Abstract
In the 19th century, Hughlings Jackson relied on clinical history, seizure semiology, and the neurologic examination as methods for seizure localization to inform the first epilepsy surgeries. In the 20th century, psychological and neuropsychological tests were first employed as both diagnostic and prognostic measures. The contemporary practice of epilepsy evaluation and management includes neuropsychology as a critical component of epilepsy care and research, and epilepsy and neuropsychology have enjoyed a very special and synergistic relationship. This paper reviews how epilepsy has shaped the practice of neuropsychology as a clinical service by asking critical questions that only neuropsychologists were in a position to answer, and how clinical care of epilepsy patients has been significantly improved based on neuropsychology's unique contributions.
Keywords: Epilepsy, Assessment
Introduction
Most historians consider the modern era of epilepsy to begin with the British neurologist John Hughlings Jackson's 1870 pamphlet “A study of convulsions.” In this monograph, Jackson describes focal motor seizures (“convulsions beginning unilaterally”) as originating in localized areas of the contralateral hemisphere rather than in the medulla, which had been thought to be the origin of epilepsy (Eadie, 2007). Jackson's 1875 description of the “march” of focal motor seizures corresponding to seizure spread along the sensorimotor homunculus is a well-known phenomenon to most neuropsychologists, even those whose practice does involve epilepsy specialization. His conceptualization of a dynamic interaction between focal seizures and distal effects outside the seizure focus not only affected the current understanding of focal perturbations in complex brain networks, but also provided the theoretical rationale for surgical treatment of epilepsy. Because Jackson believed that focal and generalized seizures both arose from a focal abnormality (“discharge lesion”), he reasoned that removing this focal abnormality would eliminate the cause of the seizures and result in a seizure cure (York & Steinberg, 2009).
Jackson's hypothesis that all epilepsy was focal in onset was critical in informing the initial epilepsy surgery cases. Sir Victor Horsley, the surgeon who performed the first epilepsy surgery in 1886 (Horsley, 1886), had conducted multiple animal procedures to refine his surgical technique and surgical methodology (Fig. 1). Horsley also developed the first stereotactic head frame for use in animals with Robert Henry Clarke in 1906 (Horsley–Clarke frame [HCF]), which was used for electrode implantation many years later in 1947 at the University of Illinois (Jensen, Stone, & Hayne, 1995). Horsley also introduced bone wax to seal skull edges and developed the c-shaped or comma-shaped (rather than cruciform) incision, both of which promoted recovery and minimized infection. Unlike most surgical interventions at that time involving emergent treatments that were primarily reactive in application, Horsley approached epilepsy surgery in a methodological and cautious fashion and benefited from the surgical technique that he developed in part from his animal procedures.
Fig. 1.

Sir Victor Horsley (1857–1916). Horsley was 29 years old when he performed his first epilepsy surgery on patient B. James, who was 22 years old.
Horsley's first epilepsy surgery patient (James B.) was a 15-year-old male who had post-traumatic epilepsy from a depressed skull fracture sustained during a traffic accident when he was 7 years old. In 1885, he developed episodes of “Jacksonian” or simple partial status epilepticus. Because the localizing value of seizure activity arising from the sensorimotor region had been well described by Jackson, and because the pattern of behavioral seizure expression corresponded to the patient's skull fracture and cortical scar, Horsley and Jackson were confident that the seizure onset occurred in the contralateral sensorimotor strip (Fig. 2). On May 25, 1886, Horsley operated to remove the cortical scar, and James B. became seizure-free. Jackson and David Ferrier, the Scottish neurologist whose work included extensive research into cortical stimulation in animals, both observed the surgery (despite the rather stodgy appearance in photographs [Fig. 1], Jackson apparently had a whimsical sense of humor—following James B.'s operation, Jackson remarked to Ferrier that Horsley missed the opportunity place a joke in a Scotsman's head! [Taylor, 1987]).
Fig. 2.

John Hughlings Jackson (1835–1911).
Horsley's second surgical case (Thomas W.) is more remarkable because seizure localization was not based on an identifiable skull defect or cortical scar. Jackson had observed Ferrier's cortical stimulation experiments in monkeys and noted that the induced motor response was identical to the movements in one of his patients during a seizure and inferred that seizure onset must have originated from the cortical hand region. Horsley operated on Thomas W. without any additional localizing information other than seizure semiology (including no neuropsychology!) and became seizure-free post-operatively following the resection of a tuberculoma (Taylor, 1987). Thus, Jackson's description of sensorimotor regions established functional localization within the cerebral cortex, and his belief that the all epilepsy had a focal onset provided the theoretical rationale to guide the initial surgical treatment of epilepsy.
Early Cognition Studies
In the 19th century and without adequate seizure treatments, epilepsy was considered to result in progressive cognitive decline and dementia. Formal studies of cognition in epilepsy did not begin to appear until the late 19th century and early 20th century. One of first English studies compared two epilepsy groups (“comparatively normal” and “marked dementia”) with healthy controls across a battery of tests that appears remarkably similar to contemporary neuropsychology practice (Smith, 1905). Cognitive measures included tests of “recognition, immediate memory, sensory discrimination, reactions involving movement and choice, as well as rhythmic movement,” and Smith made multiple observations that still resonate within contemporary neuropsychology. To test memory, both word and picture tasks were administered. Recognition memory was assessed by combining an equal number of foils with the target stimuli. Smith reported that patients not only performed more poorly recognizing words than pictures, but also that epilepsy patients with dementia showed even greater impairment in “confusion between old and new” due to their greater frequency of false-positive errors. Commenting on the ecological validity of his findings, Smith remarked “everyone forgets many things from day to day, while errors of confusion occur relatively seldom and are much more noticeable when they do occur.”
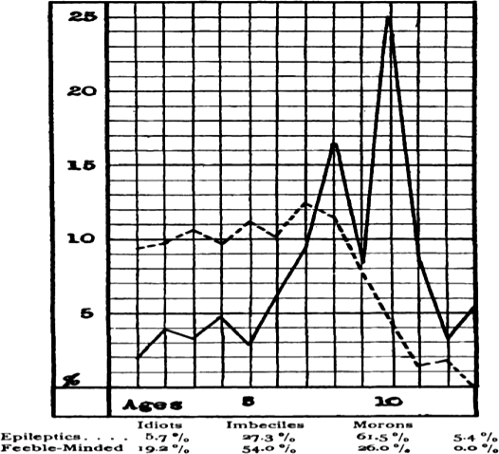
The first US study of cognition in epilepsy, and the first “psychometric” report, appeared in 1912 (Wallin, 1912a), only 4 years after Henry Goddard translated the Binet–Simon intelligence scales into English (Hermann, 2010). The Binet–Simon scale characterized intelligence by mental age (MA), with individuals with MA <3 years labeled “idiot,” those with MA between 3 and 7 years termed “imbecile,” and those with MA between 7 and 10 years classified as “moron” (a word Goddard derived from the Greek word “moros” meaning “foolish”). In addition to using standardized testing to assess cognition relative to a normative standard, this study is one of the earliest to employ a positive control group rather than healthy “normal” controls. Wallin was interested in how patients with epilepsy differed from a comparable institutionalized group and chose “feeble-minded patients,” or individuals most likely to be considered developmentally delayed in current nomenclature. In this series, there was less overall cognitive impairment in patients with epilepsy compared with feeble-minded patients based on classification frequencies (i.e., fewer “idiots” and more “morons”; Fig. 3).
Fig. 3.
The 1905 graph indicating an overall higher level of functining on the Binet–Simon test for patients with epilepsy compared with institutionalized patients with developmental disabilities (Smith, 1905).
Modern Era of Epilepsy Surgery
The maturation of epilepsy surgery as a viable treatment option for medically refractory epilepsy began at roughly the same time at three major epilepsy centers: The University of Illinois, the University of London, and the Montreal Neurological Institute (MNI)/McGill University. At all three locations, there was close collaboration among the neurosurgeon, neurologist (or electrophysiologist), and neuropsychologist. Although the MNI's contributions to the development of contemporary neuropsychology are well known, in part reflecting the continuity of their program, all three epilepsy surgery programs made important discoveries informing the neuropsychology of epilepsy.
