diego, CA). Intracellular IFN-γ staining and flow cytometry analysis were performed as described previously (22). Analysis was performed on a Becton-Dickinson FACScan with CELLQuest software (Becton Dickinson Immunocytometry System, Mountain View, CA, USA).
In vivo tumor treatment experiment
For the tumor treatment experiment, C57BL/6 mice (5 per group) were challenged with 5×104/mouse of TC-1 tumor cells by subcutaneous injection in the right leg. At 3 days after challenge with TC-1 tumor cells, mice were administered via gene gun 2 μg/mouse of each designated plasmid DNA mixture three times at 4-day intervals. Tumor growth was monitored by visual inspection and palpation twice weekly as described previously (20).
Long-term in vivo tumor protection experiment
For long-term tumor protection experiment, mice (5 per group) were vaccinated via gene gun with 2 μg/mouse of each designated plasmid DNA mixture. After 1 week, mice were boosted with the same regimen as the first vaccination. At day 60 after the last vaccination, mice were subcutaneously challenged with 1×105/mouse of TC-1 tumor cells in the right leg. Tumor growth was monitored by visual inspection and palpation twice weekly as described previously (20).
Tumor measurement and conditional survival
Three dimensional tumor sizes were measured two or three times per week with Vernier calipers. Tumor sizes were approximated by multiplying the measured lengths. From day 25 after challenging tumor cells, tumors were measured every other day, and mice with tumor sizes >19 mm in diameter or projected tumor volumes >10% body weight or >2700 mm3 were considered moribund and sacrificed. Tumor volume was calculated using the following formula: V = (L × W × D), where V is tumor volume, L is length, W is width, and D is depth. All of the animal studies were approved by the Institutional Animal Care and use Committee at Johns Hopkins Hospital (Baltimore, MD).
Statistical analysis
All data expressed as mean±s.d. are representative of at least two different experiments. Comparisons between individual data points were made using a Student's t-test. Kaplan-Meier survival curves for tumor treatment and protection experiments were applied; for differences between curves, p-values were calculated using the log-rank test. The value of p<0.05 was considered significant.
Results
DC-1 cells transfected with CIITA DNA lead to higher expression of MHC I and II molecules
We have previously developed a dendritic cell line, DC-1 that can be transfected with DNA with high efficiency (19). To characterize the expression of MHC class I and II molecules in DC-1 cells transfected with CIITA DNA, we performed flow cytometry analysis using antibodies specific for MHC I H-2 Kb, Db or MHC II I-Ab. DC-1 cells transfected with mutant CIITA, vector backbone DNA or untransfected were used as controls. The expression of MHC class I and II molecules on transfected DC-1 cells were characterized by flow cytometry, 24 hrs after transfection. As shown in Figure 1, DC-1 cells transfected with CIITA DNA expressed higher levels of MHC I and II molecules compared to DC-1 cells transfected with the control constructs or nontransfected cells. Thus, our data indicate that transfection of DC-1 cells with CIITA DNA leads to increased expression of MHC class I and II molecules.
Figure 1. Flow cytometry analysis to demonstrate the expression of murine MHC molecules in CIITA-transfected DC-1 cells.
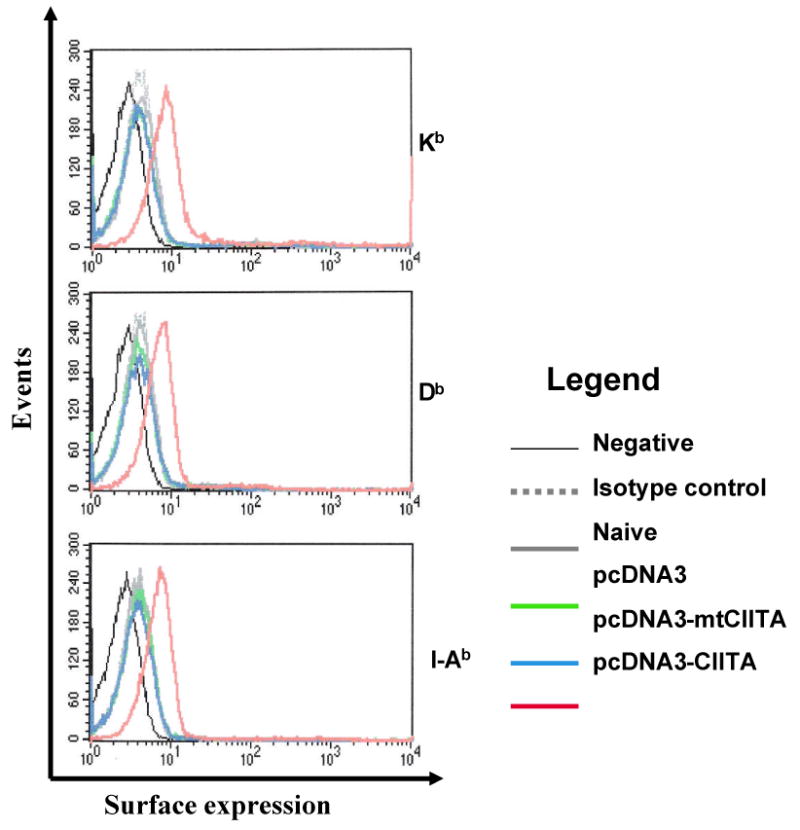
Flow cytometry data demonstrating the levels of H-2 Kb, H-2 Db, and I-Ab expression in CIITA-transfected cells and controls. The legend shows the vector plasmids with which the DCs were transfected. An immortalized dendritic cell line (DC-1) was transfected with CIITA or mutant CIITA (mtCIITA). Untransfected cells and cells transfected with the pcDNA3 vector backbone were used as a control. The expression of MHC I and II molecules was characterized using antibodies to MHC I H-2 Kb, H-2 Db, and MHC II I-Ab by flow cytometry analysis.
