Abstract
Background:
Over 50% of persons with idiopathic REM sleep behavior disorder (RBD) will develop Parkinson disease (PD) or dementia. At present, there is no way to predict who will develop disease. Since polysomnography is performed in all patients with idiopathic RBD at diagnosis, there is an opportunity to analyze if baseline sleep variables predict eventual neurodegenerative disease.
Methods:
In a longitudinally studied cohort of patients with idiopathic RBD, we identified those who had developed neurodegenerative disease. These patients were matched by age, sex, and follow-up duration to patients with RBD who remained disease-free and to controls. Polysomnographic variables at baseline (i.e., before development of neurodegenerative disease) were compared between groups.
Results:
Twenty-six patients who developed neurodegenerative disease were included (PD 12, multiple system atrophy 1, dementia 13). The interval between polysomnogram and disease onset was 6.7 years, mean age was 69.5, and 81% were male. There were no differences between groups in sleep latency, sleep time, % stages 2–4, % REM sleep, or sleep efficiency. However, patients with idiopathic RBD who developed neurodegenerative disease had increased tonic chin EMG activity during REM sleep at baseline compared to those who remained disease-free (62.7 ± 6.0% vs 41.0 ± 6.0%, p = 0.020). This effect was seen only in patients who developed PD (72.9 ± 6.0% vs 41.0 ± 6.0%, p = 0.002), and not in those who developed dementia (54.3 ± 10.3, p = 0.28). There was no difference in phasic submental REM EMG activity between groups.
Conclusions:
In patients with REM sleep behavior disorder initially free of neurodegenerative disease, the severity of REM atonia loss on baseline polysomnogram predicts the development of Parkinson disease.
GLOSSARY
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- EOG
= electrooculogram;
- PD
= Parkinson disease;
- PSG
= polysomnographic;
- RBD
= REM sleep behavior disorder.
REM sleep behavior disorder (RBD) is characterized by loss of the normal atonia of REM sleep. Affected patients talk, kick, punch, or thrash in apparent response to dream content.1 Patients with idiopathic RBD are at substantial risk of developing Parkinson disease (PD) and Lewy body dementia, with 5-year estimates of disease risk ranging from 20% to 45%, and 10-year estimates ranging from 40% to 65%.2–6 At present, there are no ways to identify which patients with idiopathic RBD will eventually develop neurodegenerative disease. One group of potential predictive markers, by definition performed on all patients with RBD at the time of diagnosis, are polysomnographic (PSG) markers. We examined whether baseline variables on PSG could predict eventual development of neurodegenerative disease.
METHODS
Patients with idiopathic RBD were recruited from the sleep disorders laboratory at the Hôpital du Sacré-Coeur, Montreal, Quebec. Ethics approval was obtained from the hospital research ethics board and all patients gave written informed consent to participate, according to the declaration of Helsinki. The presence of RBD was defined according to standard ICSD-II criteria, as an increase of tonic or phasic chin EMG activity during REM sleep,7 and either a history of elaborate motor activity during sleep associated with dream content, or behavioral manifestations occurring during REM sleep during PSG recording. All patients had a baseline neurologic examination excluding the presence of neurodegenerative disease, according to UK brain bank criteria for parkinsonism and DSM-IV criteria for dementia.8,9
From our cohort, we then selected all patients originally diagnosed with idiopathic RBD who eventually developed a defined neurodegenerative disease. These were frequency-matched according to age, sex, and duration of follow-up (since PSG) with idiopathic RBD patients who, at last visit, had not developed an identified neurodegenerative disease. Matching was performed blinded to PSG variables. Controls were selected from the general population, and were frequency-matched for age and sex. All controls had PSG documenting the absence of RBD. Duration of RBD symptoms before PSG was calculated from patient self-report, and duration of objective RBD was defined by the interval between PSG and the last follow-up examination (if well), or disease onset (if neurodegenerative disease was present).
Definition of disease.
