Summary
The phosphoinositide 3-kinase (PI 3-K) signaling pathway is frequently deregulated in cancer. Downstream of PI 3-K, Akt1 and Akt2 have opposing roles in breast cancer invasive migration leading to metastatic dissemination. Here we identify palladin, an actin-associated protein, as an Akt1-specific substrate that modulates breast cancer cell invasive migration. Akt1, but not Akt2, phosphorylates palladin at S507 in a domain that is critical for F-actin bundling. Downregulation of palladin enhances migration and invasion of breast cancer cells and induces abnormal branching morphogenesis in 3D cultures. Palladin phosphorylation at S507 is required for Akt1-mediated inhibition of breast cancer cell migration and also for F-actin bundling leading to the maintenance of an organized actin cytoskeleton. These findings identify palladin as an Akt1-specific substrate that regulates cell motility and provide a molecular mechanism that accounts for the functional distinction between Akt isoforms in breast cancer cell signaling to cell migration.
Introduction
Metastasis, one of the hallmarks of human solid tumors, is orchestrated by multiple signaling pathways that regulate cell proliferation, survival, metabolism, migration and angiogenesis. Recent studies have revealed that the phosphoinositide 3-kinase (PI 3-K)/Akt signaling cascade is one of the most frequently deregulated pathways in cancer, particularly breast carcinoma (Altomare and Testa, 2005; Engelman et al., 2006). The Akt family members, Akt1 (also known as PKBα), Akt2 (PKBβ) and Akt3 (PKBγ), play pivotal roles in cellular functions that are associated with all stages of cancer including progression to metastasis (Chin and Toker, 2009; Woodgett, 2005). Although both Akt1 and Akt2 promote cancer cell survival and growth, they exert distinct effects on breast cancer cell invasive migration and metastasis (Chin and Toker, 2009). In this context, Akt1 has been shown to promote tumor induction, but somewhat paradoxically, inhibit invasion and metastasis (Hutchinson et al., 2004; Irie et al., 2005; Liu et al., 2006; Maroulakou et al., 2007; Yoeli-Lerner et al., 2005). Conversely, Akt2 enhances invasive migration and metastasis in vivo (Arboleda et al., 2003; Irie et al., 2005). A number of distinct effector pathways have been shown to mediate the distinct effects of Akt1 and Akt2 on breast cancer cell invasion. Akt1 blocks breast cancer cell migration by promoting degradation of the transcription factor NFAT (Nuclear Factor of Activated T cells) (Yoeli-Lerner et al., 2005). Akt1 also attenuates cell migration by the regulating extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) (Irie et al., 2005) and Tuberous Sclerosis Complex 2 (TSC2) pathways (Liu et al., 2006). In contrast, Akt2 but not Akt1 up-regulates β1 integrins thereby promoting invasion of breast cancer cells in vitro as well as metastasis in vivo (Arboleda et al., 2003). However, to date the immediate isoform-specific substrates that modulate cell migration in an Akt isoform-specific manner have not been identified.
Palladin is an actin-binding and cross-linking protein that controls the organization of cellular actin networks (Dixon et al., 2008). Palladin localizes in areas of actin stress fiber-dense regions and focal adhesions (Parast and Otey, 2000). Palladin also functions as a molecular scaffold by linking several anchor proteins to actin fibers, including profilin (Boukhelifa et al., 2006), VASP (Boukhelifa et al., 2004), α-actinin (Ronty et al., 2004), Eps8 (Goicoechea et al., 2006) and ezrin (Mykkanen et al., 2001). Studies have revealed palladin overexpression in human breast tumor tissues (Goicoechea et al., 2009) and invasive rat mammary tumor cells (Wang et al., 2004). However, the mechanisms that regulate the function of palladin in cytoskeletal reorganization and cell motility remain undescribed.
Here we report the identification of palladin as a specific substrate of Akt1. Akt1, but not Akt2, phosphorylates palladin at Ser507 in vitro and in cells. Downregulation of palladin by small hairpin RNA (shRNA) enhances invasive migration and disrupts spheroid morphogenesis, indicating an anti-migratory and anti-invasive function for palladin in breast cancer cells. Phosphorylation of palladin plays a critical role in inhibiting breast cancer cell motility and promoting actin bundling activity. Taken together, these data identify palladin as the first Akt isoform-specific substrate that contributes to differential regulation of breast cancer cell migration.
Results
Akt phosphorylates palladin at Ser507 in vitro and in cells
Recent phospho-proteomic studies have revealed phosphorylation of palladin at Ser507 in a consensus sequence that conforms to the optimal Akt phosphorylation motif (RXRXXS/T) (Obata et al., 2000; Olsen et al., 2006; Villen et al., 2007) (Fig. 1A). To determine whether palladin is an Akt substrate we transfected hemagglutinin (HA)-tagged palladin into HeLa cells and stimulated cells with insulin-like growth factor-1 (IGF-1) to activate endogenous PI 3-K and Akt. Immunoprecipitated palladin was immunoblotted with an antibody that recognizes the Akt consensus phosphorylation Akt motif (Fig. 1B). Notably, pre-treatment of cells with the PI 3-K inhibitor wortmannin or the Akt inhibitor SN30978 (Defeo-Jones et al., 2005) markedly attenuates IGF-1-induced palladin phosphorylation (Fig. 1B). HeLa cells were also co-transfected with a constitutively active myristoylated Akt1 allele (Myr-Akt1) and green fluorescent protein (GFP)-tagged palladin. Palladin co-immunoprecipitates with Myr-Akt1 indicative of an association (Fig. S1A). Furthermore, GFP-palladin is phosphorylated by Myr-Akt1 in cells, suggesting that active Akt1 alone is sufficient to stimulate palladin phosphorylation. Other AGC kinases downstream of PI 3-K such as serum- and glucocorticoid-inducible kinase (SGK) (Hong et al., 2008) and S6 kinase-1 (S6K1) that share the Akt consensus phosphorylation motif do not signal to palladin since treatment with the mTOR (mammalian target of rapamycin) inhibitor rapamycin has no effect on palladin phosphorylation (Fig. S1B).
Figure 1. Akt phosphorylates palladin at Ser507.
