Abstract
Objective
Meniscal tears are a common knee injury and increased levels of interleukin-1 (IL-1) have been measured in injured and degenerated joints. Studies have shown that IL-1 decreases the shear strength, cell accumulation, and tissue formation in meniscal repair interfaces. While mechanical stress and IL-1 modulate meniscal biosynthesis and degradation, the effects of dynamic loading on meniscal repair are unknown. The purpose of this study was to determine the effects of mechanical compression on meniscal repair under normal and inflammatory conditions.
Experimental Design
Explants were harvested from porcine medial menisci. To simulate a full-thickness defect, a central core was removed and reinserted. Explants were loaded for 4 hours/day at 1Hz and 0 – 26% strain for 14 days in the presence of 0 or 100pg/mL of IL-1. Media were assessed for matrix metalloproteinase (MMP) activity, aggrecanase activity, sulfated glycosaminoglycan (S-GAG) release, and nitric oxide (NO) production. After 14 days, biomechanical testing and histological analyses were performed.
Results
IL-1 increased MMP activity, S-GAG release, and NO production, while decreasing the shear strength and tissue repair in the interface. Dynamic loading antagonized IL-1-mediated inhibition of repair at all strain amplitudes. Neither IL-1 treatment nor strain altered aggrecanase activity. Additionally, strain alone did not alter meniscal healing, except at the highest strain magnitude (26%), a level that enhanced the strength of repair.
Conclusions
Dynamic loading blocked the catabolic effects of IL-1 on meniscal repair, suggesting that joint loading through physical therapy may be beneficial in promoting healing of meniscal lesions under inflammatory conditions.
Keywords: meniscus, tissue repair, mechanical loading, interleukin-1, matrix metalloproteinase, aggrecanase, nitric oxide
Introduction
The menisci are C-shaped fibrocartilaginous tissues situated between the femoral condyles and tibial plateau. These tissues are required for normal biomechanical function of the knee, including load bearing, shock absorption, joint congruity, and joint stability1–3. The intact meniscus has been suggested to carry approximately 50% of the load applied to the joint4, resulting in approximately 10% strain in the medial meniscus5. Tears in the meniscus are a common knee injury with nearly 15% of all athletic knee injuries involving the meniscus6. In addition to the pain and loss of function associated with the initial meniscal injury, damage or loss of meniscal tissue is associated with progressive degenerative changes in the joint that ultimately lead to osteoarthritis (OA)7–11. Long term studies have shown that 50% of patients with a meniscal injury develop OA within 10 – 20 years12. Therefore, current clinical therapies for meniscal injury seek to preserve and repair damaged tissue.
The pathogenesis of osteoarthritic degeneration following knee injury appears to involve the anti-anabolic and pro-catabolic activity of inflammatory cytokines, such as interleukin-1 (IL-1), which are upregulated in injured and degenerated joints13, 14. In patients without rheumatic disease or joint effusion, less than 20 pg/mL IL-1β has been measured in the joint at necropsy15. On the other hand, in patients undergoing an arthroscopy following a meniscal tear, the concentration of synovial fluid IL-1α is between 25 – 175 pg/mL16. IL-1β levels are also elevated in patients with rheumatoid arthritis (RA; 115 pg/mL–193 pg/mL) and OA (21 pg/mL–146 pg/mL)15, 17. Additionally, full thickness meniscal tears show elevated IL-1α staining for at least 14 days18, suggesting that IL-1α may be produced for several weeks after the initial meniscal tear.
IL-1 upregulation in the joint induces many degradative and pro-inflammatory pathways13, 14, 19, 20. IL-1 increases the production of the inflammatory mediators nitric oxide (NO)21–25 and prostaglandin E2 (PGE2)21, 24, 26 in a dose-dependent manner. Additionally, IL-1 upregulates matrix metalloproteinases (MMPs)23, 24, 27, 28, increases proteoglycan release22, 24, suppresses collagen synthesis24, and upregulates tumor necrosis factor (TNF) expression23 in meniscal cells and explants. IL-1 also upregulates aggrecanase-1 (ADAMTS-4) activity but not aggrecanase-2 (ADAMTS-5) activity in bovine cartilage explants29 and porcine chondrocyte-agarose cultures30, 31. Both acute32 and chronic33, 34 exposure to IL-1 inhibits integrative meniscal repair in vitro by decreasing the shear strength of repair and suppressing cell accumulation and tissue formation in the interface. In an explant model of meniscal repair, treatment with IL-1 receptor antagonist (IL-1ra)33, MMP inhibitors28, or the anabolic growth factor TGF-β135 has shown promise in overcoming some of the degradative effects of IL-1.
In addition to changes in the biochemical environment of the joint, trauma or arthritis can also alter the biomechanical environment of the joint. In articular cartilage, many studies have shown that mechanical stress in the joint is an important factor in the regulation of chondrocyte activity36. The general consensus of these studies is that static compression suppresses matrix biosynthesis, while cyclic or intermittent loading at specific frequencies can stimulate chondrocyte metabolism (36 for review). However, little information is available regarding the response of meniscal cells to mechanical stress. Meniscal explants subjected to static compression showed decreased expression of type I and II collagen37, increased MMP-1 expression37, and decreased biosynthetic activity, as measured by 35S-sulfate and 3H-proline incorporation38. Dynamic mechanical compression increases proteoglycan and total protein synthesis22 and NO and PGE2 production by meniscal explants39, 40. Additionally 20% compressive strain of porcine explants caused increased expression of the following catabolic mediators: inducible nitric oxide synthase (NOS2), IL-1α, MMP-1, MMP-3, MMP-13, and a disintegrin and metalloproteinase with thrombospondin 4 (ADAMTS-4)41, 42. On the other hand, in the presence of IL-1, mechanical compression inhibits NO production22. Biaxial cyclic tensile stretch of isolated meniscal cells inhibited the catabolic and pro-inflammatory effects of IL-123, 43 by inhibiting NOS2, MMP expression, and NO production. Biaxial stretch also counteracted IL-1-dependent stimulation of NOS2, RANK, and RANKL in meniscal cells, potentially via inhibition of NF-κB23, 43. These findings suggest that mechanical loading can overcome many of the inflammatory effects of cytokines on meniscal cells; however, the effects of mechanical compression on repair of meniscal lesions are not known.