University of Illinois
The University of Illinois epilepsy program was led by the neurosurgeon Percival Bailey and the neurologist/neurophysiologist Frederic Gibbs. While at the University of Chicago, one of Bailey's residents was Paul Bucy, and consequently, Bailey aware the Klüver–Bucy syndrome (visual agnosia, hypersexuality, diminished emotional responsivity, and memory impairment; Klüver & Bucy, 1939). Damage to hippocampal gyrus and hippocampus were considered critical in producing the Klüver–Bucy behavioral change, and these structures were not included by Bailey in surgical resection in order to minimize the likelihood of developing the post-operative Klüver–Bucy symptoms. Bailey's decision to exclude mesial temporal lobe structures in his resections likely accounts for poorer surgical outcomes in the University of Illinois series compared with other contemporary epilepsy surgery programs (Hermann & Stone, 1989).
It is difficult for neuropsychologists relying on MRI to help inform clinical case formulations to imagine the state of neurodiagnostics during the 1950s. Surgical patients at the University of Illinois were selected based on EEG alone, and the EEG technology at that time had few resemblances to current recording capabilities. Most EEG polygraphs had a maximum of eight channels in the late 1950s, and video EEG monitoring was years away. Thus, along with the Montreal Neurological Institute, the University of Illinois transitioned epilepsy surgery away from purely lesion-directed surgery practiced by Horsley to EEG-directed surgery (Engel, 1993).
Neuropsychology
Owing to the program's proximity to the University of Chicago, Bailey had his patients evaluated pre- and post-operatively by Ward Halstead with his battery of neuropsychological measures. Although he published only a single report (Halstead, 1958), Halstead's neuropsychological findings established that epilepsy surgery did not result in generalized cognitive decline as reflected by the absence of any significant change in the Impairment Index. Further, Halstead described significant post-operative improvement in the Category Test, Tactual Form Board Memory, and Speech Discrimination Index. “An improvement in performance on most indicators is observed after anterior temporal lobectomy (ATL), but impairment in relation to normal performance is still seen on some tests.” Importantly, “no severe deficits attributable to surgical intervention are revealed in these cases.” Thus, Halstead's data demonstrated that successfully surgical treatment did not have to result in significant post-operative cognitive decline.
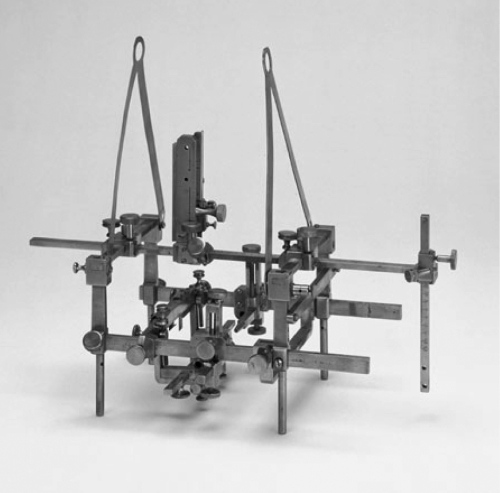
The HCF, which had been developed for animal use in 1906, was first used in humans at the University of Illinois in 1947 for depth electrode implantation (Jensen et al., 1995; Fig. 4). Subcortical depth electrodes were placed using the relationship between external landmarks and intracranial structures and confirmed by pneumoencephalography.
Fig. 4.
The Horsley–Clarke frame. Although designed for animals, it was used for depth human electrode placement in 1947 at the University of Illinois (Jensen et al., 1995).
University of London/Guy's Maudsley Hospital
The University of London is probably the least well-known program outside of epilepsy surgery circles and was directed by the neurosurgeon Murray Falconer. Falconer developed en bloc resections of the temporal lobe the facilitated pathological studies and identified hippocampal pathology as being critical for clinical outcome (Meador, Loring, & Flaningin, 1989). Victor Meyer and Aubrey Yates were the psychology members of that program.
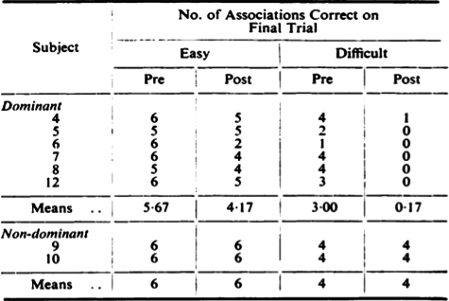
Meyer and Yates reported greater surgical effects for paired-associated learning compared with other psychological measures including a New Word Learning and Retention Test or 24 h retention (Meyer & Yates, 1955). This early report indicates the sensitivity of paired-associated learning to hippocampal dysfunction and anticipated contemporary theories of hippocampal function as a critical structure involved in “binding” associations (Fig. 5).
Fig. 5.
First reported effects illustrating sensitivity of hard-word paired-associate learning to dominant hemisphere resection. Although there is a mild post-operative decline seen in the easy-word pairs following dominant hemisphere resection, the decline in hard-word pairs is of much greater magnitude. No post-operative decline is noted in the two patients underling right temporal resection (Meyer & Yates, 1955).
Important observations by this group still reflect much of what we currently anticipate with neuropsychologist assessment. “Contrary to expectations, the group with operation on the nondominant temporal lobe failed to lower scores on nonverbal tests.” In addition, “the operation on the dominant side results in a severe deficit of auditory learning.” Important findings were also made about the distinction between linguistic capacity and verbal memory. “Despite the considerable amount of recovery from the manifest dysphasic disturbances a year after the operation, the auditory-verbal-learning impairment persists.” Unlike the MNI discussed below, there were no special precautions employed at the time of surgery such as functional stimulation mapping that were used explicitly to decrease the likelihood of post-operative language morbidity.
Montreal Neurological Institute/McGill University
The contributions of the MNI to neuropsychology and epilepsy surgery evaluation are unparalleled among epilepsy centers, with the MNI responsible for developing many techniques and approaches that continue to be widely employed. The interest in developing a multidisciplinary approach in order to better understand the effects of epilepsy and epilepsy surgery was primarily driven by their surgeon, Wilder Penfield (Penfield & Jasper, 1954), and this multidisciplinary approach to epilepsy patient evaluation and management remains perhaps his longest enduring legacy. The Montreal Neurological Institute of McGill University, co-founded by Penfield with William V. Cone (who introduced twist-drill techniques for biopsy and ventriculoperitoneal shunting techniques), opened in 1934 after a generous grant by the Rockefeller Foundation (Preul, Stratford, Bertrand, & Feindel, 1993).
The origins of many of Penfield's approaches to epilepsy surgery can be traced to the influences of Otfrid Foerster at the Brain Research Institute in Breslau, Germany (now Wroclaw, Poland; Piotrowska & Winkler, 2007). Many World War I veterans returned home with post-traumatic epilepsy, and Foerster developed cortical stimulation techniques in awake patients to reproduce their aura and provide maximal resection of scarred cortex without involving eloquent cortex (Flanigin, King, & Gallagher, 1985; Sarikcioglu, 2007). Penfield spent 6 months with Foerster in 1928 learning techniques of cortical mapping and epilepsy surgery (Sarikcioglu, 2007), and together with Foerster's published surgery outcomes of these patients only 2 years later (Foerster & Penfield, 1930). Other prominent figures in the neurology/neurosurgery who studied with Foerster include Percival Bailey and Paul Bucy.
The development of the epilepsy program at the MNI could not have occurred without the many contributions of the neurophysiologist Herbert Jasper. Before arriving at the MNI in 1938, Jasper had developed the EEG laboratory and Brown University and had published a paper in Science describing how visual stimulation would decrease the alpha rhythm, and the frequency of the alpha waves were constant over repeated evaluations, and how EEG frequency was a low as 2–3 Hz in two patients with brain injury (Feindel, 1999). “It may well be that the electroencephalograms of the sort described in this note may prove significant in psychology and clinical neurology. It is even possible that this technique may provide information in regard to brain action which will be comparable in significance to the information in regard to heart function which is provided by the electrocardiograph” (Jasper & Carmichael, 1935). After Penfield had toured Jasper's laboratory after giving a seminar at Brown University, Penfield commented that Jasper had told him that he could “localize of the focus of an epileptic seizure by the disturbance of brain rhythms outside the skull. I doubted but hoped it might be true” (Penfield & Jasper, 1972). Jasper then obtained EEGs on two of Penfield's patients, and based on Jasper's localization, both patients went to surgery where Penfield confirmed Jasper's seizure focus localization (Feindel, 1999).