A, schematic of palladin showing the position of the putative Akt consensus phosphorylation site at Ser507. Amino acid sequences of Akt motifs in other known Akt substrates are shown for comparison. The Ser507 Akt motif in palladin is evolutionarily conserved. Numbers on the left of sequences indicate the position of Ser in the Akt motif. PP, poly-proline; Ig, immunoglobulin-like domain. B, HeLa cells were transfected with HA-palladin or control vector for 9 h then serum starved for 12–16 h. Cells were then stimulated with IGF-1 (100 ng ml−1) for 20 min in the presence of wortmannin (100 nM) or Akt inhibitor SN30978 (5 μM), or DMSO. Cell extracts were immunoprecipitated with anti-HA antibody and immunoblotted with the indicated antibodies. C, HeLa cells were transfected with wild-type HA-palladin (HA-palladin WT) or HA-palladin S507A mutant or control vector for 9 h, followed by serum starving for 12–16 h. Cells were then stimulated with IGF-1 (100 ng ml−1) for 20 min. Palladin was precipitated from whole cell lysates followed by immunoblotting. D, HeLa cells were transfected with HA-palladin WT or HA-palladin S507A or control vector in serum-free media for 24 h. Anti-HA immunoprecipitates were used in in vitro assays with recombinant active Akt1 (Recom. Akt1). The kinase reaction was terminated and samples were immunoblotted. All results are representative of three independent experiments. See also Figure S1.
Akt phosphorylates palladin at Ser507, since a Ser507Ala mutant is not phosphorylated in IGF-1-stimulated cells when compared to wild type palladin (Fig. 1C). To investigate whether palladin is a direct substrate of Akt1, purified recombinant wild type and Ser507Ala palladin was incubated with purified recombinant active Akt1 protein in an in vitro protein kinase assay. Akt1 efficiently phosphorylates wild type palladin, whereas the Ser507Ala mutant is not phosphorylated (Fig. 1D).
To determine the relevance of palladin phosphorylation by Akt in breast cancer, we first evaluated palladin expression in a panel of breast cancer cell lines. Fig. 2A shows that palladin is expressed in all breast cancer cell lines examined. As with HeLa cells, palladin is phosphorylated in an IGF-1 or EGF and Akt-dependent manner in MDA-MB-231 and SKBR3 breast cancer cell lines (Fig. 2B). In immortalized non-tumorigenic MCF10A breast epithelial cells, inhibition of PI 3-K/Akt signaling by wortmannin or SN30978 impairs EGF-mediated phosphorylation of endogenous palladin (Fig. 2C). Similar results were obtained in a phosphatase and tensin homolog (PTEN)-null breast cancer cell line, BT-549. Similarly, we evaluated phosphorylation of palladin in cells expressing the two oncogenic hotspot mutations in the p110α catalytic subunit (PIK3CA) (Saal et al., 2005). Both H1047R and E545K mutant p110α alleles induce hyperactivation of Akt as measured by pSer473 phosphorylation (Fig. 2D). Importantly, this is accompanied by hyperphosphorylation of palladin with both mutant alleles. Therefore, palladin is phosphorylated in breast cancer cells in response to both physiological stimuli and oncogenic activation of the PI 3-K pathway.
Figure 2. Phosphorylation of palladin in breast cancer cells.
A, Analysis of palladin expression in various cell lines by immunoblotting. B, MDA-MB-231 and SKBR3 cells were transfected with HA-palladin for 9 h then serum starved for 12–16 h. Cells were then stimulated with IGF-1 (100 ng ml−1) for 20 min or EGF (20 ng ml−1) for 10 min in the presence of SN30978 (5 μM) or DMSO. Cell extracts were immunoprecipitated with anti-HA antibody followed by immunoblotting. C, Serum-starved MCF10A and BT-549 cells were stimulated with EGF (20 ng ml−1) for 10 min in the presence of wortmannin (100 nM) or SN30978 (5 μM), or DMSO. Endogenous palladin was immunoprecipitated with anti-palladin antibody and immunoblotted with α-pAkt-MOTIF antibody. D, MCF10A cells infected with empty vector or retroviral vectors expressing the indicated HA-p110α variants were serum-starved overnight. Phosphorylation of endogenous palladin was detected as described in C. All results are representative of three independent experiments.
Palladin is an Akt1-specific substrate
We next determined if palladin is an Akt isoform-specific substrate. First, cells were co-transfected with GFP-palladin and HA-Myr-Akt1 or HA-Myr-Akt2. In spite of the high amino-acid similarity between Akt isoforms, Akt1 but not Akt2 co-immunoprecipitates with palladin in all three cell lines tested (Fig. 3A). Consistent with this, palladin is specifically phosphorylated by Akt1 but not Akt2 in an in vitro kinase assay (Fig. 3B). In contrast, the Akt substrate GSK3β is efficiently phosphorylated by both Akt isoforms (Fig. S2A). To evaluate isoform specificity in cells, short hairpin RNA (shRNA) targeting Akt1 or Akt2 were introduced into HeLa or SKBR3 cells. Knockdown of Akt1 significantly attenuates growth factor-induced palladin phosphorylation (Fig. 3C). Conversely, depletion of Akt2 has no effect. Similar results are observed in the PTEN-deficient breast cancer cell line BT-549 (Fig. 3C). These findings demonstrate that palladin is an Akt1-specific substrate. Since Akt3 expression is minimal or below the detection limits in HeLa cells (Zinda et al., 2001) and SKBR3 cells (Fig. S2B), this Akt isoform cannot account for palladin phosphorylation in these cell lines. Conversely, although Akt3 is expressed in BT-549 cells (Fig. S2C), it is unlikely to contribute to palladin regulation since Akt1 shRNA completely eliminates phosphorylation.
Figure 3. Palladin is an Akt1-specific substrate.