The goal of this study was to investigate the effects of dynamic mechanical compression on meniscal repair in the presence or absence of the inflammatory cytokine IL-1. We hypothesized that mechanical compression at lower magnitudes (<10%) would enhance meniscal repair under both normal and inflammatory conditions, but higher magnitudes (>10%) of dynamic compression would inhibit repair. We used an in vitro model system28, 32–34, 44 to examine the effects of 0 – 26% dynamic compressive strain on the integrative repair of meniscal explants treated either with or without IL-1. Explants were dynamically loaded for 14 days and the media was assessed for MMP activity, aggrecanase activity, sulfated glycosaminoglycan (S-GAG) release, and NO production. Meniscus healing was investigated by mechanical testing of the explants to determine the interfacial shear strength, and histology was performed to visualize tissue repair.
Materials and methods
MENISCAL REPAIR MODEL SYSTEM
An explant model system was used to study integrative repair of the meniscus44. Medial menisci were harvested from 2–3 year old skeletally mature female pig knees obtained from a local abattoir. Cylindrical explants were harvested from the femoral surface of the middle one-third of the meniscus, using a 5 mm biopsy punch (Miltex, Inc, York, PA) oriented perpendicular to the meniscal surface. Using a custom built cutting device, explants were cut to an average thickness of 2.4 mm. A central 3 mm core was punched completely through each explant, using a biopsy punch (Miltex, Inc), and immediately reinserted in the same orientation to simulate a full thickness meniscal tear. Explants were placed in Costar ultra-low attachment surface 24 well plates (Corning Inc.; Corning, NY) in the center of polyacetal retaining rings. Samples were incubated in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA), containing 1000 U/mL penicillin/streptomycin (Invitrogen) for 1 hour at 37°C. This medium was removed, and explants were cultured in DMEM containing 10% heat inactivated fetal bovine serum (HyClone, Logan, UT), 0.1 mM non-essential amino acids (Invitrogen), 10 mM HEPES buffer solution (Invitrogen), 100 U/mL penicillin/streptomycin, and 37.5 µg/mL L-ascorbic acid 2-phosphate (Sigma-Aldrich, St. Louis, MO). Explants were cultured in a mechanical compression bioreactor for 14 days at 37°C and 5% CO2. Media were collected and replaced every 3 days.
MECHANICAL COMPRESSION BIOREACTOR
A closed-loop displacement controlled bioreactor was designed and manufactured to apply dynamic, unconfined compressive deformational loading to 24 specimens simultaneously. The instrument consists of a rotary stepper motor (Oriental Motor, Torrance, CA) set to microstep at 4000 steps/rev (0.09°/step) with an optical encoder for closed-loop feedback control. The stepper motor drives a linear stage (Danaher Motion, Wood Dale, IL) that is connected to a loading platform housing 24 individual polyacetal pistons. The pistons were designed such that the posts slip freely through the loading platform if not locked in place, ensuring that all pistons are in contact with their respective explant prior to being locked to the plate. The design of the instrument and the written code (LabView 6.1, National Instruments, Austin, TX) allow for precise displacement of the loading platform with sub-micron resolution.
Explants (n ≥ 9 per treatment group, each from different porcine menisci) were cultured in the mechanical compression bioreactor at 37°C/5% CO2 and loaded sinusoidally at 1 Hz to strains of 1% ± 0.2% (mean ± standard deviation), 10% ± 1%, or 26% ± 4% for 4 hours/day for 14 days. Unstrained samples were cultured in the bioreactor in parallel with strained samples; however, the pistons were locked into place approximately 1 mm above the explants. For each strain, explants received either control media or media containing 100 pg/mL recombinant porcine IL-1 α (R & D Systems, Minneapolis, MN).
MMP ACTIVITY ASSAY
Total specific MMP activity was measured in media samples from day 3 using the quenched fluorogenic substrate Dab-Gly-Pro-Leu-Gly-Met-Arg-Gly-Lys-Flu (New England Peptide; Gardner, MA) as described previously28, 32, 45.
AGGRECANASE ACTIVITY ASSAY
Aggrecanase activity was measured in media samples from day 3, using a commercially available fluorescent substrate (Anaspec; San Jose, CA) specific for ADAMTS-4, as described previously 46. Samples were run in duplicate and incubated at 37°C for 30 minutes in the dark. Fluorescence was measured at 340 nm excitation and 405 nm emission. The data is expressed as fluorescence units (measured fluorescence minus the fluorescence in the blank media) corrected for the wet weight of the tissue. Recombinant human ADAMTS-4 (5 µg/mL; Anaspec) was included as a positive control for the assay. Additionally, porcine cartilage explants that were treated for 72 hours with 1 ng/mL IL-1α were used to confirm cross-reactivity of the substrate with porcine ADAM-TS4.