DCs transfected with CIITA DNA lead to enhanced antigen presentation through the MHC class I and II pathways
We then characterized the antigen presentation through MHC class I pathways in DCs cotransfected with CRT/E6 DNA and CIITA DNA. The transfected DCs were then incubated with E6-specific CD8+ T cells overnight. The degree of MHC class I presentation of E6 would correlate with the number of IFN-γ secreting activated E6-specific T cells. The activation of E6-specific CD8+ T cells was characterized by intracellular cytokine staining followed by flow cytometry analysis. As shown in Figure 2A, DC-1 cells cotransfected with CRT/E6 DNA and CIITA DNA generated significantly higher numbers of activated E6-specific CD8+ T cells compared to DC-1 cells transfected with CRT/E6 DNA and mutant CIITA. A graphical representation of the number of activated E6-specific CD8+ T cells is depicted in Figure 2B. Taken together, our data indicate that the increased MHC class I expression mediated by CIITA has led to enhanced MHC class I presentation of E6 in DC-1 cells transfected with CRT/E6 DNA.
Figure 2. Characterization of the MHC class I and II presentation of DCs transfected with CIITA DNA.
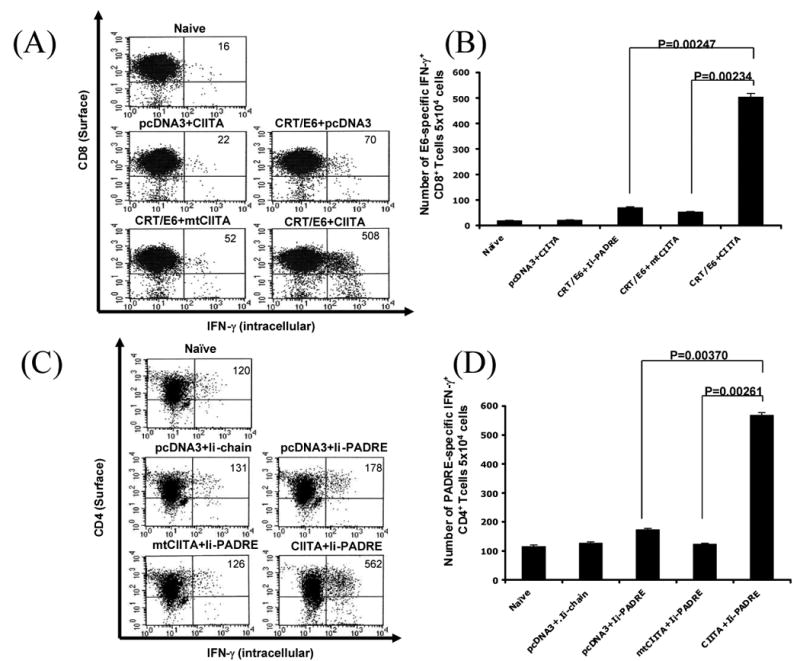
DCs were cotransfected with CIITA DNA and CRT/E6 DNA (A & B) or Ii-PADRE DNA (C & D). The DCs were then incubated with E6-specific CD8+ T cells (A & B) or PADRE-specific CD4+ T cells (C & D) overnight. The activation of antigen-specific T cells was characterized by intracellular cytokine staining followed by flow cytometry analysis using IFN-γ and CD4 or CD8-specific antibodies. A and C. Representative flow cytometry data showing the numbers of activated E6-specific CD8+ T cells (A) and PADRE-specific CD4+ T cells (C) after incubation with the cotransfected DCs. B and D. Bar graphs depicting the numbers of E6-specific CD8+ T cells (B) and PADRE-specific CD4+ T cells (D) (means±s.d.). The data presented in this figure are from one representative experiment of two performed.
