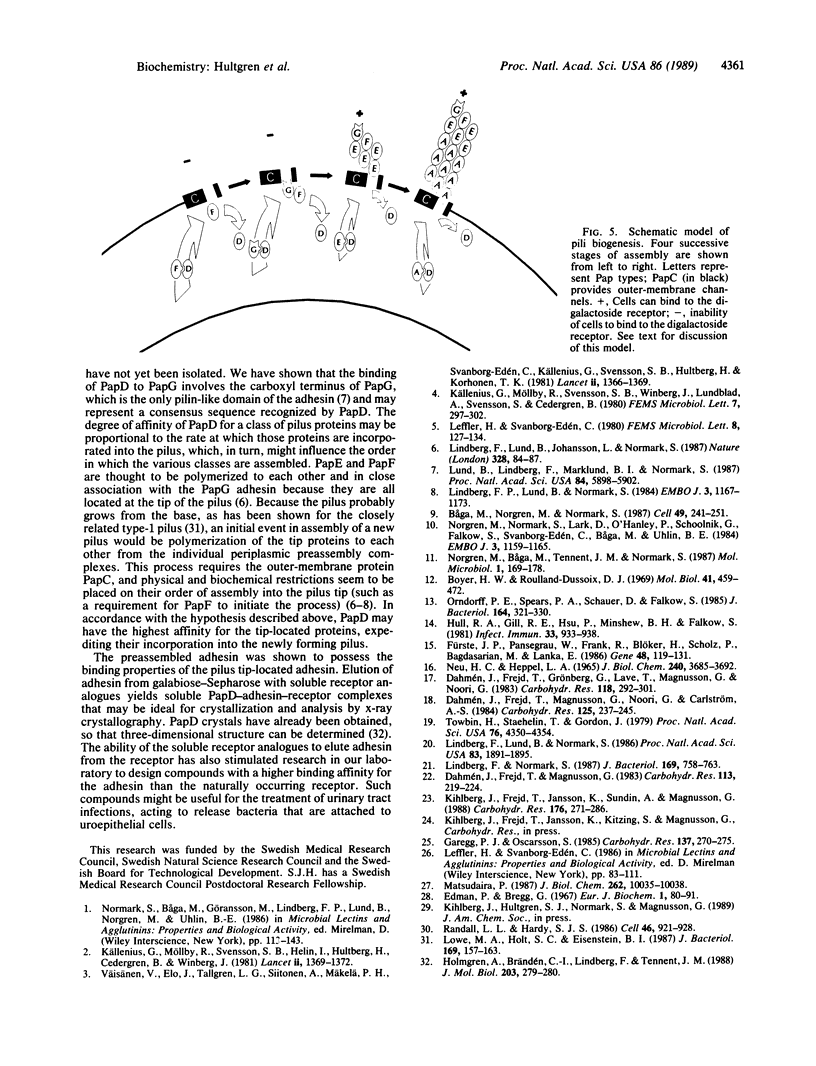
Abstract
Most uropathogenic strains of Escherichia coli produce heteropolymeric organelles, known as P pili, that bind to the globoseries of glycolipids present in the urinary tract. The formation of a P pilus is the result of a family of related proteins being coordinately assembled into the structure in a defined order with the adhesin located exclusively at the tip. The preassembled digalactoside alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin was purified to homogeneity from the periplasmic space in a complex with the periplasmic assembly protein PapD by affinity chromatography to alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-Sepharose. A receptor-binding domain was mapped to the amino-terminal half of the adhesin. The interaction of PapD with PapG, which was required for the incorporation of the adhesin into the pilus, was found to protect PapG from proteolytic cleavages and enhanced the processing of the PapG signal peptide. A preassembly domain necessary for forming a complex with PapD was mapped to the carboxyl terminus of PapG.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Båga M., Norgren M., Normark S. Biogenesis of E. coli Pap pili: papH, a minor pilin subunit involved in cell anchoring and length modulation. Cell. 1987 Apr 24;49(2):241–251. doi: 10.1016/0092-8674(87)90565-4. [DOI] [PubMed] [Google Scholar]
- Dahmén J., Frejd T., Magnusson G., Noori G., Carlström A. S. 2-Bromoethyl glycosides in glycoside synthesis: preparation of glycoproteins containing alpha-L-Fuc-(1----2)-D-Gal and beta-D-Gal-(1----4)-D-GlcNAc. Carbohydr Res. 1984 Feb 15;125(2):237–245. doi: 10.1016/0008-6215(84)85159-9. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Brändén C. I., Lindberg F., Tennent J. M. Preliminary X-ray study of papD crystals from uropathogenic Escherichia coli. J Mol Biol. 1988 Sep 5;203(1):279–280. doi: 10.1016/0022-2836(88)90109-x. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlberg J., Frejd T., Jansson K., Sundin A., Magnusson G. Synthetic receptor analogues: preparation and calculated conformations of the 2-deoxy, 6-O-methyl, 6-deoxy, and 6-deoxy-6-fluoro derivatives of methyl 4-O-alpha-D-galactopyranosyl-beta-D-galactopyranoside (methyl beta-D-galabioside). Carbohydr Res. 1988 May 15;176(2):271–286. doi: 10.1016/0008-6215(88)80138-1. [DOI] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Helin I., Hultberg H., Cedergren B., Winberg J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981 Dec 19;2(8260-61):1369–1372. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- Lindberg F. P., Lund B., Normark S. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 1984 May;3(5):1167–1173. doi: 10.1002/j.1460-2075.1984.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Normark S. Gene products specifying adhesion of uropathogenic Escherichia coli are minor components of pili. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1891–1895. doi: 10.1073/pnas.83.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Normark S. Common mechanism of ampC beta-lactamase induction in enterobacteria: regulation of the cloned Enterobacter cloacae P99 beta-lactamase gene. J Bacteriol. 1987 Feb;169(2):758–763. doi: 10.1128/jb.169.2.758-763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M. A., Holt S. C., Eisenstein B. I. Immunoelectron microscopic analysis of elongation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1987 Jan;169(1):157–163. doi: 10.1128/jb.169.1.157-163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Norgren M., Båga M., Tennent J. M., Normark S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol. 1987 Sep;1(2):169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Spears P. A., Schauer D., Falkow S. Two modes of control of pilA, the gene encoding type 1 pilin in Escherichia coli. J Bacteriol. 1985 Oct;164(1):321–330. doi: 10.1128/jb.164.1.321-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986 Sep 12;46(6):921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]