Parkinsonism was diagnosed according to UK brain bank criteria, as the presence of bradykinesia in association with rest tremor, rigidity, or postural instability.9 Once the diagnosis of parkinsonism had been determined, the most likely cause for the parkinsonism was delineated according to best clinical impression of a movement disorders specialist (R.P.) and the UK brain bank criteria for PD.9 Dementia was diagnosed based upon neuropsychological testing and/or clinical evaluation by a consensus between the neuropsychologist (J.F.G.) and the neurologist (R.P.). For those patients who underwent neuropsychological testing, dementia was defined as significant impairment on at least 2 cognitive domains (executive functions and attention, verbal learning and memory, or visuospatial abilities) in association with functional impairment due to cognitive impairment (defined as inability to self-administer medication independently or any basic or instrumental activity of daily living, due to cognitive loss).8,10 For those who refused neuropsychological testing, dementia was defined as Mini-Mental State Examination <24 in association with functional impairment due to cognitive impairment.
Polysomnographic variables.
All participants underwent 1 night of PSG recording in the sleep laboratory including a full EEG montage to rule out epilepsy. Patients were withdrawn from any medication known to affect sleep, vigilance, and motor behavior for at least 2 weeks prior to PSG recording. Respiration was monitored using an oronasal thermistor or a nasal canula and with thoracic and abdominal strain gauges, while blood oxygen saturation was continuously recorded by cutaneous finger pulse oximeter. None of the patients or controls showed an apnea index (number of apneas per hour of sleep) >10 or a respiratory event index (apneas + hypopneas) >15. For both idiopathic RBD patients and normal controls, sleep was recorded according to the method of Rechtschaffen and Kales11 which includes standard EEG leads (C3-A2, O2-A1), bilateral electrooculogram (EOG), and chin EMG recordings. Since loss of REM sleep atonia precludes use of the Rechtschaffen/Kales method for scoring REM sleep, the occurrence of the first REM was used to determine the onset of REM sleep. Termination of a REM sleep period was identified either by the occurrence of a specific EEG feature of another sleep stage (K complex, sleep spindle) or EEG sign of arousal, or by the absence of rapid eye movements for 3 consecutive minutes. Each 20-second REM epoch was scored as tonic or atonic depending if chin EMG activity (defined by amplitude >2× background, or >10 μV) was present for more or less than 50% of the epoch duration and from this, the percentage of REM periods with tone (% tonic REM) was calculated. Percent phasic chin EMG density was scored from the submental EMG activity and represented the percentage of 2-second mini-epochs containing EMG events lasting 0.1 to 10 seconds, with an amplitude exceeding 4 times the amplitude of background EMG activity. PSG variables assessing REM tone were scored by the same technician, who was blinded to eventual disease status.
Analysis.
For comparison between groups, the nonparametric Mann-Whitney U test was used to compare continuous variables. Categorical variables were compared using Fisher exact test. Because patients with dementia and patients with PD had not been matched to demographic characteristics, linear regression analysis, adjusting for age and sex, was used for comparisons of dementia and PD.
RESULTS
Patients.
A total of 29 patients with PSG recording developed a neurodegenerative disease. Three patients were excluded because PSG recording was not computerized, and REM atonia % was not calculated. Of the 26 patients included, 13 were diagnosed with dementia, 12 with idiopathic PD, and 1 with multiple system atrophy. Of the patients with dementia, 7 met consensus criteria for probable LBD,12 and 6 were diagnosed with possible LBD/AD. Age at PSG was 69.5 ± 1.5 years (mean ± standard error), and 21 (81%) were male. PSG was performed on average 6.7 years before onset of neurodegenerative disease. The mean symptom duration before PSG was 5.5 ± 0.85 years, similar to the symptom duration in those who remained disease-free (7.0 ± 2.1 years).
Sleep variables.
When comparing all patients with idiopathic RBD to controls, there were no differences in total sleep time, % of sleep in stages 1–4 and REM sleep, or sleep efficiency (table 1). As expected, there were highly significant differences between idiopathic RBD patients and controls in tonic and phasic EMG activity during REM sleep. When comparing idiopathic RBD patients who eventually developed neurodegenerative disease to idiopathic RBD patients who remained free of disease, there were no baseline differences in sleep latency, % of sleep in stages 2–4 and REM sleep, or sleep efficiency. However, patients who developed disease had a significantly increased % tonic chin EMG activity during REM sleep at baseline (i.e., more severe loss of REM atonia) than those who remained disease-free (62.7 ± 6.0% vs 41.0 ± 6.0%, p = 0.020). This effect was not found for phasic submental EMG activity during REM sleep. In addition, there was a slight increase in % of sleep in stage 1 in those who developed disease compared to those who remained disease-free (16.8 ± 2.0% vs 12.6 ± 2.5%, p = 0.034).