We also characterized the antigen presentation through MHC class II pathway in DCs cotransfected with Ii-PADRE DNA and CIITA DNA. The transfected DCs were incubated with PADRE-specific CD4+ T cells overnight. The increased in MHC class II presentation of PADRE would lead to an increased number of IFN-γ secreting activated PADRE-specific T cells. We characterized the activation of PADRE-specific CD4+ T cells using intracellular cytokine staining followed by flow cytometry analysis. As shown in Figure 2C, DC-1 cells cotransfected with CIITA DNA and Ii-PADRE DNA generated significantly higher numbers of activated PADRE-specific CD4+ T cells compared to DC-1 cells transfected with Ii-PADRE DNA and mutant CIITA. A graphical representation of the number of PADRE-specific CD4+ T cells is depicted in Figure 2D. Thus, our data similarly suggest that the increased MHC class II expression mediated by CIITA led to enhanced MHC class II presentation of PADRE in DC-1 cells transfected with Ii-PADRE DNA.
Co-administration of CRT/E6 DNA with CIITA DNA leads to enhanced E6-specific CD8+ T cell immune responses in vaccinated mice
We further characterized the antigen-specific CD8+T cell immune responses in C57BL/6 mice vaccinated with CRT/E6 DNA in conjunction with CIITA DNA or pcDNA3. Mice vaccinated with pcDNA3 with CIITA DNA were used as a negative control. One week after the last vaccination, we measured the E6-specific CD8+ cell immune responses in vaccinated mice using intracellular IFN-γ staining followed by flow cytometry analysis. As shown in Figure 3, mice vaccinated with both CRT/E6 DNA and CIITA DNA generated the highest numbers of E6-specific CD8+ T cells among the various groups. We also characterized the antigen-specific CD4+ T cell immune responses in mice vaccinated with Ii-PADRE DNA in conjunction with CIITA DNA or pcDNA3. Mice vaccinated with Ii-chain DNA with CIITA DNA were used as a negative control. We observed that mice vaccinated with Ii-PADRE DNA and CIITA DNA generated the highest numbers of PADRE-specific CD4+ T cells among all the vaccinated groups (data not shown). Thus, our data indicate that the increased MHC class I and II presentation by CIITA is capable of enhancing both the antigen-specific CD8+ and CD4+ T cell immune responses in vaccinated mice.
Figure 3. Characterization of the E6-specific CD8+ T cell immune responses in mice vaccinated with CIITA DNA and CRT/E6 DNA.
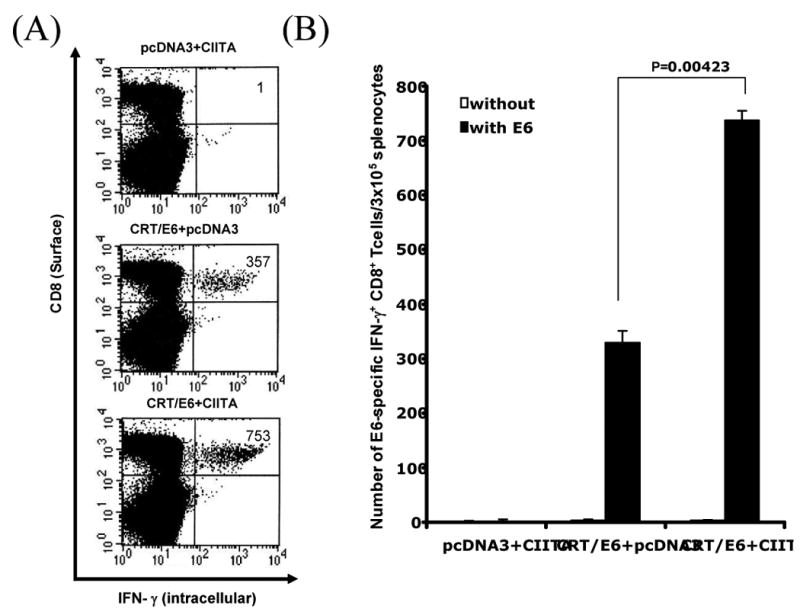
C57BL/6 mice (5 per group) were immunized with 2 μg /mouse of CIITA and/or CRT/E6 DNA twice with a 1-week interval. Splenocytes from vaccinated mice were harvested 1 week after the last vaccination and characterized for E6-specific CD8+ T cells using intracellular IFN-γ staining followed by flow cytometry analysis. A. Representative flow cytometry data for the E6-specific CD8+ T cell immune responses. The numbers in the upper right-hand corner represent the number of E6-specific IFN-γ-secreting CD8+ T cells per 5×106 pooled splenocytes. B. Bar graphs depicting the numbers of E6-specific IFN-γ-secreting CD8+ T cells per 5×106 pooled splenocytes (means±s.d.). The data presented in this figure are from one representative experiment of two performed.