Although the contributions of Brenda Milner are well known to clinical neuropsychologists, the systematic use of psychology at the MNI began years earlier. Penfield's desire for psychological input is reflected by his hire of Molly Harrower, who established what is likely the first clinical psychology service at a major hospital. Recruiting psychology into a medical environment was a novel approach at that time and began “a totally new chapter of the kinds of things [that can be done] with a rapport with medicine.” “I was really coping with really being the only woman in the hospital, the only woman fellow, the only woman on staff and the only psychologist” (http://www.uflib.ufl.edu/ufdc/?b=UF00006059&v=00001). Harrower was at Montreal from 1938–1942.
Penfield took special precautions due to his concerns about the effects of epilepsy surgery in the language dominant hemisphere (Sarikcioglu, 2007). As early as 1939, Penfield performed stimulation language mapping to identify areas of “eloquent cortex” to help guide surgical resection (Almeida, Martinez, & Feindel, 2005). In this case of a left ATL, surgery was performed using local anesthesia. The awake patient was encouraged to talk with Donald Hebb, “who drew him out on various subjects while the area of the scar was being removed.”
Hebb's contributions to our understanding of basic brain function (e.g., Hebbian circuits and cell assemblies) are well known and anticipated many contemporary concepts of neural nets. His work also included important clinical contributions to abnormal brain function, and in particular, his seminal work on the frontal lobes. Prior to the 1930s, the frontal lobes were considered the seat of all that is “noble and good” about humans. Hebb demonstrated that “frontal release” neurological signs (e.g., snout, glabellar), which at that time were considered pathognomonic of focal frontal lobe disease, did not result from large prefrontal lesions, but rather occurred due to the combination of frontal lesions with diffuse pressure effects associated with expanding mass lesions. This difference from the traditional clinical dogma resulted from the MNI's early detection of tumor and more rapid referral for cognitive evaluation compared with other centers. When reporting his early findings at the American Psychological Association, Hebb's results were met with skepticism by more senior psychologists: “I don't think I was regarded as a liar, just an incompetent” (Hebb, 1977).
Hebb was also the first to describe increased Full-Scale IQ following frontal lobe resection. Although not widely known, one of the early patients in Hebb's series on frontal lobe lesions was Penfield's sister. In fact, it was Penfield's sister who was being described as having trouble organizing family meals when describing the real-world effects in goal-directed behavior change following frontal lobe resection.
Milner was a graduate student of Hebb, and he recommended to Penfield that she evaluate his surgical patients pre- and post-operatively. The interested reader should read Milner's “autobiography” for a more thorough presentation of her many contributions to neuropsychology (Milner, 1998). On the basis of her study of patient H.M. and other patents developing post-operative amnesia, Milner demonstrated the important dissociation between episodic and procedural memory, which greatly facilitated the fractionation of “memory” into multiple and largely independent neural systems.
Because of Penfield's pursuit to document cognitive change following elective brain surgery, there are multiple ramifications for neuropsychology that I refer to as “Penfield” effects. On the broadest level, epilepsy was the first clinical disease to include neuropsychology programmatically to initially better characterize and subsequently guide the surgical treatment of epilepsy. In this context, epilepsy surgery represents the first systematic application of which is currently referred to as “Health Outcomes Research.” “Seizure free” with marked neuropsychological decline was a bad surgical outcome and constituted a surgical failure.
More narrowly, developing neuropsychology as a technique to establish surgical candidacy validated neuropsychological testing as a valuable diagnostic service. Although other approaches to neuropsychological assessment such as the Halstead Reitan Neuropsychological Battery were being used at that time, the HRNB was primarily employed to establish the presence of “organic brain disease.” Establishing “organicity” was an important contribution of neuropsychology at that time since diagnostic tools available during that era, namely EEG and pneumoencephalography, were gross measures of brain abnormalities. Pneumoencephalography was introduced in 1919 by Walter Dandy (Dandy, 1919); EEG was discovered a decade later in 1929 by Hans Berger (Berger, 1929). The goals of neuropsychological testing at MNI, however, were explicitly to identify specific neuropsychological deficits reflecting the integrity of focal brain regions so that resective surgery could be performed without significant cognitive morbidity.
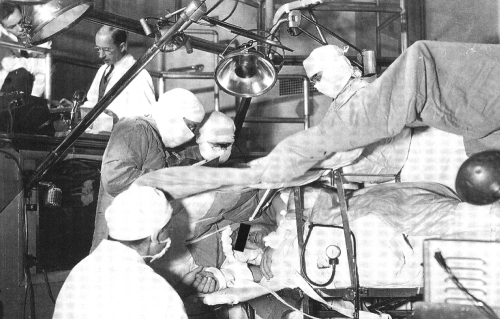
The other major contribution of the MNI is its emphasis on stimulation/language mapping, again reflecting careful appreciation of brain function, and attempts to minimize post-operative cognitive morbidity. MNI was unique in the early centers in this respect and reflected to collaborations of neuropsychology, neurology, and neurosurgery at very high levels (Fig. 6).
Fig. 6.
Operating theatre at the Montreal Neurological Institute ca. 1958. Assisting Wilder Penfield with the procedure is Herber Jasper (monitoring EEG up upper left portion of picture) and Brenda Milner (back to camera and interacting with patient).
Wada Testing
Discussion of the contributions of MNI to neuropsychology cannot be made without reference to Juhn Wada, who introduced the technique of selective hemispheric anesthesia to the MNI in 1955 (Fig. 7). Wada testing has been unique in informing our understanding of brain–behavior relationships, particularly in the days prior to functional brain imaging, and has greatly facilitated our understanding of the effects of epilepsy on alterations of cerebral language representation and handedness.
Fig. 7.

Juhn Wada (1924–).
Wada initially developed selective hemispheric barbiturization during post-World War II Japan to limit bilateral seizure effects associated with electroconvulsive therapy (ECT) (Wada, 1997). By “anesthetization through a carotid route to prevent seizure bilateralization,” Wada reasoned that the cognitive side effects associated with bilateral ECT would be minimized. Before he was able to implement this approach with patients undergoing ECT, however, Wada was presented with a patient with repeated episodes of partial status epilepticus and occasional generalization following a penetrating missile injury. Because of chloral hydrate treatment failure, the standard treatment of status at that time, Wada administered the amobarbital which had been obtained for use with ECT patients, and unilateral amobarbital injection successfully interrupted the status.
This technique as a procedure to establish cerebral language representation in epilepsy surgery candidates was introduced to the MNI in 1955 when Wada was on fellowship. Since epilepsy was known to potentially alter cerebral language dominance, Wada testing became routine when any possibility of atypical language existed and quickly became the gold standard for establishing cerebral language representation.
Wada memory testing began several years later after multiple cases of severe memory decline were observed following unilateral temporal lobectomy (Penfield & Milner, 1958). Memory declines were attributed to significant bilateral disease that went unrecognized during the presurgical evaluation. In these patients, there was insufficient residual function in the contralateral temporal lobe following surgery to sustain new memory formation (Chelune, 1995). By introducing items to be remembered during the period of hemispheric anesthesia, Milner reasoned that the effects of surgical resection on memory could be modeled and risk for significant post-surgical decline estimated (Milner, Branch, & Rasmussen, 1962).
The Wada test quickly became an indispensible clinical tool for establishing preoperative language and memory dominance. Wada testing also has provided unique brain–behavior research opportunities to investigate hemispheric specialization of mechanisms contributing to emotion (Lee, Loring, Meader, & Brooks, 1990), neglect (Meador et al., 1988), anosognosia (Durkin, Meador, Nichols, Lee, & Loring, 1994), autonomic function (Zamrini et al., 1990), and apraxia (Heilman, Meador, & Loring, 2000).