A, HeLa, SKBR3 and MDA-MB-231 cells were transfected with HA-Myr-Akt1, HA-Myr-Akt2, or empty vector, along with GFP-palladin. 24 h after transfection cells were lysed and immunoprecipitated with anti-GFP. Whole cell lysates and immunoprecipitates were subjected to immunoblotting. B, SKBR3 cells were transfected with HA-palladin WT in serum-free medium for 24 h. Anti-HA immunoprecipitates were used as substrates in in vitro kinase assays with recombinant active Akt1 or Akt2. The kinase reaction was terminated and samples were immunoblotted. C, HeLa and SKBR3 cells were co-transfected with HA-palladin and Akt1, Akt2 or control luciferase siRNA. 36 h after transfection, HeLa and SKBR3 cells were serum starved for 12 h then treated with IGF-1 (100 ng ml−1) for 20 min and EGF (20 ng ml−1) for 10 min, respectively. BT-549 cells were infected with Akt1 or Akt2 shRNA lentiviral vector or empty vector for 72 h, followed by serum starvation and then stimulation with EGF (20 ng ml−1) for 10 min. Lysates were subjected to immunoprecipitation and immunoblot analysis. D, HeLa cells were infected with retroviral vectors expressing Akt chimeras, followed by transfection with GFP-palladin for 24 h. Cell extracts were immunoprecipitated with anti-GFP antibody and immunoblotted with the indicated antibodies. Akt has 4 domains: PH, linker, catalytic and C-terminal regulatory domain. Akt chimera 1122 contains PH and linker regions of Akt1 plus catalytic and regulatory domains of Akt2. Akt chimera 2211 contains PH and linker regions of Akt2 plus catalytic and regulatory domains of Akt1. Akt chimera 2111 contains PH domain of Akt2 plus linker, catalytic and regulatory domains of Akt1. All results are representative of three independent experiments. See also Figure S2.
To determine the domain or region in Akt1 that determines isoform specificity towards palladin phosphorylation, Akt1 and Akt2 chimeras were used (Zhou et al., 2006). Palladin phosphorylation is observed in cells expressing the Akt chimera containing the PH and linker domains of Akt1 (1122), whereas phosphorylation is significantly lower in the presence of Akt chimera 2211 that contains PH and linker domains of Akt2 (Fig. 3D). This indicates that the either the PH and/or linker regions of Akt1 are important determinants for palladin phosphorylation. The Akt chimera 2111 contains the same domains as the chimera 2211, with the exception of the linker region that originates from Akt1. Palladin was phosphorylated efficiently in cells expressing the chimera 2111, but not 2211 (Fig. 3D). This indicates that the linker region of Akt1 plays an important role in determining the Akt isoform-specific function of palladin phosphorylation.
Palladin inhibits breast cancer cell invasive migration
We next determined if palladin phosphorylation regulates cellular localization. In serum-starved cells, palladin is distributed evenly in the cytoplasm with a minor colocalization with cortical actin (Fig. 4A). Upon IGF-1 stimulation, we observe an increased accumulation and colocalization of palladin and actin at sites of membrane ruffling. In contrast, this colocalization is reduced upon inhibition of PI 3-K signaling with the inhibitor LY294002. We next determined the function of palladin in modulating breast cancer cell migration. shRNA sequences specific to palladin were generated (Ronty et al., 2007) and efficient silencing was evaluated with a specific palladin antibody (Fig. S3A). Chemotactic cell motility was assessed using Transwell migration assays. In both highly invasive (MDA-MB-231 and SUM-159-PT) and poorly invasive (MCF-7) breast cancer cell lines as well as MCF10A cells, knockdown of palladin results in enhanced migration (Fig. 4B), suggestive of an anti-migratory function of palladin in breast cancer cells. Consistent with this, expression of palladin in MCF-7 cells results in decreased migration (Fig. S3B). Moreover, MCF10A cells expressing wild type or oncogenic PIK3CA exhibit increased cell migration (Fig. S3C). This is consistent with previous studies that have demonstrated enhancement of MCF10A and MDA-MB-231 cell migration mediated by oncogenic PIK3CA (Pang et al., 2009; Zhang et al., 2008). Silencing of palladin enhances migration of cells with both wild type and mutant p110α cells (Fig. S3C). Furthermore, in Matrigel invasion assays, palladin silencing also results in increased invasion of breast cancer cells (Fig. S3D). This was further corroborated using 3D cultures. Whereas control cells are able to form normal 3D spheroids, palladin knockdown cells display abnormal branching morphogenesis with protrusions invading into Matrigel (Fig. 4C).
Figure 4. Palladin inhibits breast cancer cell invasive migration.
A, MDA-MB-231 cells were stimulated with IGF-1 (100 ng ml−1) for 20 min in the presence of DMSO or LY294002 (10 μM). Immunofluorescence was performed using anti-palladin antibody and Alexa Fluor 488-conjugated phalloidin. Cell nuclei were labeled with DAPI. B, MDA-MB-231, MCF7, SUM-159-PT and MCF10A cells were infected with palladin shRNA lentiviral vector or empty vector for 48 h followed by Transwell migration assays. Relative migration (y axis) = ratio of the number of migrated cells in test versus control. Error bars represent mean ± standard error of the mean (SEM). Total cell lysates were subjected to immunoblot analysis. C, MCF10A cells infected with palladin shRNA lentiviral vector or empty pLKO vector were grown in 3D cultures for 8 days. Lysates were immunoblotted with the indicated antibodies. All results are representative of three independent experiments. See also Figure S3.
Akt1 phosphorylation of palladin blocks migration of breast cancer cells
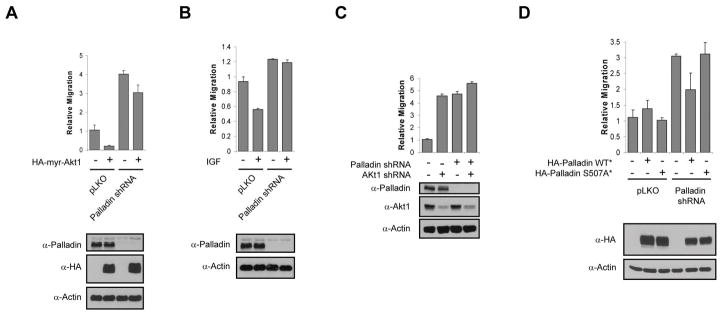
As enhanced migration upon palladin silencing phenocopies downregulation of Akt1 (Fig. S4A), we next examined if inhibition of migration by the Akt1 pathway is mediated through palladin phosphorylation. In agreement with recent studies (Yoeli-Lerner et al., 2005), expression of activated Myr-Akt1 blocks migration of MDA-MB-231 cells (Fig. 5A). This effect is rescued by silencing palladin with shRNA. To determine if this occurs under physiological signaling conditions, IGF-1 stimulation was used to activate endogenous Akt, resulting in decreased cell migration as previously demonstrated (Fig. 5B) (Yoeli-Lerner et al., 2005). Downregulation of palladin in serum-free conditions has minimal effect on migration (Fig. 5B). In contrast, IGF-1-induced inhibition of migration is completely rescued by palladin silencing. Similarly, combined downregulation of palladin and Akt1 has little or no addictive effect on migration (Fig, 5C), further demonstrating that the inhibitory effects of Akt1 on migration are mediated, at least in part, by palladin.