Table 1 Sleep parameters according to development of neurodegenerative disease
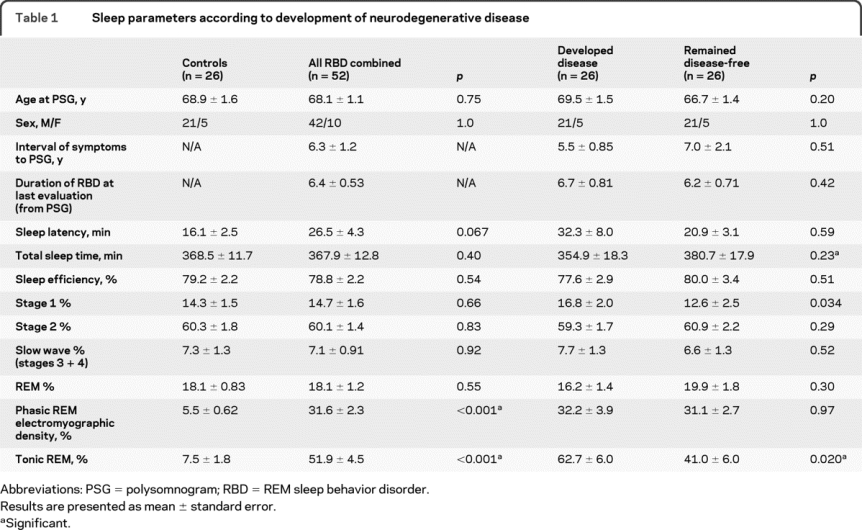
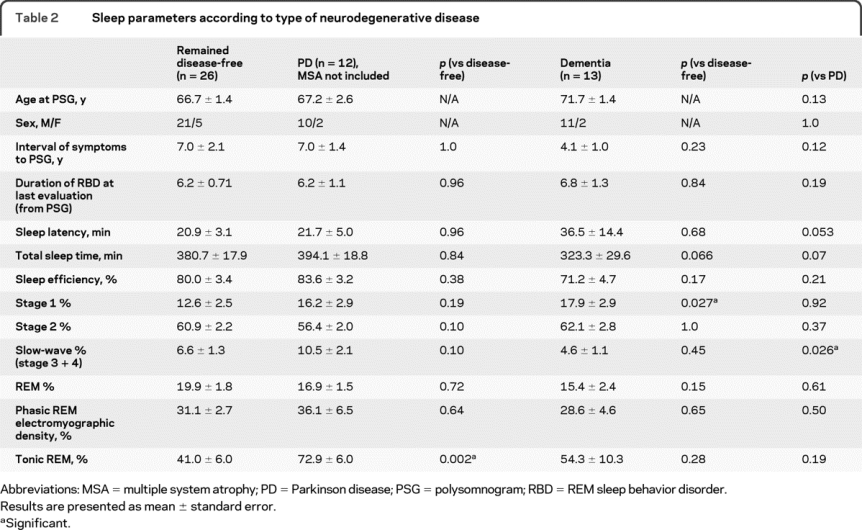
When patients were divided according to disease syndrome (PD vs dementia), baseline differences in tonic chin EMG activity during REM sleep were most striking in patients who eventually developed PD (72.9 ± 6.0% in PD vs 41.0 ± 6.0% in disease-free, p = 0.002) (table 2). The point estimate of % tonic chin EMG activity in dementia patients was intermediate between disease-free and patients with PD (54.3 ± 10.3), but these differences were not significant. Phasic submental EMG activity during REM sleep was similar in patients with dementia and patients with PD. Patients who eventually developed dementia had less slow-wave sleep at baseline compared to patients with PD (4.6 ± 1.1% vs 10.5 ± 2.1%, p = 0.026), but there was no significant difference from those who remained disease-free (p = 0.45).