S-GAG RELEASE
Total S-GAG release was measured in day 3 media, using the 1,9-dimethylmethylene blue (DMB) assay47. For this assay, standards ranging from 0 – 100 µg/mL bovine trachea chondroitin-4-sulfate type A (Sigma, St. Louis, MO) were prepared in control media. Forty microliters of standards and samples and 125 µl/well of DMB reagent were added to a 96 well plate in duplicate. Absorbance was read at 540 nm within 5 min of DMB addition and the total µg of S-GAG released from each sample was corrected for the wet weight of the tissue.
NO RELEASE
Total NO production was assessed by measuring the concentration of total nitrate and nitrite (or NOx) in day 3 media, as detailed previously48. All media samples and standards (0 – 320 µM NaNO3 in culture media) were filtered through Micron Ultracel YM-10 filters (Millipore, Bedford, MA) and diluted 1:2 in dH2O to eliminate interference of protein and media components. All nitrate was then converted to nitrite, using nitrate reductase (Roche Diagnostics; Mannheim, Germany), and total nitrite concentrations were determined using the Greiss reagent. Absorbance was read at 540 nm and total µmol NOx were normalized to the wet weight of the tissue.
BIOMECHANICAL TESTING
Interfacial shear strength of repair between the inner core and outer ring of meniscal repair model explants was determined after 14 days in culture, using a push-out test as described previously44. The peak force at failure was normalized by the area of the interface between the inner core and outer ring to determine interfacial shear strength. Measured sample thickness was used to determine the actual surface-to-surface compressive strain applied to each explant during deformational loading.
HISTOLOGICAL STAINING
Meniscal repair model explants were processed for histology after 14 days in culture. Samples were fixed overnight in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), containing 100 mM sodium cacodylate trihydrate (Electron Microscopy Sciences) at pH 7.4 and 4°C. Samples were dehydrated in EtOH, infiltrated with xylene, and paraffin embedded. Sections were stained with Harris Hematoxylin with glacial acetic acid (Poly Scientific, Bay Shore, NY), 0.02% aqueous fast green (Sigma-Aldrich), and Accustain Safranin O solution (Sigma-Aldrich).
STATISTICAL ANALYSES
Statistical analyses were performed using Statistica 7.0 (StatSoft Inc., Tulsa, OK). A two-factor ANOVA and the Newman-Keuls post hoc test were performed to determine significant differences (α=0.05) and the interactive effect of strain and IL-1. The relationship between interfacial shear strength and actual applied strain was determined using linear regression.
Results
MMP activity in the media of meniscal repair model explants was increased by IL-1 treatment in the absence of strain (Figure 1; p < 0.0005). However, application of strain at 1%, 10%, and 26% blocked the increase in MMP activity due to IL-1 (p < 0.05). Inhibition of IL-1 mediated MMP activity was most pronounced at lower strains (p < 0.0005 for 1% and 10%), as compared to 26% strain (p < 0.05). Additionally at both 1% and 10% strains, there was an interactive effect of strain and IL-1 on MMP activity (p < 0.001). In the absence of IL-1, there was no change in MMP activity at any of the strains tested.
Fig. 1.
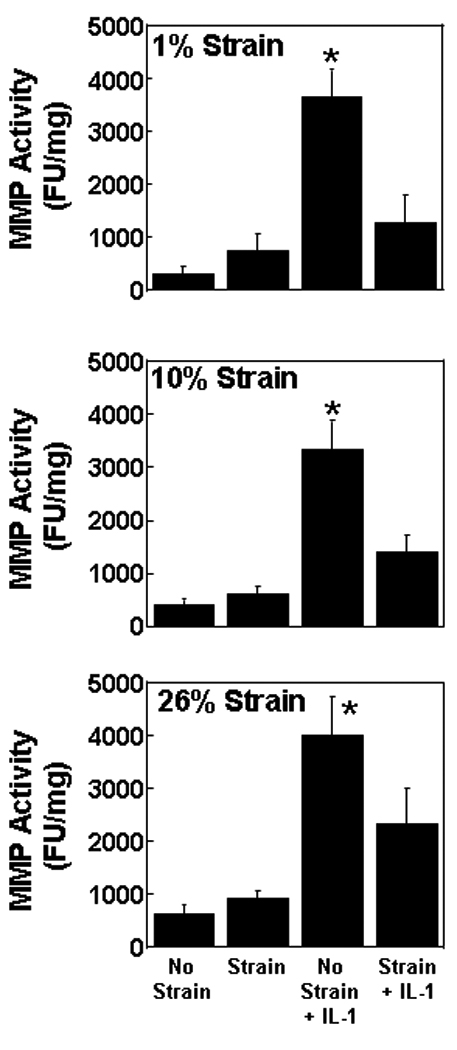
Matrix metalloproteinase (MMP) activity in the media of meniscal repair model explants that were exposed to no strain, strain, no strain + IL-1, or strain + IL-1. The strains tested were 1% (top panel), 10% (middle panel), and 26% (bottom panel). The MMP activity is expressed as fluorescence units (FU) per mg wet weight + standard error. *: p < 0.05 compared to all other treatments.
Aggrecanase activity was measurable in all treatment groups (Figure 2). However, neither IL-1 treatment nor strain altered aggrecanase activity, as compared to control samples. The combination of IL-1 and strain also did not affect aggrecanase activity.
Fig. 2.