Figure 5. Phosphorylation of palladin at Ser507 by Akt1 inhibits cell migration.
A, MDA-MB-231 cells infected with palladin shRNA or empty lentiviral vectors were transfected with HA-Myr-Akt1 or control vector. 24 h after transfection cells were subjected to Transwell migration assays and lysates were immunoblotted with the indicated antibodies. B, MDA-MB-231 cells were infected with palladin shRNA lentiviral vector or empty vector. Forty-eight hours after infection cells were serum-starved overnight then stimulated with IGF-1 (100 ng ml−1) for 18 h, followed by Transwell migration assays. Total cell lysates were subjected to immunoblot analysis. C, MDA-MB-231 cells were infected with palladin shRNA and/or Akt1 shRNA lentiviral vectors, or empty vector. Forty-eight hours after infection, cells were subjected to Transwell migration assays and lysates were immunoblotted. D, MDA-MB-231 cells were infected with palladin shRNA lentiviral vector or empty vector for 48 h. Cells were then transfected with shRNA-resistant (*) HA-palladin WT* or HA-palladin S507A mutant* or control vector. 24 h after transfection cells were subjected to Transwell migration assays. Cell lysates were immunoblotted with anti-HA and anti-actin. All Transwell migration data are represented as mean ± SEM. All results are representative of three independent experiments. See also Figure S4.
To determine if palladin phosphorylation by Akt1 is critical for the migration phenotype, an shRNA-resistant WT palladin allele was generated (HA-palladin WT*) along with an S507A mutant (HA-palladin S507A*), and re-introduced into palladin-depleted MDA-MB-231 cells. Whereas HA-palladin WT* effectively reverses the migratory effect induced by palladin shRNA, HA-palladin S507A* does not rescue (Fig. 5D). In addition, palladin phosphorylation is higher in non-tumorigenic, non-invasive MCF10A breast epithelial cells when compared to invasive and metastatic breast cancer cell lines SUM-159-PT and MDA-MB-231 (Fig. S4B). This observation is consistent with an inhibitory function of palladin phosphorylation in cell migration. Taken together, these data demonstrate that palladin phosphorylation at Ser507 is required for Akt1-mediated inhibition of migration.
Since Transwell assays measure directional migration, we next evaluated the role of palladin phosphorylation in non-directional migration using time lapse video microscopy. MDA-MB-231 cells were plated on NIH 3T3 cell-secreted extracellular matrix (ECM) and migration was monitored 1 h subsequent to plating. Control cells spread uniformly on ECM and migrate slowly (Fig. 6A, 6B and Movie S1). In contrast, silencing of palladin results in a distinct morphology with reduced areas of spreading and diminished continuity of lamellapodia. In addition, these cells have multiple protrusions resulting in a stellate appearance and also display significantly increased random migration (Fig. 6A, 6B and Movie S2). Reintroduction of wild type palladin resistant to silencing (WT*) reverses the morphology and motility to that observed with control cells (Fig. 6A, 6B and Movie S3). Conversely, cells expressing HA-palladin S507A* displaye the stellate morphology and enhanced motility that mimicks that observed in palladin-depleted cells (Fig. 6A, 6B and Movie S4). These results demonstrate that phosphorylation of palladin regulates both random and directional migration of breast cancer cells.
Figure 6. Regulation of breast cancer cell random migration by palladin phosphorylation.
A, MDA-MB-231 cells were infected with palladin shRNA lentiviral vector or empty vector for 48 h. Cells were then transfected with shRNA-resistant (*) HA-palladin WT* or HA-palladin S507A mutant* or control vector. Twenty-four hours after transfection, cell migration on NIH 3T3 cell-secreted ECM was monitored by time-lapse microscopy for 1 h. Representative phase-contrast images from the movies are shown. B, Quantification of the velocity of random migration in A. Data are represented as mean ± SEM. See also movies S1–4.
Palladin phosphorylation modulates cytoskeletal organization and F-actin bundling
Palladin has been shown to directly bind F-actin (Dixon et al., 2008). The Akt phosphorylation site Ser507 resides in a domain that is critical for cross-linking actin filaments into bundles. We therefore tested if palladin phosphorylation modulates actin organization. In control cells the actin cytoskeleton is highly organized (Fig. 7A). However, palladin silencing results in disruption of actin stress fibers. Re-introduction of wild type (WT*), but not S507A* mutant palladin reverses the highly organized actin cytoskeletal phenotype indicating an important role of palladin phosphorylation in actin dynamics (Fig. 7A). To further dissect the mechanism by which palladin modulates in actin reorganization, co-sedimentation assays were performed. Lysates of palladin-depleted MDA-MB-231 cells expressing HA-palladin variants were incubated with purified F-actin, followed by differential centrifugation to co-sediment palladin with actin filaments and actin bundles. Wild type and S507A mutant palladin co-sedimente equally efficiently with actin filaments (Fig. 7B), suggesting that phosphorylation of palladin is not essential for actin binding. Conversely, in bundling assays wild type palladin co-sediments efficiently but this is reproducibly reduced with the S507A mutant. These results indicate that palladin phosphorylation functions to control F-actin cross-linking, consistent with the enhanced migration phenotype observed upon palladin silencing.
Figure 7. Phosphorylation of palladin regulates actin organization and bundling.
A, shRNA-resistant (*) HA-palladin WT* or HA-palladin S507A mutant* was overexpressed in MDA-MB-231 cells infected with palladin shRNA lentiviral vector or empty vector. 24 h after transfection cells were fixed and stained with anti-palladin and Alexa Fluor 488-conjugated phalloidin. B, MDA-MB-231 cells were infected and transfected as described in A. Cell lysates were incubated with F-actin, and F-actin binding (left panel) and bundling (right panel) was determined by differential centrifugation at 150,000 × g for 1.5 h and 10,000 × g for 1 h, respectively. Pellet (P) and supernatant (S) fractions were immuno-blotted with anti-HA to visualize palladin and stained with Ponceau S to reveal total F-actin protein. All results are representative of three independent experiments. See also Figure S5.