Table 2 Sleep parameters according to type of neurodegenerative disease
DISCUSSION
The existence of a large cohort of patients with idiopathic RBD who have developed neurodegenerative disease provides an opportunity to define predictors of PD and dementia in patients with idiopathic RBD. In this study, we have found that the severity of abnormalities in % REM sleep atonia (the essential characteristic of RBD), assessed nearly 7 years before disease onset, can predict development of PD. This is the first reported predictor of neurodegenerative disease in patients with idiopathic RBD.
The principal finding is that % tonic chin EMG activity during REM sleep (i.e., the percentage time spent in tonic REM) predicts PD. The original article describing the elevated risk of PD among idiopathic RBD patients did not calculate % REM tone3; however, it found a nonsignificant increase in occurrence of tonic REM sleep abnormalities in those who developed PD compared to those remained idiopathic (91% vs 69%), which may accord with our findings. The only other cohort study of neurodegeneration in RBD did not find differences in either tonic or phasic REM sleep tone,4 although the point estimate of % tonic REM sleep was higher in the group who developed neurodegeneration (44.4% vs 36.7%). Of the 20 patients in this study with disease, 4 had only mild cognitive impairment, and only 9 had PD—it would be of interest to assess if % tonic REM was different between diagnostic groups in this study. A second study by the same group found no increase in % tonic REM between patients with idiopathic RBD and patients with RBD associated with PD, although there was an increase in those with multiple system atrophy.13 This study had a high proportion of mildly symptomatic RBD in the PD group, so results may not be directly comparable to our cohort, in which all patients presented with symptomatic idiopathic RBD.
The reason for the link between the severity of REM atonia loss and disease risk is not clear. One potential explanation would be that, since % tonic REM progresses with time even in established RBD,14 this could simply reflect a longer duration of RBD in the disease group. This does not seem to explain our findings because 1) patients were matched for duration of diagnosed RBD (i.e., duration since PSG) and 2) symptom interval was not different between patients who did or did not develop disease—in fact, the interval estimate was slightly longer in disease-free RBD patients (it should be noted, however, that symptom onset is often difficult for patients to define). Loss of REM atonia is the essential hallmark of RBD, so a higher % tonic EMG activity during REM sleep could be interpreted as more “severe” RBD. This could suggest that there is a subtype of “mild” RBD that is at lower risk of disease. The question of whether there are any differences in severity of degeneration in REM-controlling nuclei in patients with higher % tonic REM EMG activity awaits large-scale pathologic studies.
It should be noted that there is substantial overlap between groups and % tonic REM EMG activity by itself cannot perfectly predict disease risk. In this study, the optimal cutoff of 60% tonic REM EMG activity would allow classification of eventual disease development with a sensitivity of 64% and a specificity of 80% for all neurodegenerative disease. For predicting PD only, the optimal cutoff remains 60%, sensitivity improves to 75%, and specificity is 80%. However, calculations of this nature are completely dependent upon duration of follow-up—some of those in the “disease-free” group will almost certainly eventually develop neurodegenerative disease, changing calculations of sensitivity and specificity. In addition, our study population consisted entirely of persons who presented to our sleep disorders clinic for symptomatic complaints of dream-enactment behavior—these results may not extrapolate to general population RBD studies (i.e., asymptomatic patients screened for RBD in potential presymptomatic PD screening programs). The eventual role of PSG as a screen for asymptomatic PD remains unclear.
It is unclear why we found a difference with % tonic REM but not with phasic REM density. Abnormalities of tonic and phasic REM sleep are both clearly seen in RBD compared to controls. However, they can disconnect—for example, there are numerous unequivocal RBD cases described in which only phasic REM EMG tone was altered, with normal tonic REM sleep.15 Melatonin, a medication effective for RBD, corrects abnormalities of tonic but not phasic REM EMG, and clonazepam, the mainstay of treatment for RBD, may alter phasic but not tonic REM.5,15 There have been suggestions that tonic and phasic EMG activity in REM sleep may have separate mechanisms, with tonic EMG activity reflecting degeneration of the sublaterodorsal nucleus,16 and phasic EMG activity depending upon activation of locomotor generators during sleep and upon alteration of pathways in the intermediate ventromedial medulla.17 Therefore, it is conceivable that degeneration of the sublaterodorsal nucleus is more closely linked with risk of PD.
