Abstract
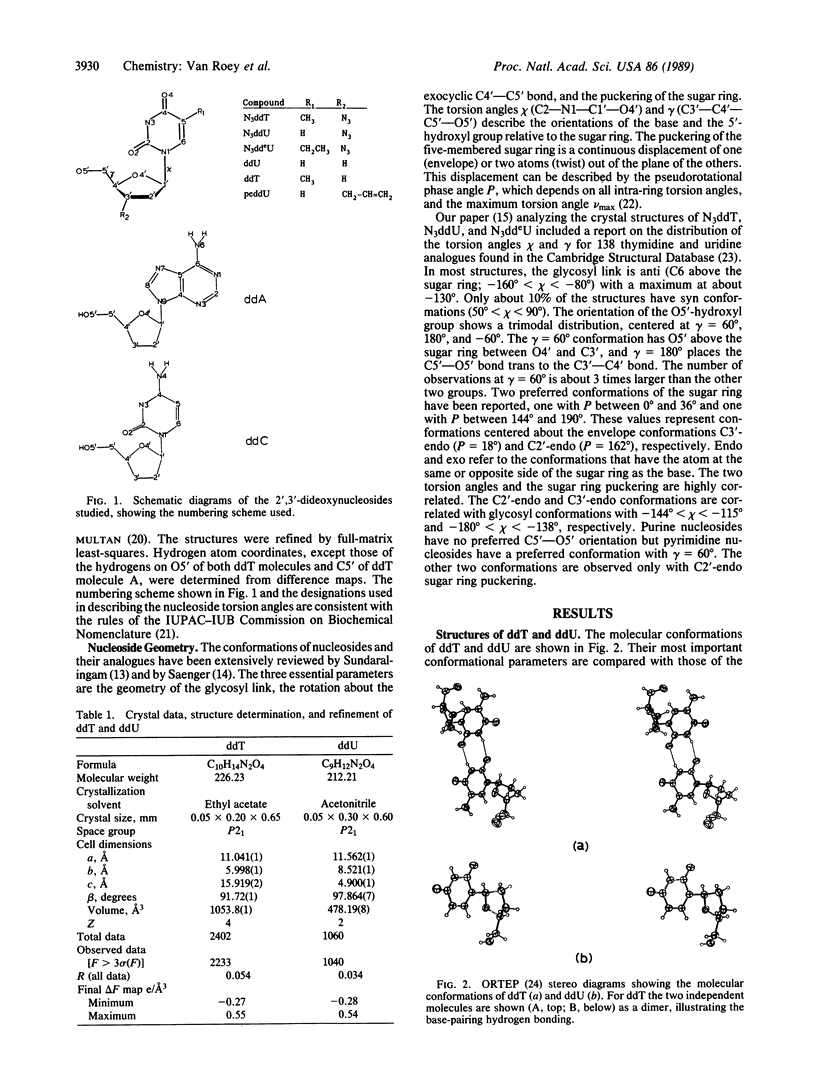
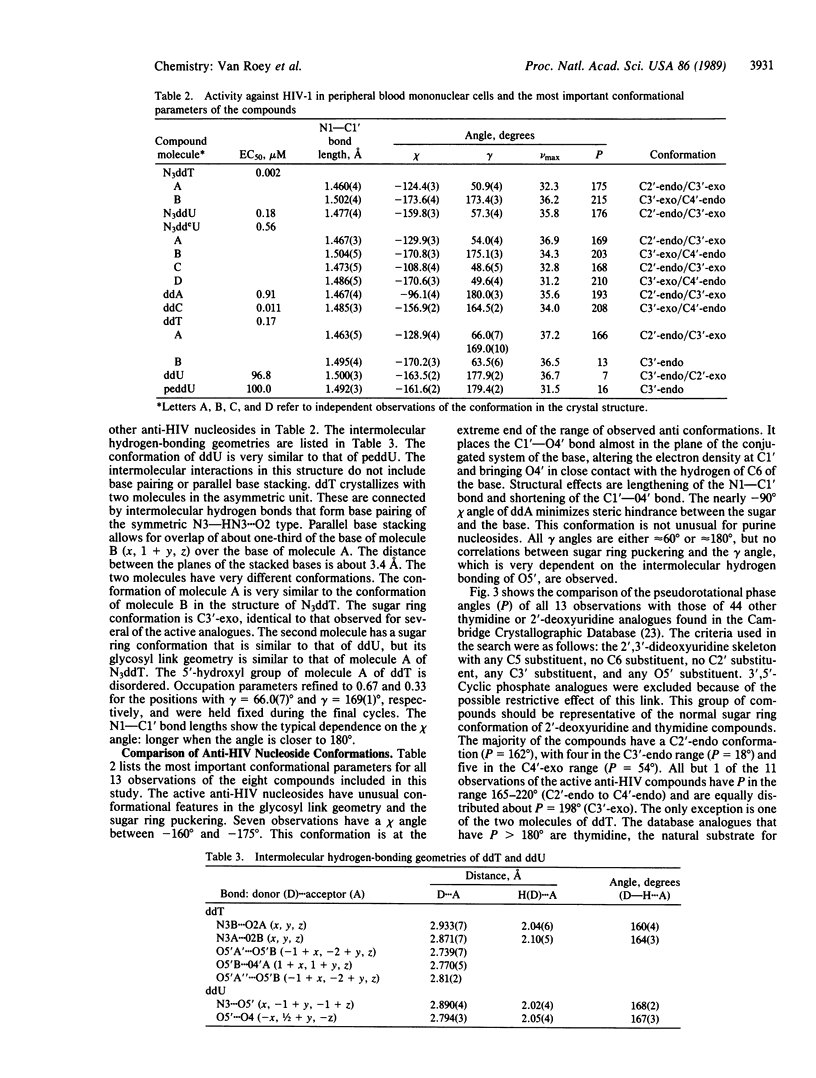
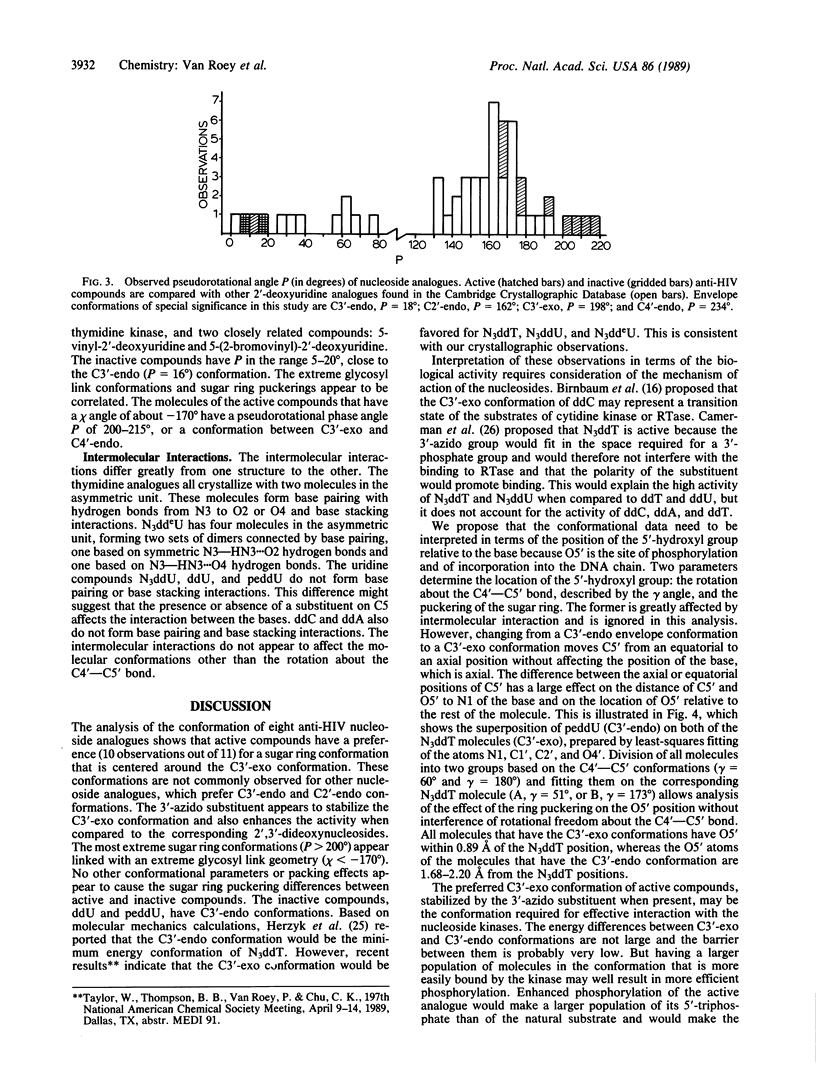
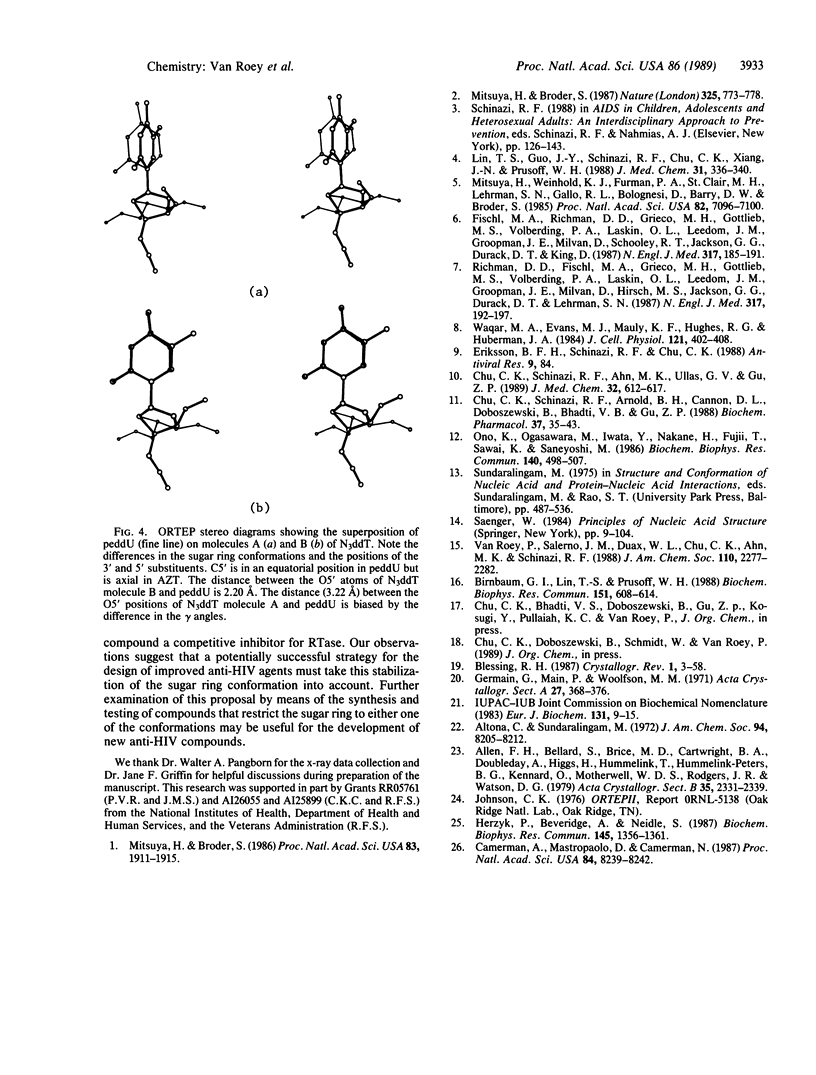
Analysis of the solid-state conformations of six active and two inactive anti-human immunodeficiency virus nucleoside analogues is used to correlate conformational features with the relative activities of the compounds. Ten of the 11 observations of the active compounds (3'-azido-3'-deoxythymidine, 3'-azido-2',3'-dideoxyuridine, 3'-azido-2',3'-dideoxy-5-ethyluridine, 2',3'-dideoxyadenosine, 2',3'-dideoxycytidine, and 3'-deoxythymidine) have C3'-exo sugar ring conformations. The only exception is one of the two molecules of 3'-deoxythymidine. Four have unusual low anti glycosyl link conformations coupled with unusual C3'-exo/C4'-endo sugar ring conformations. The inactive compounds, 2',3'-dideoxyuridine and 3'-(propyl-2-ene)-2',3'-dideoxyuridine, have C3'-endo conformations. The C3'-exo and C3'-endo conformations place C5' in