More Recent Epilepsy Surgery Centers
A second generation of epilepsy surgery programs emerged in the late 1970s and early 1980s. Notable programs (neuropsychologists) include the University of Washington (Carl Dodrill), Mayo Clinic (Robert Ivnik), Yale University (Robert Novelly), UCLA (Rebecca Rausch), and at the MNI, a passing of the neuropsychology baton from Brenda Milner to Marilyn Jones-Gotman. Important contributions to neuropsychology were made by all programs, and space does not permit a comprehensive review of each program's role in shaping neuropsychology. However, Dodrill's (1983) emphasis on psychosocial aspects of epilepsy in many ways anticipated Quality of Life as an important construct to characterize epilepsy and its treatment (Devinsky et al., 1995). Dodrill (1978) also developed a disease-specific approach to neuropsychological assessment. Rauch demonstrated subfield specialization of the hippocampus to the hard, but not easy, word pairs of the Wechsler Memory Scale (WMS) Paired-Associate Learning task (Rausch & Babb, 1993). [It is unfortunate given the strong relationship between left hippocampal function and paired-associated learning that the initial revision of the original 1945 WMS did not include individual Paired-Associate subtest norms. There were many criticisms of WMS-R as a comprehensive memory assessment protocol (Loring, 1989). Given the substantial normative improvement of the 1987 WMS-R over the 1945 WMS, however, many clinicians used the WMS-R Logical Memory (LM) and Visual Reproduction (VR) subtests alone rather than administering the entire WMS-R battery since individual subtest norms for LM and VR were included in the manual. Unfortunately, subtest norms for Paired Associates (PA) were not available in the scoring manual, and the only PA summary scores were a mean and standard deviation. Thus, clinicians relying on the presence of normative information to guide subtest selection avoided PA as a measure of verbal learning, despite evidence of its superiority to LM as a measure of hippocampal function.] Jones-Gotman's contribution to the understanding of temporal lobe epilepsy was unique in neuropsychology, with interests in temporal lobe contributions to noncognitive constructs of smell and taste (Jones-Gotman et al., 1997; Small, Jones-Gotman, Zatorre, Petrides, & Evans, 1997). Although Novelly (1992) was not an active clinical researcher, his paper in the American Psychologist remains “must reading” for anyone interested in the synergy between neuropsychology and epilepsy.
Medical College of Georgia
At the Medical College of Georgia (MCG), our contributions to understanding neuropsychological aspects of epilepsy have centered in two main areas: (a) Wada testing (Loring, Meador, Lee, & King, 1992), and (b) cognitive and behavioral effects of antiepilepsy drugs (AEDs) (Loring, Marino, & Meador, 2007). The surgical arm of the MCG program was lead by Herman Flanigin, who was the junior author with Penfield at the MNI on the landmark 1950 paper on temporal lobectomy (Penfield & Flanigin, 1950).
After arriving at MCG, I quickly realized how epilepsy physicians were in a unique position to appreciate the subtleties and sometime inconsistencies of functional measures. Early in 1986, soon after MRI was being routinely used in epilepsy surgery evaluations, Dr Flanigin (who was both a board-certified neurosurgeon trained by Wilder Penfield and a board-certified neurophysiologist trained by Herbert Jasper) was unable to find any evidence of EEG abnormality to suggest any focal abnormality across the long paper EEG record. After careful review of the MRI films, Dr Flanigin returned to the long EEG printout and quickly identified a small but consistent focal abnormality based upon the location of subtle MRI brain change. Dr Flanigin smiled and stated that this was an “MRI enhanced EEG,” informally illustrating the importance of interpreting the EEG (and by extension, neuropsychological results) within the entire clinical context and available laboratory findings.
Wada Testing
Because Wada protocols are not standardized, there is ongoing controversy over its clinical utility given the reported variability of Wada protocols to predict memory outcome (Loring & Meador, 2010) This concern has grown stronger as reliable noninvasive alternatives to Wada testing for establishing language and memory representation (e.g., fMRI) continue to be developed and refined (Loring & Meador, 2009).
At MCG, we demonstrated how method variance in Wada memory protocols produces significantly different Wada memory results. Factors such as stimulus type (pictures vs. real objects) (Loring et al., 1997), timing of stimulus presentation (Loring, Meador, Lee, King, et al., 1994), mixed stimuli (Lee, Park, Westerveld, Hempel, & Loring, 2002), and amobarbital dose (Loring, Meador, & Lee, 1992) all affect Wada memory correlations with seizure onset laterality. Further, different sensitivities to seizure onset laterality, with corresponding differences in diagnostic sensitivity and specificity, result from different criteria used to infer interhemispheric memory asymmetries (Loring, Bowden, Lee, & Meador, 2009).
Even within our own epilepsy center, we reported different “utility” of Wada memory results based upon evolving Wada technique. For example, we observed that successful post-operative memory outcomes could be observed following Wada memory failure. Our Wada protocol at that time (Loring et al., 1990) employed relatively large total doses of amobarbital (up to 250 mg). However, after we demonstrated an adverse dose effect on Wada memory results (Loring, Meador, Lee, et al., 1992) and following the introduction of real objects as our primary memory stimuli (Loring, Meador, Lee, et al., 1992), we observed a case of post-operative amnesia in which strongly asymmetric Wada memory results appeared to predict that neuropsychological outcome (Loring, Hermann, et al., 1994). Across a larger series of patients testing using lower dosing and real-object Wada memory stimuli, memory performance is related to verbal memory decline following left ATL (Loring et al., 1995). Wada memory performances are similarly correlated with seizure outcomes following ATL (Loring, Meador, Lee, Nichols, et al., 1994).
Cognitive and Behavioral AED Effects
Among the many factors affecting cognition in patients with epilepsy (e.g., age of seizures onset, disease substrate, type of seizure, seizure frequency), AEDs are unique since medication choice is under the control of the physician and patient. Since AEDs decrease membrane excitability, increase post-synaptic inhibition, or alter synchronization of neural networks, it is not surprising that they are often associated with neuropsychological side effects including decreased motor/psychomotor speed and attention (Meador, 2005). After seizure control, the most important aspect of AED treatment is the side effect profile including problems with cognition, energy level, school performance, childbearing, coordination, and sexual function.
Across our MCG studies of older AEDs, we developed a core neuropsychological AED test battery that emphasizes measures of psychomotor speed, attention, and memory (Meador et al., 1991, 1995). Using this protocol, we demonstrated poorer graphomotor performance associated with phenobarbital compared with carbamazepine (Tegretol) or phenytoin (Dilantin) or valproate (Depakote), despite relatively low serum phenobarbital levels (Meador, Loring, Huh, Gallagher, & King, 1990; Meador et al., 1995). Both lamotrigine (Lamictal) and levetiracetam (Keppra) are associated with less cognitive impairment than carbamazepine (Meador, Loring, Ray, & Motamedi, 2006; Meador et al., 2001). We have established greater neuropsychological risk in epilepsy patients with topiramate (Topamax) compared with valproate (Depakote) or lamotrigine (Lamictal) in adjunctive therapy and in healthy volunteers (Blum et al., 2006; Meador, Loring, Hulihan, Kamin, & Karim, 2003; Meador et al., 2005), and have introduced reliable change indices as a way to operationalize AED-associated cognitive decline (Loring, Meador, Williamson, Wiegand, & Hulihan, 2007). Cognitive AED effects are increasingly appreciated—in one recent pediatric trial of overall AED effectiveness in the treatment of childhood absence epilepsy, ethosuximide was considered superior to valproate since, despite comparable efficacy in treating seizures and normalizing the EEG, ethosuximide was associated with less impairment of attention (Glauser et al., 2010). As is the case with epilepsy surgery outcomes, poor neuropsychological treatment effects with AEDs is a well-recognized potential treatment risk, and measuring this risk as a formal treatment outcome in clinical drug trials is increasingly being employed to establish treatment effectiveness, even in the context of good treatment efficacy.
Bozeman Epilepsy Consortium
In 1990, the NIH issued a consensus statement asserting that neuropsychology not only was essential for preoperative evaluation of epilepsy surgery candidacy, but also was necessary for evaluating surgical outcomes (http://consensus.nih.gov/1990/1990SurgeryEpilepsy077html.htm). Partially in response to this initiative, a group of neuropsychologists from major epilepsy surgical centers formed an informal (and unfunded) research collaboration. This group included Bill Barr (Long Island Jewish Hospital), Gordon Chelune (Cleveland Clinic), Bruce Hermann (Baptist Memorial Hospital), Ken Perrine (New York University), Esther Strauss (University of Victoria), Max Trenerry (Mayo Clinic), Mike Westerveld (Yale University), and MCG. Because the initial meetings were held in Bozeman, Montana, this collaboration became known as the Bozeman Epilepsy Consortium.
This small group of neuropsychologists with specialized interests in epilepsy provided the forum for frank exchanges about test selection and utilization that were unencumbered by pathological historical inertia (i.e., “that's the way we've always done it”) or pathological training inertia (i.e., that's the way we did it during internship/fellowship). In response to these discussions, some centers modified their assessment protocols; although in some instances, institutional considerations for database continuity overrode the desire for implementing new and potentially better assessment approaches. With the widespread availability of MRI, introduction of the personal computer and subsequent availability of the Internet, and maturation of video/EEG recording technologies, the zeitgeist was right for multicenter collaboration that could answer specific clinical epilepsy research questions.