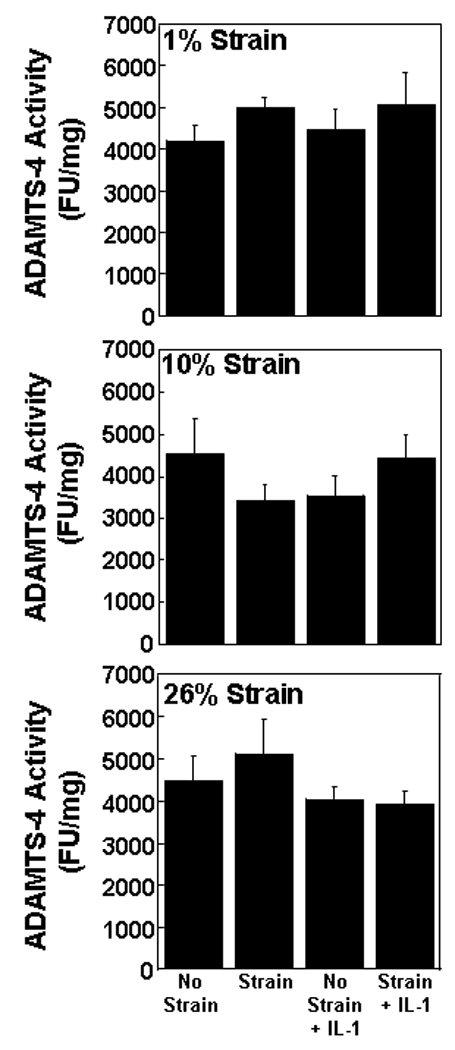
ADAMTS-4 activity in the media of meniscal repair model explants that were exposed to no strain, strain, no strain + IL-1, or strain + IL-1. The strains tested were 1% (top panel), 10% (middle panel), and 26% (bottom panel). The aggrecanase activity is expressed as fluorescence units (FU) per mg wet weight + standard error.
In the absence of strain, S-GAG release into the media was enhanced approximately 5-fold by IL-1 treatment (Figure 3; p < 0.0005). Strains of both 1% and 10% were effective in reducing IL-1 mediated release of S-GAG (p ≤ 0.005). However, 26% strain had no effect on the S-GAG release in the presence of IL-1. At both 1% and 10% strains there was an interactive effect of strain and IL-1 (p < 0.05). For all strains there was a significant effect of IL-1 that contributed to S-GAG release into the media from the meniscal repair explants (p < 0.00001). None of the strains tested altered the S-GAG release into the media in the absence of IL-1.
Fig. 3.
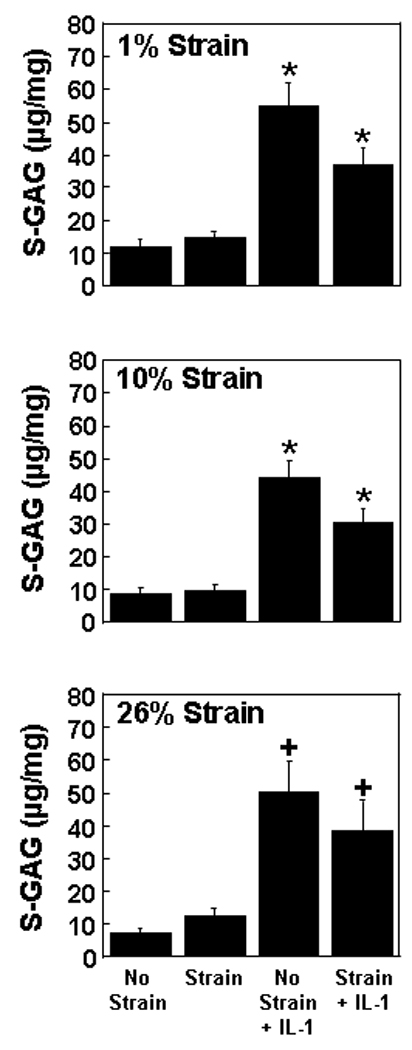
Sulfated glycosaminoglycan (S-GAG) release into the media of meniscal repair model explants that were exposed to no strain, strain, no strain + IL-1, or strain + IL-1. The strains tested were 1% (top panel), 10% (middle panel), and 26% (bottom panel). The S-GAG release is expressed as µg S-GAG per mg wet weight + standard error. *: p ≤ 0.005 compared to all other treatments. +: p < 0.01 compared to no strain and strain.
NO release into the media was increased by IL-1 treatment in the absence of strain (Figure 4; p < 0.0005). However, all tested strains reduced the IL-1 mediated NO release (p < 0.005). At all strains, there was an interactive effect of strain and IL-1 that contributed to NO release into the media (p < 0.05). In the absence of IL-1, none of the strains tested altered the NO release into the media from meniscal repair explants.
Fig. 4.
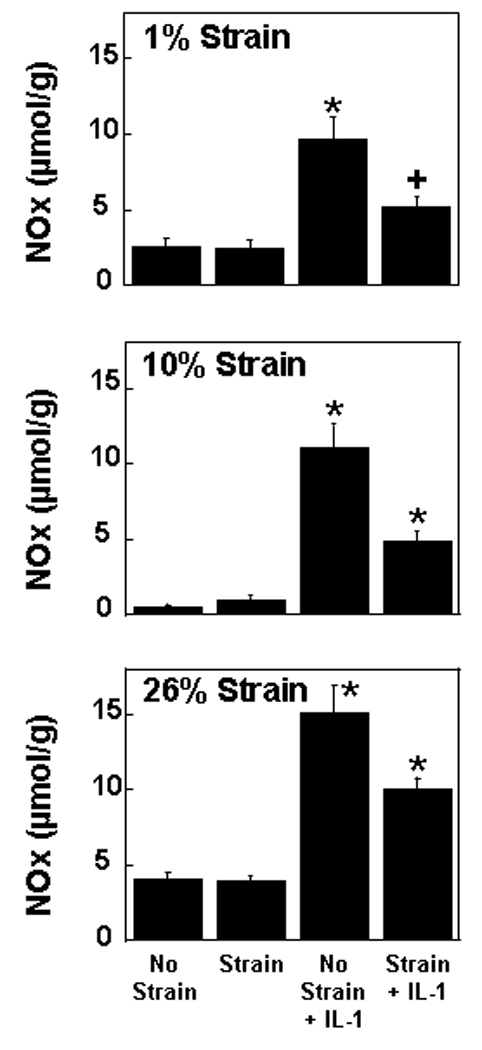
Nitric oxide (NO) release into the media of meniscal repair model explants that were exposed to no strain, strain, no strain + IL-1, or strain + IL-1. The strains tested were 1% (top panel), 10% (middle panel), and 26% (bottom panel). The NO release is expressed as µmol per g wet weight + standard error. *: p < 0.005 compared to all other treatments. +: p < 0.05 compared to no strain.