It is also unclear why there was a relationship between tonic REM and risk of PD, but no relationship with dementia. According to Braak's suggested staging system of PD, there is progressive synuclein accumulation in an ascending pattern in the brainstem, such that pontine structures (involved in REM control) should be severely affected by the time clinical threshold for PD develops.18 In dementia, such a staging system may not apply; that is, there may be alternative progression patterns of neurodegeneration. This concept is supported by pathologic studies of patients with a malignant phenotype of PD who presented with early dementia, in which Braak stages were not followed.19 Therefore, one could propose that patients who eventually develop dementia may have less consistently severe degeneration of pontine structures at baseline. However, this hypothesis would require confirmation in pathologic studies.
Finally, we have found some equivocal evidence that there is a shift in sleep patterns from slow wave (stage 3 and 4) to lighter stages of sleep (stage 1) in those who will eventually develop dementia, compared to those who developed PD (although there were no significant differences between patients with dementia and controls). This cannot be explained by age or sex differences, as these were adjusted for in regression analysis. Differences were subtle, and scoring of slow-wave sleep can be difficult, so it is possible that these results represented a chance finding. Slow-wave sleep progressively declines with normal aging, particularly among men.20 Some studies have demonstrated decreased slow wave sleep in patients with AD compared to controls,21–23 although other studies have found no differences.24–26 There were very few patients in these studies with Lewy body dementia. It would be important to study sleep patterns in dementia subtypes, including LBD.
It is important to note some limitations of this study. Although sample size was relatively large, there would not be sufficient power for subgroup analysis. Diagnosis of neurodegenerative disease was clinical. In particular, diagnostic criteria for Lewy body dementia may be insensitive—we suspect that most patients with possible LBD/clinical AD in fact had LBD.27 Phasic muscle activity was measured in the chin EMG only, and it is possible that estimations in the limbs may provide different results. The study is exploratory in nature, with no adjustment for multiple comparisons.28 It is conceivable that some of our results were due to chance—this would be less likely for the findings of REM atonia, in which consistent significant differences were seen in all patient groups. This study was restricted to evaluation of polysomnographic markers because these were generally available for all patients. There are numerous other potential markers of interest which are actively being studied in an ongoing prospective protocol, by our group29 and other groups—this will yield results in the next few years.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. R.B. Postuma.
DISCLOSURE
Dr. Postuma serves on a scientific advisory board for Teva Pharmaceutical Industries Ltd. and receives research support from the CIHR [201317 (PI), 192205 (Co-PI), 166206 (Co-I)], Fonds de Recherche de la Sante Quebec [9965 (PI)], the Weston Foundation, and the Drummond Foundation. Dr. Gagnon receives research support from the CIHR [MOP-84482 (PI), MOP-62955 (Co-PI), and MOP-93802 (Co-PI)] and the Fonds de la Recherche en Santé du Québec [11834 (PI)]. S. Rompré reports no disclosures. Dr. Montplaisir serves on scientific advisory boards for Boehringer Ingelheim, Servier, and Merck Serono; has received funding for travel from GlaxoSmithKline, Sanofi-Aventis, and Boehringer Ingelheim; serves as an Associate Editor of Sleep Medicine and Sleep Medicine Reviews; has received speaker honoraria from Valeant Pharmaceuticals International, GlaxoSmithKline, Sanofi-Aventis, and Boehringer Ingelheim; and receives research support from Sanofi-Aventis, Boehringer Ingelheim, and the CIHR [MOP-62955 (PI), NFP-88373 (PI), MGC-77493 (PI), MOP-74542 (PI), MOP-173519 (Co-PI), MOP-64221 (Co-PI), and MOP-11701 (Co-PI)].