Test administration was discussed at each Bozeman meeting for database quality control, and surprisingly, several small but potentially relevant differences in test administration were identified. Also discussed were practical matters about how neuropsychological data were presented, both in formal clinical reports and during the epilepsy surgery conference. A much greater appreciation was obtained, not only for the variability in neuropsychological assessment approaches designed to answer a common clinical question in epilepsy, but also for broader institutional differences in preoperative evaluation including the use of intracranial EEG monitoring, language mapping, and surgical technique. The Bozeman collaboration provided a way to quickly amass a meaningful sample size to investigate the sensitivity of common neuropsychological measures of language and material-specific memory measures as well as important brain–behavior relationships.
Bozeman Summary
Despite the impressive research output from this collaboration (summarized in Table 1), the importance of the Bozeman collaboration extends far beyond the incremental knowledge that was generated. The Bozeman group demonstrated the power of collaborative research initiatives and the power of data pooling across sites. This group also discussed how a variety of programmatic or non-assessment questions could be addressed in a collaborative research setting. Bozeman participants also formed the first members of NPSYCH, the first Internet neuropsychology listserv group. NPSYCH membership was extended to the larger epilepsy neuropsychology community in 1994 in New Orleans, and then to the general neuropsychology community in 1995 in Seattle.
Table 1.
Peer-reviewed publications produced by the Bozeman Epilepsy Consortium
| Reference | Study topic |
|---|---|
| Barr and colleagues (1997) | Sensitivity of figural fluency tests to right temporal lobe dysfunction |
| Chelune and colleagues (1998) | Relationship of IQ to seizure outcome after temporal lobectomy |
| Hermann, Connell, Barr, and Wyler (1995) | Sensitivity of the Warrington Memory Test to lateralized temporal lobe epilepsy |
| Hermann, Trenerry, and Colligan (1996) | Contributors to depression in TLE |
| Hermann and colleagues (1999) | Relationship of surgical approach in left ATL to post-operative naming outcomes |
| Loring and colleagues (1997) | Memory stimulus type and Wada memory outcomes |
| Loring and colleagues (1999) | “Crowding” associated with nonspace occupying lesions |
| Loring, Hermann, Lee, Drane, and Meador (2000) | Sensitivity of the Memory Assessment Scales to lateralized temporal lobe epilepsy |
| Loring and colleagues (2008) | Sensitivity of common naming and verbal memory measures to left TLE |
| Perrine, Hermann, and colleagues (1995) | Relationship of quality of life to neuropsychological performance in epilepsy |
| Perrine, Westerveld, and colleagues (1995) | Relationship of Wada memory asymmetries predict post-operative seizure outcome |
| Strauss and colleagues (1995) | Factors are related to impaired neuropsychological profiles in epilepsy |
| Strauss and colleagues (1997) | Differential rates of age of seizure onset between sexes and between hemispheres |
| Strauss and colleagues (2000) | Category-specific naming risks associated with left ATL |
| Trenerry and colleagues (1996) | MMPI profiles differences between left and right TLE patients and differential changes following ATL |
| Westerveld and colleagues (2000) | Cognitive outcomes in pediatric ATL |
| Wilde and colleagues (2001) | Sensitivity of WMS-III index discrepancies in patients with TLE |
| Wilde and colleagues (2003) | Confirmatory factor analysis of WMS-III in patients with TLE |
Notes: ATL = anterior temporal lobectomy; TLE = temporal lobe epilepsy; MMPI = Minnesota Multiphasic Personality Inventory; WMS = Wechsler Memory Scale.
The Bozeman collaboration provided the opportunity for reasoned discussions about a rational approach to assessment and anticipated the NINDS initiative to establish “common data elements” that began at the 2008 meeting of the American Epilepsy Society. Given the long history of cross-disciplinary collaborations in epilepsy management and epilepsy research, epilepsy was the first clinical group selected by NINDS to participate in the CDE project.
One goal of the NINDS CDE project is to permit comparison across studies using the same constructs and the same operational measures of those constructs. Although this will facilitate data pooling across sites, a larger goal of this initiative will hopefully include NIH funding for database creation and maintenance as a research repository for both funded and non-funded neuropsychology epilepsy studies.
Evidence-based Neuropsychology
As part of the increasing emphasis on evidence-based medicine, neuropsychology will need to continue to demonstrate its relevance not only to patient diagnosis and disease progression, but also to health outcomes. As previously discussed, in a recent study studying AED effectiveness in children with absence seizures, comparable efficacy of controlling seizures was present in two of the three medications studied (Glauser et al., 2010). However, one drug (ethosuximide) was recommended as being superior based on the absence of neuropsychological impairment of attention compared with the other (valproate). It is not surprising that there is a new emphasis on cognitive and behavioral outcomes by the FDA when evaluating drugs for pediatric indications.
Because funded disease-based research by the NIH will contain the highest level of evidence in clinical studies (e.g., randomized trials, double blind treatments, longitudinal outcomes), CDEs will provide the best scientific basis for neuropsychological test sensitivity and specificity. This may have the unfortunate effect of diminishing innovative test development. For example, the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983) is considered the “gold standard” naming test and is include in the epilepsy NP CDEs. However, responsive auditory naming assessment appears to be a more appropriate method for assessing “tip of the tongue” or word finding difficulty. Further, auditory naming sites are distributed more anteriorly in the temporal lobe than visual naming sites, and consequently, appear more related to post-operative naming decline (Hamberger, McClelland, McKhann, Williams, & Goodman, 2007; Hamberger, Seidel, Mckhann, Perrine, & Goodman, 2005). Thus, to the degree that CDEs decrease the degrees of freedom in neuropsychological test selection, advances in the assessment development to develop and implement better measures for the neuropsychological constructs of interest may be impeded.
CDEs, however, will also have the benefit of decreasing the introduction of new tests or test revisions without ample data for important clinical endpoints prior to test publication (Loring & Bauer, 2010). This will more easily allow cross-disease comparisons if common assessment metrics are used, as well as facilitate data pooling across centers for both funded and non-funded projects—an effective way to rapidly acquire large sample sizes to address a variety of clinical research questions and which has been demonstrated to be highly effective based on the success of the Bozeman Epilepsy Consortium. Finally, major medical journals (e.g., Neurology) now require levels of evidence to be published with their clinical trials, and it seems likely that neuropsychology journals will soon follow this trend in evidence-based assessment for the quality of published research reports. Common metrics will permit greater comparisons across results, which will ultimately eliminate some of the variability in neuropsychological findings across reports.
Conclusions
The neuropsychology of epilepsy continues to be a robust area of clinical research, with the next generation (or half generation) of epilepsy neuropsychologists making very important contributions (e.g., Sallie Baxendale at Queen Square, Christoph Helmstaedter at the University Hospital of Bonn, Michael Saling at the University of Melbourne to name just a few). Epilepsy will continue to be a major clinical disease on which the evidence-base for neuropsychological test utility is established. Neuropsychology will continue to play a critical role in establishing epilepsy phenotypes (and perhaps endophenotypes) as part of translational research initiatives to identify both genetic disease risks and possible pharmacogenomic predictors of treatment outcomes. Neuropsychology will also be used with more traditional clinical endpoints to characterize disease severity and functional impairments associated with new and evolving measures of brain structure and function, their interconnectivity, and patterns of complex neuronal networks (e.g., diffusion tensor imaging, functional MRI, Granger causality, local field potentials). As observed by Professor John M. Richardson, Jr, “when it comes to the future, there are three kinds of people: Those who let it happen, those who make it happen, and those who wonder what happened.” Neuropsychology will be well represented in the second group.
Author Notes
This paper is based on a presentation delivered to the 2009 National Academy of Neuropsychology meeting in New Orleans, Louisiana, for the Distinguished Lifetime Contributions to Neuropsychology Award. I am honored to receive this award given the long and intimate relationship enjoyed between epilepsy and neuropsychology and one that continues to be recognized both by our neuropsychology colleagues and by the larger clinical research community. In this context, Brenda Milner received the Distinguished Lifetime Contributions to Neuropsychology Award in 1994. Two other epilepsy neuropsychologists, both members of the Bozeman Epilepsy Consortium, have also been recognized by the National Academy of Neuropsychology for their contributions to our field—Bruce Hermann received the Lifetime Contributions to Neuropsychology Award in 2006, and Gordon Chelune served as NAN President in 1994. This paper is dedicated to the memory of Esther Strauss, a trusted friend and valuable Bozeman collaborator. I thank Bruce Hermann, Kim Meador, and Juhn Wada for their helpful comments on earlier drafts of this manuscript.