In the absence of strain, IL-1 decreased the integrative shear strength of repair in meniscal repair model samples (Figure 5; p < 0.05). Dynamic compression at 1%, 10% or 26% strain increased the shear strength of IL-1 treated explants to strengths that were comparable to that measured in unstrained explants (p ≤ 0.01). Additionally, there was a positive correlation between increasing shear strength in the presence of IL-1 and actual applied strain (r2 = 0.25; p < 0.00005). At 1% and 26% strains, there was a significant effect of cytokine (p < 0.05) with an average 47 % decrease in strength of repair, and at all strains there was a significant effect of loading (p < 0.005) that contributed to the integrative shear strength of repair. In the absence of IL-1, at 26% strain, there was a 2-fold increase in the shear strength of repair compared to unstrained samples (p < 0.005), but there was no effect of either 1% or 10% strains alone. Furthermore, there was also a positive correlation between increasing shear strength of repair and actual applied strain (r2 = 0.14, p < 0.005).
Fig. 5.
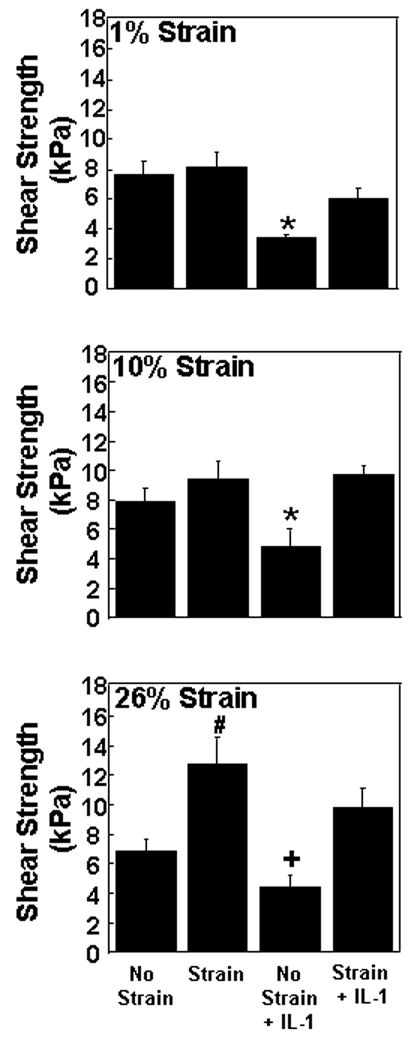
Integrative shear strength of repair for meniscal repair model explants that were exposed to no strain, strain, no strain + IL-1, or strain + IL-1. The strains tested were 1% (top panel), 10% (middle panel), and 26% (bottom panel). The shear strength of repair is expressed as kPa + standard error. *: p < 0.05 compared to all other treatments. #: p < 0.005 compared to no strain and no strain + IL-1. +: p < 0.01 compared to 26% strain + IL-1.
Histological analysis of unstrained and all strained meniscal repair model explants revealed healing of the meniscal defect at the interface over 14 days (Figure 6). The interface was filled with an extracellular matrix that stained strongly with fast green, revealing the presence of collagen fibers, and moderately with safranin O, indicating the presence of proteoglycans. On the other hand, no visible tissue repair was detected in IL-1 treated explants that were not strained. Additionally, IL-1 treatment resulted in loss of proteoglycan staining in the meniscal extracellular matrix. However, IL-1 treated explants that received 1%, 10%, or 26% strain showed increased tissue repair at the interface.
Fig. 6.
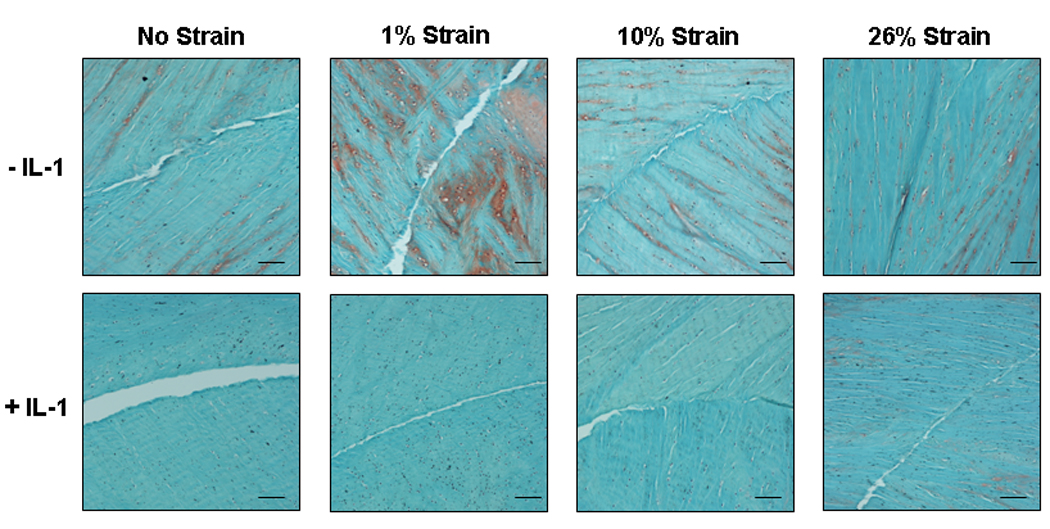
Histological images of paraffin embedded meniscal repair model explants stained with hematoxylin to identify the cell nuclei (black), fast green (green) to identify the collagen fibers, and safranin O (red) to stain proteoglycans. Samples were incubated with no IL-1 or 100 pg/mL IL-1 and subjected to 0%, 1%, 10%, or 26% strain. Scale bar is equal to 100µm.