Address correspondence and reprint requests to Dr. Jacques Montplaisir, Centre d'Étude du Sommeil et des Rythmes Biologiques, Hôpital du Sacré-Cœur de Montréal, 5400 Boul. Gouin Ouest, Montréal, Québec, Canada, H4J 1C5 JY.Montplaisir@UMontreal.CA
Study funding: Supported by the Canadian Institutes of Health Research and by the Fonds de la Recherche en Santé du Québec.
Disclosure: Author disclosures are provided at the end of the article.
Received August 24, 2009. Accepted in final form October 28, 2009.
REFERENCES
- 1.Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol 2006;5:424–432. [DOI] [PubMed] [Google Scholar]
- 2.Schenck CH, Mahowald MW. REM behavior disorder (RBD): delayed emergence of parkinsonism and/or dementia in 65% of older men initially diagnosed with idiopathic RBD, and an analysis of the minimum & maximum tonic and/or phasic electromyographic abnormalities found during REM sleep. Sleep 2003;26:A316Abs. [Google Scholar]
- 3.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 1996;46:388–393. [DOI] [PubMed] [Google Scholar]
- 4.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006;5:572–577. [DOI] [PubMed] [Google Scholar]
- 5.Iranzo A, Molinuevo J, Santamaria J, et al. Sixty-four percent of patients with idiopathic REM sleep behavior disorder developed a neurological disorder after a mean clinical follow-up of seven years. Sleep 2008;31:A280Abs. [Google Scholar]
- 6.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009;72:1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology 1992;42:1371–1374. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord 2007;22:2314–2324. [DOI] [PubMed] [Google Scholar]
- 11.Rechtschaffen A, Kales AA. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: US Government Printing Office, Public Health Service; 1968. [Google Scholar]
- 12.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 13.Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology 2005;65:247–252. [DOI] [PubMed] [Google Scholar]
- 14.Iranzo A, Ratti P, Casanova-Molla J, Serradell M, Vilaseca I, Santamaria J. Excessive muscular activity increases over time in idiopathic REM sleep behavior disorder. Sleep 2009;32:1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002;25:120–138. [DOI] [PubMed] [Google Scholar]
- 16.Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry 2008;79:387–391. [DOI] [PubMed] [Google Scholar]
- 17.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007;130:2770–2788. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 19.Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson's disease. Acta Neuropathol 2008;115:409–415. [DOI] [PubMed] [Google Scholar]
- 20.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med 2004;164:406–418. [DOI] [PubMed] [Google Scholar]
- 21.Bliwise DL, Tinklenberg J, Yesavage JA, et al. REM latency in Alzheimer's disease. Biol Psychiatry 1989;25:320–328. [DOI] [PubMed] [Google Scholar]
- 22.Loewenstein RJ, Weingartner H, Gillin JC, Kaye W, Ebert M, Mendelson WB. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol Aging 1982;3:371–377. [DOI] [PubMed] [Google Scholar]
- 23.Gagnon JF, Petit D, Latreille V, Montplaisir J. Neurobiology of sleep disturbances in neurodegenerative disorders. Curr Pharm Des 2008;14:3430–3445. [DOI] [PubMed] [Google Scholar]
- 24.Allen SR, Seiler WO, Stahelin HB, Spiegel R. Seventy-two hour polygraphic and behavioral recordings of wakefulness and sleep in a hospital geriatric unit: comparison between demented and nondemented patients. Sleep 1987;10:143–159. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel R, Herzog A, Koberle S. Polygraphic sleep criteria as predictors of successful aging: an exploratory longitudinal study. Biol Psychiatry 1999;45:435–442. [DOI] [PubMed] [Google Scholar]
- 26.McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Teri L. Factors associated with concordance and variability of sleep quality in persons with Alzheimer's disease and their caregivers. Sleep 2008;31:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postuma RB, Gagnon JF, Vendette M, Montplaisir J. Idiopathic REM sleep behavior disorder in the transition to degenerative disease. Mov Disord 2009;24:2225–2232. [DOI] [PubMed] [Google Scholar]
- 28.Bender R, Lange S. Adjusting for multiple testing: when and how? J Clin Epidemiol 2001;54:343–349. [DOI] [PubMed] [Google Scholar]
- 29.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology 2006;66:845–851. [DOI] [PubMed] [Google Scholar]