Funding
This manuscript was supported in part by NIH R01038455.
Conflict of Interest
None declared.
References
- Almeida A. N., Martinez V., Feindel W. The first case of invasive EEG monitoring for the surgical treatment of epilepsy: Historical significance and context. Epilepsia. 2005;46(7):1082–1085. doi: 10.1111/j.1528-1167.2005.66404.x. doi:10.1111/j.1528-1167.2005.66404.x. [DOI] [PubMed] [Google Scholar]
- Barr W. B., Chelune G. J., Hermann B. P., Loring D. W., Perrine K., Strauss E., et al. The use of figural reproduction tests as measures of nonverbal memory in epilepsy surgery candidates. Journal of the International Neuropsychological Society. 1997;3(5):435–443. [PubMed] [Google Scholar]
- Berger H. Über das Elektroenkephalogramm des Menschen. Archiv für Psychiatrie und Nervenkrankheiten. 1929;87:527–570. doi:10.1007/BF01797193. [Google Scholar]
- Blum D., Meador K., Biton V., Fakhoury T., Shneker B., Chung S., et al. Cognitive effects of lamotrigine compared with topiramate in patients with epilepsy. Neurology. 2006;67(3):400–406. doi: 10.1212/01.wnl.0000232737.72555.06. doi:10.1212/01.wnl.0000232737.72555.06. [DOI] [PubMed] [Google Scholar]
- Chelune G. J. Hippocampal adequacy versus functional reserve: Predicting memory functions following temporal lobectomy. Archives of Clinical Neuropsychology. 1995;10:413–432. [PubMed] [Google Scholar]
- Chelune G. J., Naugle R. I., Hermann B. P., Barr W. B., Trenerry M. R., Loring D. W., et al. Does presurgical IQ predict seizure outcome after temporal lobectomy? Evidence from the Bozeman Epilepsy Consortium. Epilepsia. 1998;39(3):314–318. doi: 10.1111/j.1528-1157.1998.tb01379.x. doi:10.1111/j.1528-1157.1998.tb01379.x. [DOI] [PubMed] [Google Scholar]
- Dandy W. E. Rontgenography of the Brain after the Injection of Air into the Spinal Canal. Annals of Surgery. 1919;70(4):397–403. doi: 10.1097/00000658-191910000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Vickrey B. G., Cramer J., Perrine K., Hermann B., Meador K., et al. Development of the quality of life in epilepsy inventory. Epilepsia. 1995;36(11):1089–1104. doi: 10.1111/j.1528-1157.1995.tb00467.x. doi:10.1111/j.1528-1157.1995.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Dodrill C. B. A neuropsychological battery for epilepsy. Epilepsia. 1978;19(6):611–623. doi: 10.1111/j.1528-1157.1978.tb05041.x. doi:10.1111/j.1528-1157.1978.tb05041.x. [DOI] [PubMed] [Google Scholar]
- Dodrill C. B. Psychosocial characteristics of epileptic patients. Research Publications: Association for Research in Nervous and Mental Disease. 1983;61:341–353. [PubMed] [Google Scholar]
- Durkin M. W., Meador K. J., Nichols M. E., Lee G. P., Loring D. W. Anosognosia and the intracarotid amobarbital procedure (Wada test) Neurology. 1994;44(5):978–979. doi: 10.1212/wnl.44.5.978. [DOI] [PubMed] [Google Scholar]
- Eadie M. J. Cortical epileptogenesis—Hughlings Jackson and his predecessors. Epilepsia. 2007;48(11):2010–2015. doi: 10.1111/j.1528-1167.2007.01163.x. doi:10.1111/j.1528-1167.2007.01163.x. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr . Historical perspectives. In: Engel, J. Jr, editor. Surgical treatment of the epilepsie. New York: Raven Press; 1993. pp. 693–705. [Google Scholar]
- Feindel W. Herbert Henri Jasper (1906–1999): An appreciation. Canadian Journal of Neurological Sciences. 1999;26(3):224–229. doi: 10.1017/s0317167100000299. [DOI] [PubMed] [Google Scholar]
- Flanigin H., King D., Gallagher B. Surgical treatment of epilepsy. In: Pedley T. A., Meldrum B. S., editors. Recent advances in epilepsy. Vol. 2. Edinburgh: Churchill-Livingstone; 1985. pp. 297–339. [Google Scholar]
- Foerster O., Penfield W. The structural basis of traumatic epilepsy and results of radical operation. Brain. 1930;53:99–119. doi:10.1093/brain/53.2.99. [Google Scholar]
- Glauser T. A., Cnaan A., Shinnar S., Hirtz D. G., Dlugos D., Masur D., et al. the Childhood Absence Epilepsy Study Group. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. New England Journal of Medicine. 2010;362(9):790–799. doi: 10.1056/NEJMoa0902014. doi:10.1056/NEJMoa0902014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead W. C. Proceedings of the Association for Research in Nervous and Mental Disease. Baltimore: Williams & Wilkins; 1958. Some behavioral aspects of partial temporal lobectomy in man; pp. 478–489. [PubMed] [Google Scholar]
- Hamberger M. J., McClelland S., 3rd, McKhann G. M., 2nd, Williams A. C., Goodman R. R. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions. Epilepsia. 2007;48(3):531–538. doi: 10.1111/j.1528-1167.2006.00955.x. doi:10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Hamberger M. J., Seidel W. T., Mckhann G. M., II, Perrine K., Goodman R. R. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128(11):2742–2749. doi: 10.1093/brain/awh621. doi:10.1093/brain/awh621. [DOI] [PubMed] [Google Scholar]
- Hebb D. The frontal lobe. CMA Journal. 1977;116:1373–1374. [PMC free article] [PubMed] [Google Scholar]
- Hermann B. 100 years of Epilepsia: Landmark papers and their influence in neuropsychology and psychiatry. Epilepsia. 2010 doi: 10.1111/j.1528-1167.2009.02486.x. doi:10.1111/j.1528-1167.2009.02486.x, February 3. Epub ahead of print doi:10.1111/j.1528-1167.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- Heilman K. M., Meador K. J., Loring D. W. Hemispheric asymmetries of limb-kinetic apraxia: A loss of deftness. Neurology. 2000;55(4):523–526. doi: 10.1212/wnl.55.4.523. [DOI] [PubMed] [Google Scholar]
- Hermann B. P., Connell B., Barr W. B., Wyler A. R. The utility of the Warrington Recognition Memory Test for temporal lobe epilepsy: Pre- and postoperative results. Journal of Epilepsy. 1995;8:139–145. doi:10.1016/0896-6974(95)00022-6. [Google Scholar]
- Hermann B. P., Perrine K., Chelune G. J., Barr W., Loring D. W., Strauss E., et al. Visual confrontation naming following left anterior temporal lobectomy: A comparison of surgical approaches. Neuropsychology. 1999;13(1):3–9. doi: 10.1037//0894-4105.13.1.3. doi:10.1037/0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- Hermann B. P., Stone J. L. A historical review of the epilepsy surgery program at the University of Illinois Medical Center: The contributions of Bailey, Gibbs, and collaborators to the refinement of anterior temporal lobectomy. Journal of Epilepsy. 1989;2(3):155–163. [Google Scholar]
- Hermann B. P., Trenerry M. R., Colligan R. C. Learned helplessness, attributional style, and depression in epilepsy. Bozeman Epilepsy Surgery Consortium. Epilepsia. 1996;37(7):680–686. doi: 10.1111/j.1528-1157.1996.tb00633.x. doi:10.1111/j.1528-1157.1996.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Horsley V. Brain-surgery. British Medical Journal. 1886;2:670–675. [Google Scholar]
- Jasper H. H., Carmichael L. Electrical potentials from the intact human brain. Science. 1935;81(2089):51–53. doi: 10.1126/science.81.2089.51. doi:10.1126/science.81.2089.51. [DOI] [PubMed] [Google Scholar]
- Jensen R. L., Stone J. L., Hayne R. Use of the Horsley–Clarke stereotactic frame in humans. Stereotactic and Functional Neurosurgery. 1995;65(1–4):194–197. doi: 10.1159/000098694. doi:10.1159/000098694. [DOI] [PubMed] [Google Scholar]
- Jones-Gotman M., Zatorre R. J., Cendes F., Olivier A., Andermann F., McMackin D., et al. Contribution of medial versus lateral temporal-lobe structures to human odour identification. Brain. 1997;120(Pt. 10):1845–1856. doi: 10.1093/brain/120.10.1845. [DOI] [PubMed] [Google Scholar]