Since invasive cancer cells form actin-rich invadopodia to degrade the extracellular matrix during invasion (Artym et al., 2009), we examined if palladin phosphorylation regulates formation of invadopodia. Palladin silencing significantly increases the percentage of MDA-MB-231 cells with invadopodia (Fig. S5), indicating that palladin inhibits invadopodial formation. However, introduction of both wild type (WT*) and S507A* mutant palladin alleles reverses the percentage of invadopodia-containing cells to values observed with control cells (Fig. S5). These results indicate that although palladin functions to control invadopodia formation, Ser507 phosphorylation does not play a role in this phenotype. Instead, the role of palladin phosphorylation by Akt1 may be restricted to the regulation of F-actin cross-linking as discussed above.
Discussion
The essential role of Akt in cancer cell growth and survival has made it an attractive target for the development of anti-cancer therapeutics (Hennessy et al., 2005). However recent studies that have revealed opposing functions of Akt1 and Akt2 in the regulation of carcinoma migration leading to metastatic dissemination have necessitated a reevaluation of inhibition of Akt in cancer therapy (Chin and Toker, 2009; Sawyers, 2006). Thus far, Akt isoform-specific substrates and molecular mechanisms that are responsible for this distinction have remained elusive. Indeed, despite over 150 Akt substrates that have been characterized to date, only a few have been evaluated for isoform specificity. These include the cell cycle regulators p21 CIP1 (Heron-Milhavet et al., 2006) and SKP2 (Gao et al., 2009) that are Akt1-specific targets, whereas MDM2 (Brognard et al., 2007) and AS160 (Bouzakri et al., 2006; Gonzalez and McGraw, 2009) are specifically phosphorylated by Akt2. However, none of these account for the differential effects of Akt isoforms on invasive migration.
In the present study we have identified palladin, an actin-associated protein, as an Akt1 substrate. Phosphorylation of palladin by PI 3-K and Akt1 signaling by physiological stimuli such as IGF-1 as well as by genetic mutations in the pathway, such as PTEN loss and oncogenic PIK3CA supports a role for this Akt1 target in both physiological and pathophysiological signaling. The results presented here also provide evidence for a functional role for palladin in invasive migration of breast cancer cells, and highlight the critical role of palladin phosphorylation in regulating migration and actin bundling.
Despite the high sequence and structural homology among Akt isoforms, Akt1 but not Akt2 interacts with and phosphorylates palladin. Substrate selectivity of isoforms can be achieved by several potential mechanisms, including subcellular compartmentalization, binding to distinct scaffolding molecules and differential activation or regulation by extracellular stimuli. Indeed, it has recently been shown that in insulin-stimulated adipocytes Akt2 preferentially accumulates at the plasma membrane and specifically phosphorylates the substrate AS160 to regulate GLUT4 trafficking (Gonzalez and McGraw, 2009). However, differential compartmentalization of Akt isoforms is unlikely to account for the exclusivity of palladin phosphorylation by Akt1, since purified Akt1 but not Akt2 phosphorylates palladin in a cell-free system. It is more likely that an intrinsic molecular determinant or ‘docking motif’ on Akt1 promotes its association with palladin. The data from the Akt chimeras studies indicate that the linker region of Akt1 plays an important role in determining the isoform-specificity of palladin phosphorylation. Interestingly, Field and colleagues have identified the same region as an important determinant in the ability of Akt1 and Akt2 to form dorsal ruffles during fibroblast migration (Zhou et al., 2006). The precise residues within the linker region that are responsible for this isoform-specificity are yet to be determined. Regardless, these data point to an important function of the linker region in modulating palladin phosphorylation by the Akt1 pathway.
Our findings that palladin has an anti-migratory role in breast cancer cells differ from a recent study in which palladin knockdown by siRNA inhibited invasive migration in Transwell assays (Goicoechea et al., 2009). The distinction between the two studies could be explained by the different migration conditions or chemoattractants used. In the present study results obtained with Transwell assays were corroborated using time-lapse video microscopy as well as 3-D cultures. In all cases, cells expressing palladin shRNA exhibited highly migratory and invasive phenotypes in four distinct breast cancer cell lines. Most importantly, rescue experiments revealed that overexpression of palladin is able to reverse the migratory effect induced by palladin shRNA, further demonstrating the specificity of the approach and confirming the inhibitory function of palladin phosphorylation on invasive migration of breast cancer cells.
Our studies also point to a new mechanism by which palladin phosphorylation modulates its actin regulatory activity. We show that phosphorylation of palladin promotes actin bundling, consistent with the observation that the Akt motif at Ser507 resides in a domain that critical for cross-linking actin (Dixon et al., 2008). We propose that by promoting the formation of actin bundles and re-organizing the actin cytoskeleton, palladin phosphorylation inhibits cell migration. It is also worth noting that a link between actin filaments and integrin receptors plays a crucial role in dictating the organization and stability of adhesions during cell migration (Vicente-Manzanares et al., 2009). Polymerization of actin fibers regulates clustering of activated β1 integrins at the leading edge of migrating fibroblasts (Galbraith et al., 2007). Interestingly, palladin can stabilize β1 integrins in fibroblasts (Liu et al., 2007). Since palladin phosphorylation modulates the organization of actin cytoskeleton, it is possible that Ser507 phosphorylation by Akt1 could regulate β1 integrin signaling to the migration phenotype.
Coordinated up-regulation of both the stimulatory and inhibitory branch of actin motility machinery has been observed in invasive carcinoma cells (Wang et al., 2004). Accordingly, expression of palladin is increased in human breast tumor tissues (Goicoechea et al., 2009) and invasive mammary tumors in rats (Wang et al., 2004). Since our data show that palladin phosphorylation functions to inhibit invasive migration, it will be interesting to determine the relative phosphorylation levels of palladin in breast tumor tissues. This will only be possible with the use of specific phospho Ser507 palladin antibodies.