Coadministration of CRT/E6 DNA with CIITA DNA and Ii-PADRE further enhances E6-specific CD8+ T cell immune responses in vaccinated mice
We recently demonstrated that DNA vaccines encoding HPV-16 E6 and/or E7 antigens co-administered with Ii-PADRE could improve the HPV antigen-specific CD8+ T cell immune responses in vaccinated mice (12). Since the strategy to enhance CD4+ T cell help represents a different strategy to enhance DNA vaccine potency, we explored if this strategy (Ii-PADRE) can be combined with the strategies to enhance MHC class I/II presentation (CIITA) to further enhance the antigen-specific T cell immune responses in vaccinated mice. Thus, we vaccinated C57BL/6 mice with the combinations of DNA constructs illustrated in Table 1. One week after the last vaccination, we measured the E6-specific CD8+ cell immune responses in vaccinated mice using intracellular IFN-γ staining followed by flow cytometry analysis. As shown in Figure 4, mice vaccinated with CRT/E6, Ii-PADRE and CIITA DNA generated significantly higher numbers of E6-specific CD8+ T cells compared to mice vaccinated with CRT/E6, Ii-PADRE and with pcDNA3 DNA (p= 0.00343) or with mutant CIITA DNA (p= 0.00201). We also characterized the PADRE-specific CD4+ T cell immune responses in vaccinated mice. We found that mice vaccinated with CRT/E6, Ii-PADRE and CIITA DNA generated significantly higher number of PADRE-specific CD4+ T cells compared to vaccination with CRT/E6, Ii-PADRE and mtCIITA DNA (data not shown). Thus, our results suggest that co-administration of CRT/E6 DNA with CIITA DNA and Ii-PADRE DNA further enhances E6-specific CD8+ T cell immune responses in vaccinated mice.
Figure 4. Characterization of the E6-specific CD8+ T cells in mice vaccinated with CRT/E6, CIITA DNA and Ii-PADRE DNA vaccines.
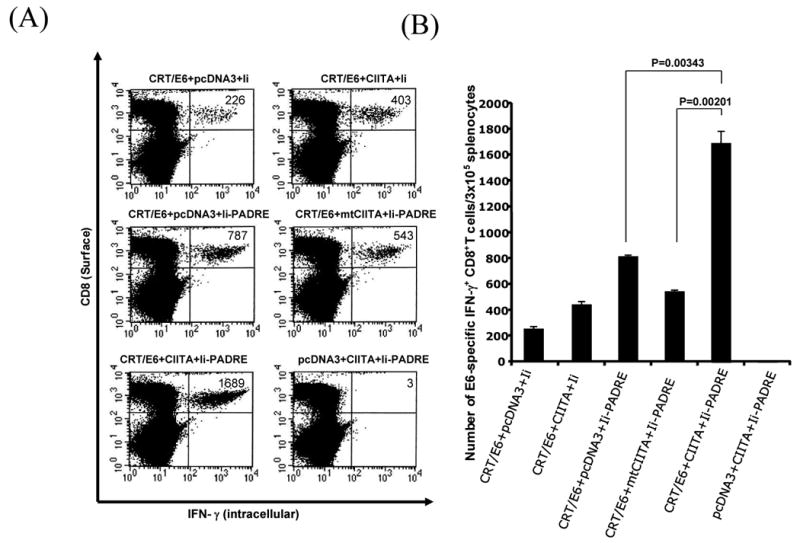
C57BL/6 mice (5 per group) were immunized with 2 μg /mouse twice with a 1-wk interval of the DNA combinations listed in Table 1. Splenocytes from vaccinated mice were harvested 1 week after the last vaccination and characterized for E6-specific CD8+ T cells using intracellular IFN-γ staining followed by flow cytometry analysis. A. Representative flow cytometry data. The numbers in the upper right-hand corner represent the number of E6-specific IFN-γ-secreting CD8+ T cells per 5×106 pooled splenocytes. B. Bar graphs depicting the numbers of E6-specific IFN-γ-secreting CD8+ T cells per 5×106 pooled splenocytes (means±s.d.). The data presented in this figure are from one representative experiment of two performed.