- Kaplan E. F., Goodglass H., Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Klüver H., Bucy P. C. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Lee G. P., Loring D. W., Meader K. J., Brooks B. B. Hemispheric specialization for emotional expression: A reexamination of results from intracarotid administration of sodium amobarbital. Brain and Cognition. 1990;12(2):267–280. doi: 10.1016/0278-2626(90)90019-k. doi:10.1016/0278-2626(90)90019-K. [DOI] [PubMed] [Google Scholar]
- Lee G. P., Park Y. D., Westerveld M., Hempel A., Loring D. W. Effect of Wada methodology in predicting lateralized memory impairment in pediatric epilepsy surgery candidates. Epilepsy and Behavior. 2002;3(5):439–447. doi: 10.1016/s1525-5050(02)00514-0. doi:10.1016/S1525-5050(02)00514-0. [DOI] [PubMed] [Google Scholar]
- Loring D. W. The Wechsler Memory Scale-Revised, or the Wechsler Memory Scale-Revisited? The Clinical Neuropsychologist. 1989;3(1):59–69. doi:10.1080/13854048908404077. [Google Scholar]
- Loring D. W., Bauer R. M. Testing the limits: Cautions and concerns regarding the new Wechsler IQ and Memory scales. Neurology. 2010;74(8):685–690. doi: 10.1212/WNL.0b013e3181d0cd12. doi:10.1212/WNL.0b013e3181d0cd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring D. W., Bowden S. C., Lee G. P., Meador K. J. Diagnostic utility of Wada Memory Asymmetries: Sensitivity, specificity, and likelihood ratio characterization. Neuropsychology. 2009;23(6):687–693. doi: 10.1037/a0016528. doi:10.1037/a0016528. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Hermann B. P., Lee G. P., Drane D. L., Meador K. J. The Memory Assessment Scales and lateralized temporal lobe epilepsy. Journal of Clinical Psychology. 2000;56(4):563–570. doi: 10.1002/(sici)1097-4679(200004)56:4<563::aid-jclp9>3.0.co;2-k. doi:10.1002/(SICI)1097-4679(200004)56:4<563::AID-JCLP9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Hermann B. P., Meador K. J., Lee G. P., Gallagher B. B., King D. W., et al. Amnesia after unilateral temporal lobectomy: A case report. Epilepsia. 1994;35(4):757–763. doi: 10.1111/j.1528-1157.1994.tb02507.x. doi:10.1111/j.1528-1157.1994.tb02507.x. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Hermann B. P., Perrine K., Plenger P. M., Lee G. P., Meador K. J. Effect of Wada memory stimulus type in discriminating lateralized temporal lobe impairment. Epilepsia. 1997;38(2):219–224. doi: 10.1111/j.1528-1157.1997.tb01100.x. doi:10.1111/j.1528-1157.1997.tb01100.x. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Lee G. P., Meador K. J., Flanigin H. F., Smith J. R., Figueroa R. E., et al. The intracarotid amobarbital procedure as a predictor of memory failure following unilateral temporal lobectomy. Neurology. 1990;40(4):605–610. doi: 10.1212/wnl.40.4.605. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Marino S., Meador K. J. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychological Review. 2007;17(4):413–425. doi: 10.1007/s11065-007-9043-9. doi:10.1007/s11065-007-9043-9. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Meador K. J. Language and Memory Testing. In: Fisch B., editor. Principles and practices of electrophysiological and video monitoring in epilepsy and intensive care. New York: Demos Medical Publishing; 2009. pp. 369–378. [Google Scholar]
- Loring D. W., Meador K. J. The Wada test: Current perspectives and applications. In: Barr W. B., Morrison C., editors. Handbook on the neuropsychology of epilepsy. New York: Springer; 2010. [Google Scholar]
- Loring D. W., Meador K. J., Lee G. P. Amobarbital dose effects on Wada memory testing. Journal of Epilepsy. 1992;5:171–174. doi:10.1016/S0896-6974(05)80136-7. [Google Scholar]
- Loring D. W., Meador K. J., Lee G. P., King D. W. Amobarbital effects and lateralized brain function: The Wada test. New York, NY: Springer; 1992. [Google Scholar]
- Loring D. W., Meador K. J., Lee G. P., King D. W., Gallagher B. B., Murro A. M., et al. Stimulus timing effects on Wada memory testing. Archives of Neurology. 1994;51(8):806–810. doi: 10.1001/archneur.1994.00540200086020. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Meador K. J., Lee G. P., King D. W., Nichols M. E., Park Y. D., et al. Wada memory asymmetries predict verbal memory decline after anterior temporal lobectomy. Neurology. 1995;45(7):1329–1333. doi: 10.1212/wnl.45.7.1329. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Meador K. J., Lee G. P., Nichols M. E., King D. W., Gallagher B. B., et al. Wada memory performance predicts seizure outcome following anterior temporal lobectomy. Neurology. 1994;44(12):2322–2324. doi: 10.1212/wnl.44.12.2322. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Meador K. J., Williamson D. J., Wiegand F., Hulihan J. F. Topiramate Dose Effects on Neuropsychological Function: Analysis from a Randomized Double-Blind Placebo-Controlled Study; Presented at the Annual Meeting of the American Epilepsy Society; Philadelphia. 2007. [Google Scholar]
- Loring D. W., Strauss E., Hermann B. P., Barr W. B., Perrine K., Trenerry M. R., et al. Differential Neuropsychological Test sensitivity to left temporal lobe epilepsy. Journal of the International Neuropsychological Society. 2008;14(3):394–400. doi: 10.1017/S1355617708080582. [DOI] [PubMed] [Google Scholar]
- Loring D. W., Strauss E., Hermann B. P., Perrine K., Trenerry M. R., Barr W. B., et al. Effects of anomalous language representation on neuropsychological performance in temporal lobe epilepsy. Neurology. 1999;53(2):260–264. doi: 10.1212/wnl.53.2.260. [DOI] [PubMed] [Google Scholar]
- Meador K. J. Cognitive effects of epilepsy and of antiepileptic medications. In: Wyllie E., editor. The treatment of epilepsy. Baltimore: Williams & Wilkins; 2005. pp. 1215–1226. [Google Scholar]
- Meador K. J., Loring D. W., Allen M. E., Zamrini E. Y., Moore E. E., Abney O. L., et al. Comparative cognitive effects of carbamazepine and phenytoin in healthy adults. Neurology. 1991;41(10):1537–1540. doi: 10.1212/wnl.41.10.1537. [DOI] [PubMed] [Google Scholar]
- Meador K. J., Loring D. W., Flaningin H. F. History of epilepsy surgery. Journal of Epilepsy. 1989;2 doi:10.1016/0896-6974(89)90054-6. [Google Scholar]
- Meador K. J., Loring D. W., Huh K., Gallagher B. B., King D. W. Comparative cognitive effects of anticonvulsants. Neurology. 1990;40(3 Pt 1):391–394. doi: 10.1212/wnl.40.3_part_1.391. [DOI] [PubMed] [Google Scholar]
- Meador K. J., Loring D. W., Hulihan J. F., Kamin M., Karim R. Differential cognitive and behavioral effects of topiramate and valproate. Neurology. 2003;60(9):1483–1488. doi: 10.1212/01.wnl.0000063308.22506.19. [DOI] [PubMed] [Google Scholar]
- Meador K. J., Loring D. W., Lee G. P., Brooks B. S., Thompson E. E., Thompson W. O., et al. Right cerebral specialization for tactile attention as evidenced by intracarotid sodium amytal. Neurology. 1988;38(11):1763–1766. doi: 10.1212/wnl.38.11.1763. [DOI] [PubMed] [Google Scholar]
- Meador K. J., Loring D. W., Moore E. E., Thompson W. O., Nichols M. E., Oberzan R. E., et al. Comparative cognitive effects of phenobarbital, phenytoin, and valproate in healthy adults. Neurology. 1995;45(8):1494–1499. doi: 10.1212/wnl.45.8.1494. [DOI] [PubMed] [Google Scholar]