In summary, the present study defines a mechanism that links palladin to Akt1-mediated inhibition of breast cancer cell migration. We show that Akt1, but not Akt2, phosphorylates palladin at Ser507. In turn this induces reorganization of the actin cytoskeleton through actin bundling activity and ultimately leads to inhibition of migration of breast cancer cells. To our knowledge, this is the first specific substrate that accounts for the distinct roles of Akt isoforms in cell migration. Undoubtedly, more isoform-specific substrates that confer functional selectivity remain to be identified. Given the fact that Akt isoforms are frequently hyperactivated in breast cancer and that considerable effort is underway to develop pharmacological Akt inhibitors as therapeutic agents, these findings underscore the importance of dissecting the precise mechanisms by which the PI 3-K and Akt pathway regulates breast cancer invasive migration leading to metastatic dissemination.
Experimental Procedures
Cell Culture
HEK293T, HeLa, MCF7, MDA-MB-231 and MDA-MB-468 cells were maintained in Dulbecco’s modified Eagle medium (DMEM; Cellgro) supplemented with 10% Fetal Bovine Serum (FBS; HyClone). SKBR3 cells were cultured in McCoy’s 5A medium (Cambrex) supplemented with 10% FBS. BT-549 cells were grown in RPMI 1640 medium supplemented with 10% FBS. SUM-159-PT cells were cultured in Ham’s F12 medium (Cellgro) supplemented with 5% FBS, 1 μg ml−1 hydrocortisone (Sigma-Aldrich) and 5 μg ml−1 insulin (Sigma-Aldrich). MCF10A were grown in DMEM/Ham’s F12 medium supplemented with 5% equine serum (Gibco-brl), 10 μg ml−1 insulin, 500 ng ml−1 hydrocortisone (Sigma-Aldrich), 20 ng ml−1 EGF (R&D systems) and 100 ng ml−1 cholera toxin (List Biological Labs).
Growth factors and inhibitors
Cells were stimulated with recombinant human IGF-1 (R&D systems) at a final concentration of 100 ng ml−1 for 20 min (except for migration assays in Fig. 5B). Recombinant human EGF was added to cells at 20 ng ml−1 for 10 min. Wortmannin (Sigma-Aldrich) and SN30978 (referred to Akti-1/2 in (Defeo-Jones et al., 2005); gift from Dr. Peter Shepherd (Symansis and University of Auckland, New Zealand)) were added to cells 15 min prior growth factor stimulation at final concentrations of 100 nM and 5 μM, respectively. Rapamycin (Sigma-Aldrich) was added to cells 15 min prior stimulation at 100 nM. LY294002 (Alexis Biochemicals) was added to cells 15 min prior growth factor stimulation at a final concentration of 10 μM.
Antibodies
Anti-Akt1 monoclonal antibody, anti-Akt2 polyclonal antibody, anti-phospho-Akt S473 (pAkt) monoclonal antibody, anti-phospho-Akt substrate (pAkt-motif) monoclonal antibody, anti-phospho-GSK3β monoclonal antibody, anti-GSK3β monoclonal antibody, anti-p110α monoclonal antibody and anti-phospho S6K1 polyclonal antibody were obtained from Cell Signaling Technology. Anti-Akt polyclonal antibody and anti-green fluorescent protein (GFP) monoclonal antibody were purchased from Santa Cruz Biotechnology. Anti-palladin polyclonal antibody was from ProteinTech Group. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit immunoglobulin G (IgG) antibody were purchased from Chemicon. Cy3-conjugated anti-rabbit IgG antibody was from Jackson laboratory. Anti-β-actin monoclonal antibody was purchased from Sigma-Aldrich. Anti-Akt1 polyclonal antibody was raised against a synthetic peptide (VDSERRPHFPQFSYSASGTA) and generated in house. Anti-HA monoclonal antibody was purified from the 12CA5 hybridoma.
Plasmids
HA-Myr-Akt1 (Yoeli-Lerner et al., 2005) and HA-GSK3β (Ding et al., 2000) plasmids have been described previously. HA-Myr-Akt2, HA-p110α/pBABE-puro, HA-p110α H1047R/pBABE-puro and HA-p110α E545K/pBABE-puro plasmids were obtained from Addgene. Retroviral vectors expressing Akt chimeras 1122, 2211 and 2111 were gifts from Dr. Jeffrey Field (Zhou et al., 2006) (University of Pennsylvania). HA-Palladin and GFP-Palladin plasmids were gifts from Dr. Mikko Ronty (Ronty et al., 2007) (University of Helsinki, Finland). HA-Palladin S507A was constructed by site-directed mutagenesis with the following primers: sense, 5′-AGGCCTCGTTCTAGAGCAAGGGACAGTGGAG-3′; anti-sense, 5′-CTCCACTGTCCCTTGCTCTAGAACGAGGCCT-3′. shRNA-resistant variants of palladin were constructed by site-directed mutagenesis with the following primers: Sense, 5′-CCTCCGATGAGGAAATTCAAGGAACCAAAGACGCTGTTATTCAAGACCTGGAAC-3′; anti-sense, 5′-GTTCCAGGTCTTGAATAACAGCGTCTTTGGTTCCTTGAATTTCCTCATCGGAGG-3′. All sequences were verified by DNA sequencing.
RNA interference
For shRNA-mediated knockdown of Akt isoforms, a set of single-stranded oligonucleotides encoding the Akt1 or Akt2 target shRNA and its complement were synthesized. The hairpin sequences have been validated previously (Irie et al., 2005). Akt1, sense, 5′-CCGGGAGTTTGAGTACCTGAAGCTGCTCGAGCAGCTTCAGGTACTCAAACTCTTTT TG–3′; Akt1, anti-sense, 5′–AATTCAAAAAGAGTTTGAGTACCTGAAGCTGCTCGAGCAGCTTCAGGTACTCAAACTC–3′; Akt2, sense, 5′–CCGGGCGTGGTGAATACATCAAGACCTCGAGGTCTTGATGTATTCACCACGCTTTTTG–3′; Akt2, anti-sense, 5′–AATTCAAAAAGCGTGGTGAATACATCAAGACCTCGAGGTCTTGATGTATTCACCAC GC–3′. The oligonucleotide pair was annealed and inserted into pLKO. To produce lentiviral supernatants, 293T cells were co-transfected with control or Akt1 or Akt2 shRNA-containing pLKO vectors, VSVG and psPAX2 for 48 h. Palladin shRNA sequence (sense, 5′-CCGGGGCACAAAGGATGCTGTTATTCTCGAGAATAACAGCATCCTTTGTGCCTTTT TG-3′; anti-sense, 5′-AATTCAAAAAGGCACAAAGGATGCTGTTATTCTCGAGAATAACAGCATCCTTTGTG CC-3′) has been validated previously (Ronty et al., 2007) and was cloned into the pLKO lentiviral expression system as described above. For siRNA-mediated knockdown of Akt isoforms, Akt1 siRNA oligos (sense, 5′-GAGUUUGAGUACCUGAAGCUGUU-3′; anti-sense, 5′-CAGCUUCAGGUACUCAAACUCUU-3′) and Akt2 siRNA oligos (sense, 5′-GCGUGGUGAAUACAUCAAGACUU-3′; anti-sense, 5′-GUCUUGAUGUAUUCACCACGCUU-3′) were purchased from Dharmacon. Cells were transfected with Akt1, Akt2 or control luciferase GL2 siRNA (Dharmacon) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
In vitro kinase assays
HeLa or SKBR3 cells were transfected with HA-Palladin or HA-Palladin S507A. 7 h post-transfection, cells were serum-starved for an additional 16 h before harvesting. Palladin was immunoprecipitated from cell extracts and incubated with 500 ng recombinant Akt1 (Cell Signaling Technology) or Akt2 (Cell Signaling Technology) in the presence of 250 μM cold ATP in a kinase buffer for 1 h at 30°C. The kinase reaction was stopped by the addition of SDS-PAGE loading buffer and the samples were assayed by immunoblotting.