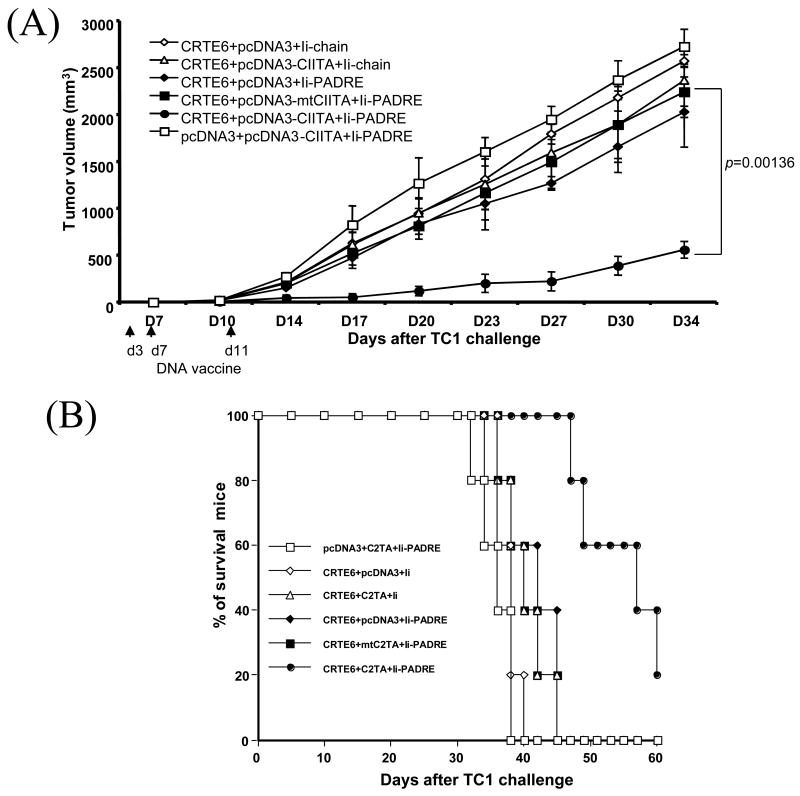
Treatment with a combination of CIITA DNA, CRT/E6 and Ii-PADRE DNA leads to enhanced antitumor effects and prolonged survival in TC-1 tumor-bearing mice
In order to determine if the enhanced E6-specific T cell response generated by co-administration of the combination of CIITA, CRT/E6 and Ii-PADRE DNA translates into therapeutic antitumor effects, we performed in vivo tumor treatment experiments using an HPV-16 E6/E7-expressing tumor model, TC-1. C57BL/6 mice were first challenged subcutaneously with TC-1 tumor cells and then, three days later, treated with the combinations of DNA constructs illustrated in Table 1. The treated mice were monitored for tumor growth. As shown in Figure 5A, tumor-bearing mice treated with the combination of CRT/E6, CIITA DNA and Ii-PADRE DNA exhibited significantly decreased tumor growth compared to the tumor-bearing mice treated with CRT/E6, Ii-PADRE DNA and the mutant CIITA DNA (p= 0.00136). We also performed Kaplan-Meier survival analysis of the treated mice. As shown in Figure 5B, tumor-challenged mice treated with the combination of CRT/E6, CIITA DNA and Ii-PADRE DNA also exhibited significantly prolonged survival compared to the other treatment groups. Thus, our data indicates that treatment with a combination of CIITA DNA, CRT/E6 and Ii-PADRE DNA leads to enhanced antitumor effects and prolonged survival in TC-1 tumor-bearing mice.
Figure 5. In vivo tumor treatment experiments.
C57BL/6 mice (5 per group) were first challenged with 5×104/mouse of TC-1 tumor cells by subcutaneous injection. Three days after tumor challenge, the mice were administered 2 μg DNA/mouse 3 times with 4-day intervals of the various DNA vaccine mixtures listed in Table 1. The mice were monitored for evidence of tumor growth by inspection and palpation twice a week. Tumor volumes were measured starting from day 7 after tumor challenge. (A) Line graph depicting the tumor volumes in mice of different tumor treatments (means±s.d.). (B) Kaplan & Meier survival analysis in mice of the tumor treatment experiments. The data shown here are from one representative experiment of two performed.
Co-administration of CRT/E6 DNA with CIITA DNA and Ii-PADRE leads to enhanced E6-specific CD8+ memory T cells in vaccinated mice
We also characterized the long-term antigen specific immune responses to vaccination with the combination of CIITA, CRT/E6 and Ii-PADRE DNA. C57BL/6 mice were vaccinated with the combinations of DNA constructs illustrated in Table 1. Sixty days later, we measured the E6-specific CD8+ cell immune responses in vaccinated mice using intracellular IFN-γ staining followed by flow cytometry analysis. As shown in Figure 6, vaccination with CRT/E6, Ii-PADRE and CIITA DNA generated higher numbers of E6-specific CD8+ memory T cells compared to vaccination with CRT/E6 and Ii-PADRE with mutant CIITA DNA (p= 0.00756). We also determined the PADRE-specific CD4+ T cell long-term immune responses in mice vaccinated with CRT/E6, Ii-PADRE and CIITA DNA compared to the other DNA construct combinations. Mice vaccinated with CRT/E6, Ii-PADRE and CIITA DNA generated significantly higher number of PADRE-specific CD4+ T memory cells compared to vaccination with CRT/E6, Ii-PADRE and mtCIITA DNA (data not shown). Thus, our data indicate that co-administration of CRT/E6 DNA with CIITA DNA and Ii-PADRE leads to enhanced E6-specific CD8+ memory T cells in vaccinated mice.