- Meador K. J., Loring D. W., Ray P. G., Motamedi G. Cognitive and behavioral effects of carbamazepine and levetiracetam in healthy volunteers. Neurology. 2006;66((Suppl. 2)):A72. [Google Scholar]
- Meador K. J., Loring D. W., Ray P. G., Murro A. M., King D. W., Perrine K. R., et al. Differential cognitive and behavioral effects of carbamazepine and lamotrigine. Neurology. 2001;56:1177–1182. doi: 10.1212/wnl.56.9.1177. [DOI] [PubMed] [Google Scholar]
- Meador K. J., Loring D. W., Vahle V. J., Ray P. G., Werz M. A., Fessler A. J., et al. Cognitive and behavioral effects of lamotrigine and topiramate in healthy volunteers. Neurology. 2005;64(12):2108–2114. doi: 10.1212/01.WNL.0000165994.46777.BE. doi:10.1212/01.WNL.0000165994.46777.BE. [DOI] [PubMed] [Google Scholar]
- Meyer V., Yates A. J. Intellectual changes following temporal lobectomy for psychomotor epilepsy: Preliminary communication. Journal of Neurology, Neurosurgery and Psychiatry. 1955;18:44–52. doi: 10.1136/jnnp.18.1.44. doi:10.1136/jnnp.18.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Brenda Milner. In: Squire L. R., editor. Neuroscience in autobiography. New York: Academic Press; 1998. pp. 178–395. [Google Scholar]
- Milner B., Branch C., Rasmussen T. Study of short-term memory after intracarotid injection of sodium amytal. Transactions of the American Neurological Association. 1962;87:224–226. [Google Scholar]
- Novelly R. A. The debt of neuropsychology to the epilepsies. American Psychologist. 1992;47(9):1126–1129. doi: 10.1037//0003-066x.47.9.1126. doi:10.1037/0003-066X.47.9.1126. [DOI] [PubMed] [Google Scholar]
- Penfield W., Flanigin H. Surgical therapy of temporal lobe seizures. AMA Arch Neurol Psychiatry. 1950;64(4):491–500. doi: 10.1001/archneurpsyc.1950.02310280003001. [DOI] [PubMed] [Google Scholar]
- Penfield W., Jasper H. Epilepsy and the functional anatomy of the human brain. Boston: Little, Brown; 1954. [Google Scholar]
- Penfield W., Jasper H. In J. P. Cordeau & P. Gloor (Eds.). Recent contributions to neurophysiology: International Symposium in Neurosciences in Honor of Herbert H. Jasper. EEG and Clinical Neurophysiology. 1972;Suppl. 31:9–12. [Google Scholar]
- Penfield W., Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. Archives of Neurology and Psychiatry. 1958;79:475–497. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- Perrine K., Hermann B. P., Meador K. J., Vickrey B. G., Cramer J. A., Hays R. D., et al. The relationship of neuropsychological functioning to quality of life in epilepsy. Archives of Neurology. 1995;52(10):997–1003. doi: 10.1001/archneur.1995.00540340089017. [DOI] [PubMed] [Google Scholar]
- Perrine K., Westerveld M., Sass K. J., Devinsky O., Dogali M., Spencer D. D., et al. Wada memory disparities predict seizure laterality and postoperative seizure control. Epilepsia. 1995;36(9):851–856. doi: 10.1111/j.1528-1157.1995.tb01627.x. doi:10.1111/j.1528-1157.1995.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Piotrowska N., Winkler P. A. Otfrid Foerster, the great neurologist and neurosurgeon from Breslau (Wroclaw): His influence on early neurosurgeons and legacy to present-day neurosurgery. Journal of Neurosurgery. 2007;107(2):451–456. doi: 10.3171/JNS-07/08/0451. [DOI] [PubMed] [Google Scholar]
- Preul M. C., Stratford J., Bertrand G., Feindel W. Neurosurgeon as innovator: William V. Cone (1897–1959) Journal of Neurosurgery. 1993;79(4):619–631. doi: 10.3171/jns.1993.79.4.0619. [DOI] [PubMed] [Google Scholar]
- Rausch R., Babb T. L. Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Archives of Neurology. 1993;50(8):812–817. doi: 10.1001/archneur.1993.00540080023008. [DOI] [PubMed] [Google Scholar]
- Sarikcioglu L. Otfrid Foerster (1873–1941): One of the distinguished neuroscientists of his time. Journal of Neurology, Neurosurgery and Psychiatry. 2007;78(6):650. doi: 10.1136/jnnp.2006.112680. doi:10.1136/jnnp.2006.112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M., Jones-Gotman M., Zatorre R. J., Petrides M., Evans A. C. A role for the right anterior temporal lobe in taste quality recognition. Journal of Neuroscience. 1997;17(13):5136–5142. doi: 10.1523/JNEUROSCI.17-13-05136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. G. A comparison of some mental and physical tests in their application to epileptic and to normal subjects. British Journal of Psychology. 1905;2:240–260. [Google Scholar]
- Strauss E., Hunter M., Hermann B. P., Loring D. W., Trenerry M. R., Barr W. B., et al. Differential rates of age of seizure onset between sexes and between hemispheres? Journal of the International Neuropsychological Society. 1997;3(5):428–434. [PubMed] [Google Scholar]
- Strauss E., Loring D., Chelune G., Hunter M., Hermann B., Perrine K., et al. Predicting cognitive impairment in epilepsy: Findings from the Bozeman epilepsy consortium. Journal of Clinical and Experimental Neuropsychology. 1995;17(6):909–917. doi: 10.1080/01688639508402439. doi:10.1080/01688639508402439. [DOI] [PubMed] [Google Scholar]
- Strauss E., Semenza C., Hunter M., Hermann B., Barr W., Chelune G., et al. Left anterior lobectomy and category-specific naming. Brain and Cognition. 2000;43(1–3):403–406. [PubMed] [Google Scholar]
- Taylor D. C. One hundred years of epilepsy surgery: Sir Victor Horsley's contribution. In: Engel, J. Jr, editor. Surgical treatment of the epilepsies. New York: Raven Press; 1987. pp. 7–11. [Google Scholar]
- Trenerry M. R., Herman B. P., Barr W. B., Chelune G. J., Loring D. W., Perrine K., et al. MMPI scale elevations before and after right and left temporal lobectomy. Assessment. 1996;3:307–315. doi:10.1177/1073191196003003010. [Google Scholar]
- Wada J. A. Youthful season revisited. Brain and Cognition. 1997;33:7–13. doi: 10.1006/brcg.1997.0879. doi:10.1006/brcg.1997.0879. [DOI] [PubMed] [Google Scholar]
- Wallin J. E. W. Eight months of psycho-clinical research at the New Jersey State Village for Epileptics, with some results from the Binet–Simon testing. Epilepsia. 1912a;A3:366–380. doi:10.1111/j.1528-1157.1912.tb03046.x. [Google Scholar]
- Westerveld M., Sass K. J., Chelune G. J., Hermann B. P., Barr W. B., Loring D. W., et al. Temporal lobectomy in children: Cognitive outcome. Journal of Neurosurgery. 2000;92(1):24–30. doi: 10.3171/jns.2000.92.1.0024. doi:10.3171/jns.2000.92.1.0024. [DOI] [PubMed] [Google Scholar]
- Wilde N., Strauss E., Chelune G. J., Loring D. W., Martin R. C., Hermann B. P., et al. WMS-III performance in patients with temporal lobe epilepsy: Group differences and individual classification. Journal of the International Neuropsychological Society. 2001;7(7):881–891. [PubMed] [Google Scholar]
- Wilde N. J., Strauss E., Chelune G. J., Hermann B. P., Hunter M., Loring D. W., et al. Confirmatory factor analysis of the WMS-III in patients with temporal lobe epilepsy. Psychological Assessment. 2003;215(1):56–63. doi: 10.1037/1040-3590.15.1.56. [DOI] [PubMed] [Google Scholar]
- York G. K., 3rd, Steinberg D. A. Hughlings Jackson's suggestion for the treatment of epilepsy. Neurology. 2009;73(14):1155–1158. doi: 10.1212/WNL.0b013e3181bacec7. doi:10.1212/WNL.0b013e3181bacec7. [DOI] [PubMed] [Google Scholar]
- Zamrini E. Y., Meador K. J., Loring D. W., Nichols F. T., Lee G. P., Figueroa R. E., et al. Unilateral cerebral inactivation produces differential left/right heart rate responses. Neurology. 1990;40(9):1408–1411. doi: 10.1212/wnl.40.9.1408. [DOI] [PubMed] [Google Scholar]