Discussion
Our results demonstrate that dynamic mechanical loading can overcome some of the degradative effects of IL-1 on meniscal repair. Pathophysiologic concentrations of IL-1 caused increases in MMP activity, S-GAG release, NO production, decreases in tissue formation in the interface, and consequently decreases in the shear strength of repair. However, dynamic compression at 1%, 10%, or 26% strain blocked the IL-1 mediated inhibition of meniscal repair. Interestingly, mechanical loading under noninflammatory conditions did not alter the healing of meniscal lesions. These findings support our hypothesis that, in an inflammatory environment, meniscal repair was enhanced by low magnitudes of dynamic loading, but refute our hypothesis that high magnitudes of strain would inhibit meniscal repair. Additionally, our results refute the hypothesis that dynamic loading enhances repair under control (non-inflammatory) conditions, with the exception of the highest applied strain, a level that led to increased integrative shear strength of repair in the absence of IL-1. Our findings suggest that dynamic loading of the knee following a meniscal injury may be a beneficial physical therapy regime to promote healing of meniscal lesions and to combat the degradative effects of inflammation. However, further studies are necessary to translate this work in vivo and to elucidate the exact nature of the therapy.
The effects of biomechanical factors on cartilaginous tissues have been shown to be dependent on the type of load, magnitude, and frequency of loading. It has been estimated that in situ compressive strains range from approximately 10 – 15% in normal menisci4 and increase to 20% following 30 – 60% meniscectomy5. Using a similar compression device, McHenry et al. have shown that the application of 0.05 MPa or 0.1 MPa of dynamic compressive stress to porcine meniscal explants resulted in final measured strains of 10% or 20%, respectively49. At higher magnitudes of loading, meniscal explants appear to exhibit a pro-inflammatory response, characterized by increased production of NOS2 and prostaglandin E240. Thus, to examine a range of physiologic and hyper-physiologic conditions, we assessed the effects of dynamic compression at strains ranging from 0 – 26% at a frequency of 1 Hz.
In this study, loading of meniscal repair explants in the absence of IL-1 had no effect on MMP activity. Experiments on isolated chondrocytes and fibrochondrocytes have also shown that 6% cyclic tensile strain alone does not alter MMP activity50, 51. Other studies in superficial porcine meniscal explants have shown that expression of MMP-1, MMP-3, and MMP-13 was increased by 2 hours of 20% strain42. Additionally, bovine cartilage explants loaded at 0.5 MPa and 1 Hz for 3 hours showed increased MMP-2 and MMP-9 expression, activation, and activity52. Dynamic compression of porcine tibial plateau cartilage explants at 0.1 MPa and 0.5 Hz for 6 hours increased expression of both MMP-3 and its inhibitor the tissue inhibitor of metalloproteinase 2 (TIMP-2)53. Differences in our results and previous studies may be due to intrinsic tissue differences, duration of loading, type of loading, actual applied strain, frequency, and/or assessed outcome measures (enzyme activity versus gene expression).
In this study, dynamic compressive loading blocked IL-1 mediated increases in MMP activity in the media of meniscal repair model explants. In agreement with our results, 2% cyclic biaxial tensile strain of isolated rheumatoid synovial cells reduced the mRNA and protein levels of MMP-1 and MMP-13 and decreased the activity of these enzymes in the presence of IL-1β and use of a transcriptional inhibitor suggested that mechanical loading reduced the activity of the MMPs at a post-transcriptional level54. Biaxial cyclic tensile strain has also been shown to antagonize IL-1β effects in chondrocytes and temporomandibular fibrochondrocytes by reducing IL-1-mediated increases in MMP-1 mRNA and protein levels and increasing TIMP-2 expression50, 51. Loading-induced decreases in MMP expression and increases in TIMP expression are consistent with our results demonstrating a net decrease in MMP activity with loading in the presence of IL-1. However, the specific MMPs involved in IL-1 mediated degradation of meniscal repair explants are currently unknown. In a previous study, we have shown that the inhibition of individual MMPs was not sufficient to overcome the effects of IL-1; however, there was no specific inhibitor of MMP-1 activity available at the time28. In contrast, broad spectrum inhibition of MMP activity increased repair in the presence of IL-1. Thus either MMP-1 is driving the degradation due to IL-1 or a combination of MMPs are necessary to mediate these effects.
IL-1 increased S-GAG release into the media, presumably through the upregulation of MMPs, aggrecanases, and other degradative enzymes. ADAMTS-4 activity was not upregulated by IL-1, suggesting that this enzyme is not mediating the increase in S-GAG release. Our data is consistent with a recent study in immature bovine meniscus showing that inhibition of MMPs but not ADAMTS-4 and ADAMTS-5 was effective at blocking IL-1 mediated release of S-GAGs and decreases in mechanical properties55. However, bovine meniscus has been shown to upregulate aggrecanases in response to TNF-α treatment56. Previous studies have shown that ADAMTS-4 is the major aggrecanase upregulated by IL-1 in porcine chondrocytes 30. Therefore, in the presence of IL-1, MMPs are likely the predominant degradative enzymes causing the release of S-GAGs. However, it is possible that other aggrecanases or degradative enzymes may also be involved in the IL-1 mediated catabolism of the extracellular matrix.