Transwell migration and invasion assays
Transwell filters (8 μm pore size; Corning) were left uncoated for migration assays or coated with 1–10 μg Matrigel (BD Biosciences) for invasion assays. 3 × 105 cells in serum-free medium containing 0.1% BSA were added to the upper chambers in triplicate. NIH 3T3-cell-conditioned medium was added to the lower chambers. After 2–22 h incubation at 37°C, non-migrated cells at the top of the filters were removed. Cells that had migrated to the bottom of the filters were fixed and stained using the Hema-3® stain set (Protocol) or X-gal.
Time-lapse microscopy
Cells were plated on tissue culture dishes containing NIH 3T3-cell-secreted ECM for 1 h before time-lapse microscopy. Phase-contrast images of live cells were taken every 2.5 min for 1 h with an inverted microscope (Eclipse2000; Nikon) equipped with a heated stage and digital image analysis software (IPLab; Scanalytics). Velocity of random migration was quantified using NIS-Elements AR software (Nikon).
3D cultures
3D culture assay was performed as previously described (Debnath et al., 2003). Briefly, chamber slides were coated with a 50:50 mixture of growth factor-reduced Matrigel (BD Biosciences) and bovine dermal collagen I (Matrigel/collagen; Vitrogen Cohesion Technologies) and allowed to solidify for 30 min. 2000–4000 MCF10A cells in assay medium (DMEM/Ham’s F12 medium supplemented with 2% equine serum, 10 μg ml−1 insulin, 500 ng ml−1 hydrocortisone, 5 ng ml−1 EGF, 100 ng ml−1 cholera toxin, 2 μg ml−1 puromycin and 2% Matrigel/collagen) were seeded to the coated chamber slides. The assay medium was replaced every 4 days.
Actin binding and bundling assays
Cells were lysed 24 h post-transfection in ice-cold lysis buffer (1% NP-40, 150 mM NaCl, 10 mM KCl, 20 mM Tris-HCl (pH 7.5), 0.1% SDC, 0.1% SDS, proteinase inhibitor cocktail (Sigma-Aldrich), 50 nM calyculin (Sigma-Aldrich), 1 mM sodium pyrophosphate, 20 mM sodium fluoride). Actin binding and bundling assays were performed according to the manufacturer’s protocol (Cytoskeleton). Briefly, actin filaments (F-actin) was prepared by incubation of human non-muscle monomeric G-actin in polymerization buffer (50 mM KCl, 2 mM MgCl2, 1 mM ATP, 0.18 mM CaCl2, 5 mM Tris (pH 8)) for 1 h at room temperature. Lysates were pre-cleared by centrifugation at 150,000 × g for 1 h at 4°C. Pre-cleared lysates were then incubated with 10 μM purified F-actin for 30 min at room temperature. The reaction mixture were centrifuged at 150,000 × g for 1.5 h and 10,000 × g for 1 h to sediment actin filaments and bundles, respectively. The amount of palladin and F-actin in both the supernatant and precipitant were detected by immunoblotting and Ponceau S staining.
Immunofluorescence analysis
Cells plated on coverslips were fixed with 2% paraformaldehyde for 10 min and permeabilized with 0.5% Triton X-100 for 1 min. Cells were then blocked with 1% BSA in 20 mM Tris-HCl (pH 7.5) for 20 min and incubated with anti-palladin polyclonal antibody for 1 h. After washing twice with phosphate-buffered saline (PBS), cells were incubated with Cy3-conjugated anti-rabbit IgG antibody for 1 h. F-actin was visualized with Alexa Fluor 488-conjugated phalloidin (Invitrogen). Cells were then rinsed twice with PBS and mounted with Prolong Gold antifade reagent/4,6-diamidino-2-phenylindole (DAPI) (Pierce). Images of cells were acquired using a fluorescence microscope (Eclipse TE300; Nikon) and digital image analysis software (IPLab; Scanalytics).
Immunoblots and immunoprecipitation
Cells were washed with ice-cold PBS and lysed in EBC buffer (0.5% NP-40, 120 mM NaCl, 50 mM Tris-HCl (pH 7.4), proteinase inhibitor cocktail, 50 nM calyculin, 1 mM sodium pyrophosphate, 20 mM sodium fluoride, 2 mM EDTA, 2 mM EGTA) for 25 min on ice. Cell extracts were pre-cleared by centrifugation at 13,000 rpm for 10 min at 4°C and protein concentration was measured with the Bio-Rad protein assay reagent using a Beckman Coulter DU-800 machine. Lysates were then resolved on 8% acrylamide gels by SDS-PAGE and transferred electrophoretically to nitrocellulose membrane (BioRad) at 160 mA for 80 min. The blots were blocked in TBST buffer (10 mM Tris-HCl, pH 8, 150 mM NaCl, 0.2% Tween 20) containing 5% (w/v) nonfat dry milk for 30 min, and then incubated with the specific primary antibody diluted in blocking buffer at 4°C overnight. Membranes were washed three times in TBST, and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Membranes were washed 3 times and developed using enhanced chemiluminescence substrate (Pierce). For immunoprecipitation, lysates were incubated with 1–2 μg of antibody for 2–4 h at 4°C followed by incubation with 15 μl protein A/G sepharose beads (Amersham Biosciences) for another 2 h. Immune complexes were washed 4 times with NETN buffer (0.5% NP-40, 1 mM EDTA, 20 mM Tris-HCl (pH 8), 100 mM NaCl) and once with PBS. Precipitates were resolved by SDS-PAGE and the separated proteins were analyzed by Western blot.