Figure 6. Characterization of the long-term E6-specific CD8+ T cell immune response in mice vaccinated with CRT/E6, CIITA DNA and Ii-PADRE DNA vaccines.
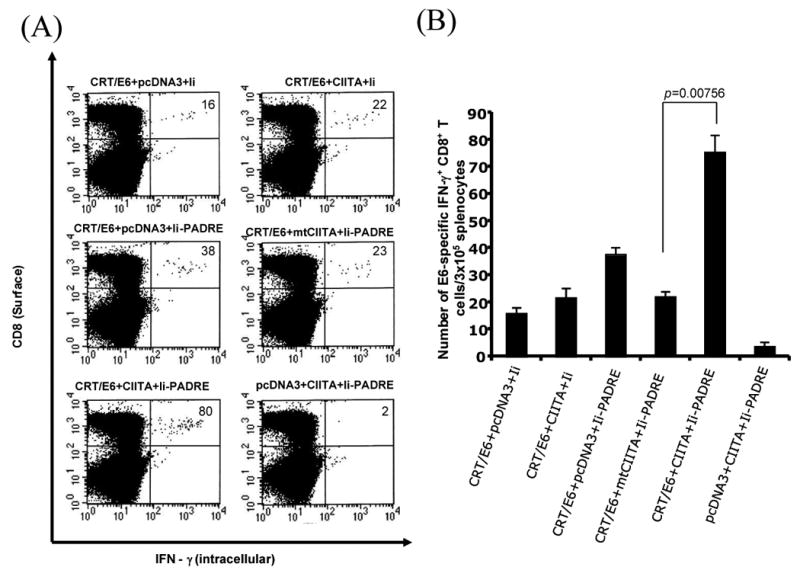
C57BL/6 mice (5 per group) were immunized with 2 μg /mouse twice with a 1-wk interval of the DNA combinations listed in Table 1. Splenocytes from vaccinated mice were harvested 60 days after the last vaccination and characterized for E6-specific CD8+ T cells using intracellular IFN-γ staining followed by flow cytometry analysis. A. Representative flow cytometry data. The numbers in the upper right-hand corner represent the number of memory E6-specific IFN-γ-secreting CD8+ T per 5×106 pooled splenocytes. B. Bar graphs depicting the numbers of memory E6-specific IFN-γ-secreting CD8+ T cells per 5×106 pooled splenocytes (means±s.d.). The data presented in this figure are from one representative experiment of two performed.
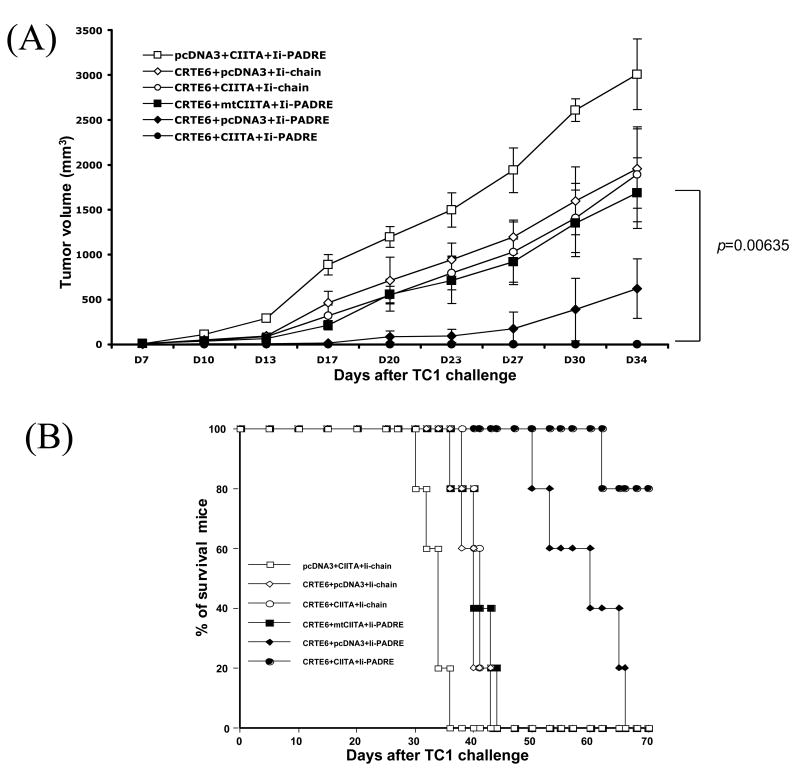
Co-administration of CRT/E6 DNA with CIITA DNA and Ii-PADRE leads to long-term protection against TC-1 tumors and prolonged survival in vaccinated mice
To determine whether the observed increase in E6-specific CD8+ memory T cells generated by the combination of CIITA DNA, CRT/E6 and Ii-PADRE DNA could be translated into long-term protective anti-tumor effects, we performed long-term in vivo tumor protection experiments. C57BL/6 mice were vaccinated with the various DNA constructs illustrated in Table 1. Two months after the last vaccination, immunized mice were subcutaneously challenged with TC-1 tumor cells and then monitored for tumor growth. As shown in Figure 7A, mice vaccinated with CIITA DNA, CRT/E6 DNA and Ii-PADRE DNA demonstrated almost complete inhibition of tumor growth compared to mice vaccinated with CRT/E6 DNA, Ii-PADRE DNA and the mutant CIITA DNA. We also performed Kaplan Meier survival analysis. As shown in Figure 7B, we observed significantly prolonged survival in these mice. These data suggest that the co-administration of the combination of CIITA DNA and Ii-PADRE DNA can further enhance the ability of CRT/E6 DNA to generate long-term protective antitumor effects against TC-1 tumors in vaccinated mice.