axial and equatorial positions, respectively. This affects the location of the 5'-hydroxyl group in relation to the location of the base. The 5'-hydroxyl group is the site of phosphorylation of the nucleoside and the observation of this conformational preference of the active compounds may be of importance for the development of new nucleosides with activity against human immunodeficiency virus.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Birnbaum G. I., Lin T. S., Prusoff W. H. Unusual structural features of 2',3'-dideoxycytidine, an inhibitor of the HIV (AIDS) virus. Biochem Biophys Res Commun. 1988 Feb 29;151(1):608–614. doi: 10.1016/0006-291x(88)90637-7. [DOI] [PubMed] [Google Scholar]
- Camerman A., Mastropaolo D., Camerman N. Azidothymidine: crystal structure and possible functional role of the azido group. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8239–8242. doi: 10.1073/pnas.84.23.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. K., Schinazi R. F., Ahn M. K., Ullas G. V., Gu Z. P. Structure-activity relationships of pyrimidine nucleosides as antiviral agents for human immunodeficiency virus type 1 in peripheral blood mononuclear cells. J Med Chem. 1989 Mar;32(3):612–617. doi: 10.1021/jm00123a018. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Herzyk P., Beveridge A., Neidle S. Conformational properties of 3'-azido-3'deoxy-thymidine (AZT), an inhibitor of HIV reverse transcriptase. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1356–1361. doi: 10.1016/0006-291x(87)91587-7. [DOI] [PubMed] [Google Scholar]
- Lin T. S., Guo J. Y., Schinazi R. F., Chu C. K., Xiang J. N., Prusoff W. H. Synthesis and antiviral activity of various 3'-azido analogues of pyrimidine deoxyribonucleosides against human immunodeficiency virus (HIV-1, HTLV-III/LAV). J Med Chem. 1988 Feb;31(2):336–340. doi: 10.1021/jm00397a011. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Broder S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2',3'-dideoxynucleosides. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1911–1915. doi: 10.1073/pnas.83.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H., Broder S. Strategies for antiviral therapy in AIDS. 1987 Feb 26-Mar 4Nature. 325(6107):773–778. doi: 10.1038/325773a0. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Ogasawara M., Iwata Y., Nakane H., Fujii T., Sawai K., Saneyoshi M. Inhibition of reverse transcriptase activity by 2',3'-dideoxythymidine 5'-triphosphate and its derivatives modified on the 3' position. Biochem Biophys Res Commun. 1986 Oct 30;140(2):498–507. doi: 10.1016/0006-291x(86)90760-6. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Fischl M. A., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Hirsch M. S. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- Waqar M. A., Evans M. J., Manly K. F., Hughes R. G., Huberman J. A. Effects of 2',3'-dideoxynucleosides on mammalian cells and viruses. J Cell Physiol. 1984 Nov;121(2):402–408. doi: 10.1002/jcp.1041210218. [DOI] [PubMed] [Google Scholar]