Supplementary Material
Acknowledgments
We thank Mikko Ronty, Jeffrey Field, Peter Shepherd and Symansis for providing reagents; Isaac Rabinovitz for his technical support with time-lapse microscopy; Shiva Kazerounian for assistance with 3D cultures; and members of the Toker laboratory for discussions. This study was supported in part by grants from the National Institutes of Health (A.T., CA122099) and the Susan G. Komen Breast Cancer Foundation (Y.R.C., PDF0706963).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- Artym VV, Yamada KM, Mueller SC. ECM degradation assays for analyzing local cell invasion. Methods Mol Biol. 2009;522:211–219. doi: 10.1007/978-1-59745-413-1_15. [DOI] [PubMed] [Google Scholar]
- Boukhelifa M, Moza M, Johansson T, Rachlin A, Parast M, Huttelmaier S, Roy P, Jockusch BM, Carpen O, Karlsson R, Otey CA. The proline-rich protein palladin is a binding partner for profilin. Febs J. 2006;273:26–33. doi: 10.1111/j.1742-4658.2005.05036.x. [DOI] [PubMed] [Google Scholar]
- Boukhelifa M, Parast MM, Bear JE, Gertler FB, Otey CA. Palladin is a novel binding partner for Ena/VASP family members. Cell Motil Cytoskeleton. 2004;58:17–29. doi: 10.1002/cm.10173. [DOI] [PubMed] [Google Scholar]
- Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, Zierath JR. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab. 2006;4:89–96. doi: 10.1016/j.cmet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal. 2009;21:470–476. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Defeo-Jones D, Barnett SF, Fu S, Hancock PJ, Haskell KM, Leander KR, McAvoy E, Robinson RG, Duggan ME, Lindsley CW, et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol Cancer Ther. 2005;4:271–279. [PubMed] [Google Scholar]
- Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- Dixon RD, Arneman DK, Rachlin AS, Sundaresan NR, Costello MJ, Campbell SL, Otey CA. Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem. 2008;283:6222–6231. doi: 10.1074/jbc.M707694200. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. NatRevGenet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Galbraith JA. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science. 2007;315:992–995. doi: 10.1126/science.1137904. [DOI] [PubMed] [Google Scholar]
- Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea S, Arneman D, Disanza A, Garcia-Mata R, Scita G, Otey CA. Palladin binds to Eps8 and enhances the formation of dorsal ruffles and podosomes in vascular smooth muscle cells. J Cell Sci. 2006;119:3316–3324. doi: 10.1242/jcs.03076. [DOI] [PubMed] [Google Scholar]
- Goicoechea SM, Bednarski B, Garcia-Mata R, Prentice-Dunn H, Kim HJ, Otey CA. Palladin contributes to invasive motility in human breast cancer cells. Oncogene. 2009;28:587–598. doi: 10.1038/onc.2008.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci U S A. 2009;106:7004–7009. doi: 10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- Heron-Milhavet L, Franckhauser C, Rana V, Berthenet C, Fisher D, Hemmings BA, Fernandez A, Lamb NJ. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol Cell Biol. 2006;26:8267–8280. doi: 10.1128/MCB.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 2004;64:3171–3178. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA, Bissell MJ. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc Natl Acad Sci U S A. 2006;103:4134–4139. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Luo HJ, Yang H, Wang L, Kong H, Jin YE, Wang F, Gu MM, Chen Z, Lu ZY, Wang ZG. Palladin regulates cell and extracellular matrix interaction through maintaining normal actin cytoskeleton architecture and stabilizing beta1-integrin. J Cell Biochem. 2007;100:1288–1300. doi: 10.1002/jcb.21126. [DOI] [PubMed] [Google Scholar]
- Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- Mykkanen OM, Gronholm M, Ronty M, Lalowski M, Salmikangas P, Suila H, Carpen O. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–3073. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J Biol Chem. 2000;275:36108–36115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Pang H, Flinn R, Patsialou A, Wyckoff J, Roussos ET, Wu H, Pozzuto M, Goswami S, Condeelis JS, Bresnick AR, et al. Differential enhancement of breast cancer cell motility and metastasis by helical and kinase domain mutations of class IA phosphoinositide 3-kinase. Cancer Res. 2009;69:8868–8876. doi: 10.1158/0008-5472.CAN-09-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–656. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronty M, Taivainen A, Heiska L, Otey C, Ehler E, Song WK, Carpen O. Palladin interacts with SH3 domains of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling. Exp Cell Res. 2007;313:2575–2585. doi: 10.1016/j.yexcr.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronty M, Taivainen A, Moza M, Otey CA, Carpen O. Molecular analysis of the interaction between palladin and alpha-actinin. FEBS Lett. 2004;566:30–34. doi: 10.1016/j.febslet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmstrom PO, Mansukhani M, Enoksson J, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- Sawyers CL. Will kinase inhibitors have a dark side? N Engl J Med. 2006;355:313–315. doi: 10.1056/NEJMcibr062354. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. J Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci U S A. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu G, Dziubinski M, Yang Z, Ethier SP, Wu G. Comprehensive analysis of oncogenic effects of PIK3CA mutations in human mammary epithelial cells. Breast Cancer Res Treat. 2008;112:217–227. doi: 10.1007/s10549-007-9847-6. [DOI] [PubMed] [Google Scholar]
- Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. JBiolChem. 2006;281:36443–36453. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]
- Zinda MJ, Johnson MA, Paul JD, Horn C, Konicek BW, Lu ZH, Sandusky G, Thomas JE, Neubauer BL, Lai MT, Graff JR. AKT-1, -2, and -3 are expressed in both normal and tumor tissues of the lung, breast, prostate, and colon. Clin Cancer Res. 2001;7:2475–2479. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.