Figure 7. Long-term in vivo tumor protection experiments.
C57BL/6 mice (5 per group) were immunized with 2 μg DNA/mouse twice with a 1-week interval of the various DNA vaccine mixtures listed in Table 1. Two months after the last vaccination, the mice were challenged by subcutaneous injection of 1×105/mouse of TC-1 cells. The mice were monitored for evidence of tumor growth by inspection and palpation twice a week. Tumor volumes were measured starting from day 7 after tumor challenge. (A) Line graph depicting tumor volume in mice challenged with TC-1 cells (means±s.d.). (B) Kaplan & Meier survival analysis in mice challenged with TC-1 cells. The data shown here are from one representative experiment of two performed.
Discussion
In the current study, we employed a combination of DNA vaccines encoding CRT/E6, Ii-PADRE DNA and CIITA DNA to further improve DNA vaccine potency. We showed that DC-1 cells transfected with CIITA DNA exhibited enhanced MHC I/II expression leading to improved antigen presentation through the MHC class I/II pathways. Co-administration of the combination of CRT/E6 DNA with CIITA DNA and Ii-PADRE further enhanced E6-specific CD8+ T cell immune responses and the antitumor effects in TC-1 tumor-bearing mice. Vaccination with the combination vaccine also led to enhanced E6-specific CD8+ memory T cell response, long-term protection against TC-1 tumors and prolonged survival in vaccinated mice. Thus, the combination of CIITA DNA with CRT/E6 and Ii-PADRE DNA vaccines represents a promising approach to further enhance the potency of DNA vaccines.
The employment of gene gun administration is important for the success of the current strategy. All these strategies most likely require the direct delivery of DNA into the DCs in order to effectively influence the priming of the T cells. For example, intracellular targeting strategies using CRT require the linked antigen to be directly targeted to the endoplasmic reticulum in order enhance the antigen processing. Furthermore, the Ii-PADRE DNA strategy requires the induction of CD4+ T helper cells in the vicinity of antigen-specific CD8+ T cells in order to enhance T cell activation. Finally, the strategy employing CIITA DNA is required to be delivered directly to the DCs so that it can increased expression of MHC class I/II molecules on its surface, leading to enhanced MHC class I/II antigen processing and presentation. Thus, all the strategies employed in the current study rely heavily on the intradermal delivery of antigen via gene gun.
In our study, we have successfully employed DNA vaccines encoding the CIITA DNA to enhance DNA vaccine potency. Previously, the CIITA has been used in other vaccine systems, particularly tumor-cell based vaccines to successfully improve vaccine potency (23-26). Furthermore, tumor cells transfected with CIITA and/or CD80 has been shown to activate tumor-specific CD4+ T cells (25, 26). Thus, the employment CIITA has been shown to be promising in the enhancement of cancer vaccine potency.
It is important to identify the best vaccine for future clinical translation. DNA vaccines employing different strategies to improve vaccine potency modify the properties of DCs through different, complementary mechanisms. In our study, we showed that the intradermal administration of the combination of CRT/E6, Ii-PADRE and CIITA DNA vaccines generated the best E6-specific CD8+ T cell immune responses and antitumor effects against TC-1 tumors. Each of the DNA constructs employs strategies to modify the properties of DCs through different mechanisms. It is likely that the potency of this combination of DNA vaccines can be further enhanced by the addition of a DNA vaccine employing a strategy that operates through another mechanism. For example, one potential strategy is to modify the properties of DCs using co-administration of DNA encoding antiapoptotic proteins. We have previously shown that co-administration of DNA vaccines with DNA encoding antiapoptotic proteins such Bcl-xL has led to the prolonged life of DCs, resulting in significant enhancement of antigen-specific CD8+ T cell immune responses (27). It would be of interest to see if this antiapoptotic strategy can be combined with the aforementioned DNA vaccine strategies to further enhance DNA vaccine potency for eventual clinical translation.