Physiologic magnitudes (1% or 10%) of dynamic compressive strain blocked the IL-1-mediated release of S-GAG, while hyperphysiologic strain (26%) did not have a significant effect on GAG release in the presence of IL-1. High magnitudes of strain have previously been shown to be catabolic57, suggesting that 26% strain may be activating additional pathways that prevent loading from antagonizing the IL-1 mediated release of S-GAGs. Other studies have shown that 10% dynamic strain at 1 Hz for 3 hours/day of young bovine chondrocyte-seeded agarose constructs was not sufficient to block the IL-1 induced GAG loss58. The differences in our results are likely due to assessment of meniscal explants versus isolated chondrocytes in agarose. In our study, dynamic loading alone had no effect on S-GAG release into the media. However, other studies have shown that 2 hours of 20% strain at 1 Hz is sufficient to increase S-GAG release from porcine meniscal explants49. This difference may be due to the short term loading regime (only 1 day for 2 hours versus 14 days for 4 hours/day), as previous studies have shown that under normal culture conditions, meniscus explants show significant loss of S-GAG, which may obscure additional increases caused by mechanical loading or other stimuli33.
Dynamic loading of meniscal repair explants also had no effect on NO release. Experiments performed by Agarwal and colleagues using a variety of different cell types have consistently shown no effect of dynamic tensile strain on NOS2 expression23, 51 or NO production50. Furthermore, porcine meniscal explants subjected to 0 – 20% strain have shown no change in NO release41. However, other studies have shown conflicting results from decreased NO release by 15% strain of chondrocyte-agarose constructs59, 60 to increased NO production by porcine meniscal explants subjected to dynamic compressive stresses of 0.0125 – 0.5 MPa at 1 Hz for 24 hours39, 40. These differences are likely attributable to the different model systems and magnitudes of the applied stress or strain, particularly the overall duration of loading. In this study, dynamic loading suppressed the IL-1-mediated upregulation of NO release. In agreement with our results, many other studies have also demonstrated that dynamic compression and dynamic strain antagonize IL-1-induced NOS2 expression23, 51 and production of NO22, 50, 59, 60. Strain-induced suppression of the catabolic effects of IL-1 prevents degradation of meniscal extracellular matrix following meniscal injury, perhaps providing an opportunity for the intrinsic repair capacity of the tissue33, 34 to heal the meniscal lesion.
Interestingly, loading alone increased the shear strength of repair at the highest strain tested. In addition, the strain applied to each meniscal repair explant correlated with increased shear strength, suggesting that higher strains would be most beneficial to promote integrative meniscal repair. In the absence of strain, meniscal explants exhibited an intrinsic repair response that is consistent with previous studies in meniscus33, 44, 61 and articular cartilage62–65. The integrative repair of cartilage is dependent upon the migration and proliferation of viable cells into the defect62 and cell-mediated biosynthesis, including collagen production62, 64 and cross-linking65.
Many studies have analyzed the effects of mechanical loading and/or IL-1 on the expression and synthesis of both collagen and proteoglycans with mixed results. In explants of cartilage or meniscus, dynamic loading alone has been shown to have a wide range of effects from no change in aggrecan transcript levels37 or proteoglycan synthesis50, 51 to upregulation in their expression42, 53, 59 and synthesis60, 66. A similar range of effects have been reported with respect to collagen. Dynamic loading alone has been shown to cause no change in collagen I mRNA levels42, decreased type II collagen transcript levels37, no effect on collagen II transcription51, 59, increased collagen II expression53, and enhanced [3H]-thymidine incorporation60, 66. However, in the presence of IL-1, dynamic loading is generally anabolic, resulting in an overwhelming increase in expression59 and synthesis of aggrecan50, 51, 60, 66. For collagen, dynamic loading in the presence of IL-1 had no effect on collagen II transcript levels in one experiment59, but showed an increase in collagen II expression51 and [3H]-thymidine incorporation60, 66 in several other studies. All of these results suggest that loading alone can have disparate effects depending on the experimental parameters.
In this study, we demonstrated that dynamic loading blocks the pro-inflammatory effects that are mediated by IL-1 and promotes integrative meniscal repair in an inflammatory microenvironment. Mechanical loading of articular cartilage can also inhibit IL-1 induced matrix degradation67. Interestingly, mechanical loading of meniscal repair model explants was more effective than IL-1ra33, MMP inhibitors28, or the anabolic growth factor TGFβ135 in promoting integrative meniscal repair in the presence of IL-1. The mechanism(s) by which dynamic loading antagonizes the effects of IL-1 need to be investigated further. Mechanical loading may regulate IL-1 expression by meniscal fibrochondrocytes. A slight induction of IL-1α but not IL-1β transcript levels have been measured in porcine menisci subjected to 20% strain41 but cyclic hydrostatic pressure suppresses IL-1β expression in rabbit menisci68. It is also possible that mechanical loading caused a downregulation in the IL-1 type I receptor and/or an upregulation in the type II IL-1 decoy receptor66, 69. Under 50% static compression, IL-1ra blocks decreases in cartilage explant proteoglycan synthesis70, suggesting a role for the IL-1 receptors in mediating the effects of load. Preloading for 1 hour with cyclic tensile strain was unable to significantly decrease NOS2 expression and NO production in chondrocytes treated with IL-1, suggesting that strain alone is unable to downregulate the cell surface IL-1 receptors51. However, these experiments did not directly assess receptor number and did not address the idea that a synergism between mechanical loading and IL-1 may be required to diminish the cell surface receptors. Alternatively, mechanical loading may regulate IL-1 signaling mediators that are downstream of the IL-1 receptor, such as NF-κB57, RANK, and RANKL43. Dynamic loading and IL-1 have been shown to be anti-inflammatory by suppressing not only the catabolic effects of IL-1 but also by diminishing expression of other pro-inflammatory factors, such as TNF-α23.