In summary, we have identified an innovative strategy to increase the expression of MHC class I/II molecules on DCs to enhance DNA vaccine potency. This strategy can potentially be used in other antigenic systems for the control of infectious diseases and cancer.
Footnotes
This work was supported by the Flight Attendant Medical Research Institute and National Cancer Institute SPORE in Cervical Cancer P50 CA098252 and the 1 RO1 CA114425-01.
References
- 1.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 3.Hung CF, Yang M, Wu TC. Modifying professional antigen-presenting cells to enhance DNA vaccine potency. Methods Mol Med. 2006;127:199–220. doi: 10.1385/1-59745-168-1:199. [DOI] [PubMed] [Google Scholar]
- 4.Tsen SW, Paik AH, Hung CF, Wu TC. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Rev Vaccines. 2007;6:227–239. doi: 10.1586/14760584.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, Wu TC. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–678. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash PD, Opas M, Michalak M. Calreticulin: not just another calcium-binding protein. Mol Cell Biochem. 1994;135:71–78. doi: 10.1007/BF00925962. [DOI] [PubMed] [Google Scholar]
- 7.Spee P, Neefjes J. TAP-translocated peptides specifically bind proteins in the endoplasmic reticulum, including gp96, protein disulfide isomerase and calreticulin. Eur J Immunol. 1997;27:2441–2449. doi: 10.1002/eji.1830270944. [DOI] [PubMed] [Google Scholar]
- 8.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 9.Peng S, Ji H, Trimble C, He L, Tsai YC, Yeatermeyer J, Boyd DA, Hung CF, Wu TC. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol. 2004;78:8468–8476. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 11.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snoke K, Serra HM, Kubo RT, Sette A, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 12.Hung CF, Tsai YC, He L, Wu TC. DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4+ T-cell immune responses and enhances vaccine potency. Mol Ther. 2007;15:1211–1219. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D, Hoory T, Wu TC, Hung CF. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life and intracellular targeting strategies with a strategy to boost CD4+ T cell. Human gene therapy. 2007;18:1129–1139. doi: 10.1089/hum.2007.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109 S21-33 doi: 10.1016/s0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 15.Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 16.Martin BK, Chin KC, Olsen JC, Skinner CA, Dey A, Ozato K, Ting JP. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591–600. doi: 10.1016/s1074-7613(00)80347-7. [DOI] [PubMed] [Google Scholar]
- 17.Gobin SJ, Peijnenburg A, Keijsers V, van den Elsen PJ. Site alpha is crucial for two routes of IFN gamma-induced MHC class I transactivation: the ISRE-mediated route and a novel pathway involving CIITA. Immunity. 1997;6:601–611. doi: 10.1016/s1074-7613(00)80348-9. [DOI] [PubMed] [Google Scholar]
- 18.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 19.Kim TW, Hung CF, Boyd DA, He L, Lin CT, Kaiserman D, Bird PI, Wu TC. Enhancement of DNA vaccine potency by coadministration of a tumor antigen gene and DNA encoding serine protease inhibitor-6. Cancer Res. 2004;64:400–405. doi: 10.1158/0008-5472.can-03-1475. [DOI] [PubMed] [Google Scholar]
- 20.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 21.Greer SF, Harton JA, Linhoff MW, Janczak CA, Ting JP, Cressman DE. Serine residues 286, 288, and 293 within the CIITA: a mechanism for down-regulating CIITA activity through phosphorylation. J Immunol. 2004;173:376–383. doi: 10.4049/jimmunol.173.1.376. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, Wu TC. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 23.Armstrong TD, Clements VK, Martin BK, Ting JP, Ostrand-Rosenberg S. Major histocompatibility complex class II-transfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6886–6891. doi: 10.1073/pnas.94.13.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dissanayake SK, Thompson JA, Bosch JJ, Clements VK, Chen PW, Ksander BR, Ostrand-Rosenberg S. Activation of tumor-specific CD4(+) T lymphocytes by major histocompatibility complex class II tumor cell vaccines: a novel cell-based immunotherapy. Cancer Res. 2004;64:1867–1874. doi: 10.1158/0008-5472.can-03-2634. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JA, Dissanayake SK, Ksander BR, Knutson KL, Disis ML, Ostrand-Rosenberg S. Tumor cells transduced with the MHC class II Transactivator and CD80 activate tumor-specific CD4+ T cells whether or not they are silenced for invariant chain. Cancer Res. 2006;66:1147–1154. doi: 10.1158/0008-5472.CAN-05-2289. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JA, Srivastava MK, Bosch JJ, Clements VK, Ksander BR, Ostrand-Rosenberg S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4(+) T lymphocytes. Cancer Immunol Immunother. 2008;57:389–398. doi: 10.1007/s00262-007-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, Kumar S, Wu TC. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109–117. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]