In summary, our data suggest a beneficial effect of dynamic loading in promoting repair of meniscal lesions in an inflammatory microenvironment, such as a knee joint following meniscal injury, meniscal repair surgery, or arthritis. Our data further suggest the potential usefulness of physical rehabilitation treatment for meniscal lesions, but more research is needed to fully elucidate the mechanism(s) of IL-1 inhibition and translation of this work into clinical practice.
Acknowledgments
We thank Poston Pritchett, Dr. Franklin Moutos, and Bridgette Furman for technical assistance. We also thank our funding sources the Arthritis Foundation, VA Rehabilitation Research, and NIH Grants AR50245, AG15768, AR48182, AR48852, and AR55434.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors have any financial or personal relationships with other people or organizations that influence this work.
References
- 1.Markolf KL, Bargar WL, Shoemaker SC, Amstutz HC. The role of joint load in knee stability. J Bone Joint Surg Am. 1981;63:570–585. [PubMed] [Google Scholar]
- 2.Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints--Part I: Tibial surface of the knee. J Biomech Eng. 1983;105:216–225. doi: 10.1115/1.3138409. [DOI] [PubMed] [Google Scholar]
- 3.Wojtys EM, Chan DB. Meniscus Structure and Function. AAOS Instructional Course Lectures. 2005;54:323–330. [PubMed] [Google Scholar]
- 4.Spilker RL, Donzelli PS, Mow VC. A transversely isotropic biphasic finite element model of the meniscus. J Biomech. 1992;25:1027–1045. doi: 10.1016/0021-9290(92)90038-3. [DOI] [PubMed] [Google Scholar]
- 5.Zielinska B, Donahue TL. 3D finite element model of meniscectomy: changes in joint contact behavior. J Biomech Eng. 2006;128:115–123. doi: 10.1115/1.2132370. [DOI] [PubMed] [Google Scholar]
- 6.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: A 10-year study. Knee. 2006;13:184–188. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Wyland DJ, Guilak F, Elliott DM, Setton LA, Vail TP. Chondropathy after meniscal tear or partial meniscectomy in a canine model. J Orthop Res. 2002;20:996–1002. doi: 10.1016/S0736-0266(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 8.Christoforakis J, Pradhan R, Sanchez-Ballester J, Hunt N, Strachan RK. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy-the Journal of Arthroscopic and Related Surgery. 2005;21:1366–1369. doi: 10.1016/j.arthro.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 11.Burger C, Kabir K, Mueller M, Rangger C, Minor T, Tolba RH. Retropatellar chondromalacia associated with medial osteoarthritis after meniscus injury. One year of observations in sheep. Eur Surg Res. 2006;38:102–108. doi: 10.1159/000093281. [DOI] [PubMed] [Google Scholar]
- 12.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 13.Lotz M. Cytokines in cartilage injury and repair. Clinical Orthopaedics & Related Research. 2001;391 Suppl:S108–S115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 15.Kahle P, Saal JG, Schaudt K, Zacher J, Fritz P, Pawelec G. Determination of cytokines in synovial fluids: correlation with diagnosis and histomorphological characteristics of synovial tissue. Annals of the Rheumatic Diseases. 1992;51:731–734. doi: 10.1136/ard.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vangsness CT, Jr, Burke WS, MacPhee RD, Narvy SHuman. International Cartilage Repair Society. San Diego, CA: 2006. Knee Synovial Fluid Analysis (Abstract) p. 87. [Google Scholar]
- 17.Hopkins SJ, Humphreys M, Jayson MI. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clinical & Experimental Immunology. 1988;72:422–427. [PMC free article] [PubMed] [Google Scholar]
- 18.Ochi M, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing.[see comment] Arthroscopy. 2001;17:724–731. doi: 10.1053/jars.2001.23583. [DOI] [PubMed] [Google Scholar]
- 19.van den Berg WB, Joosten LA, van de Loo FA. TNF alpha and IL-1 beta are separate targets in chronic arthritis. Clin Exp Rheumatol. 1999;17:S105–S114. [PubMed] [Google Scholar]
- 20.Lotz M, Blanco FJ, von Kempis J, Dudler J, Maier R, Villiger PM, et al. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995;43:104–108. [PubMed] [Google Scholar]
- 21.LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44:2078–2083. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 22.Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. Journal of Applied Physiology. 2003;95:308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti M, Madhavan S, Deschner J, Rath-Deschner B, Wypasek E, Agarwal S. Dynamic biophysical strain modulates proinflammatory gene induction in meniscal fibrochondrocytes. Am J Physiol Cell Physiol. 2006;290:C1610–C1615. doi: 10.1152/ajpcell.00529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao M, Stefanovic-Racic M, Georgescu HI, Miller LA, Evans CH. Generation of nitric oxide by lapine meniscal cells and its effect on matrix metabolism: stimulation of collagen production by arginine. J Orthop Res. 1998;16:104–111. doi: 10.1002/jor.1100160118. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto S, Takahashi K, Ochs RL, Coutts RD, Amiel D, Lotz M. Nitric oxide production and apoptosis in cells of the meniscus during experimental osteoarthritis. Arthritis Rheum. 1999;42:2123–2131. doi: 10.1002/1529-0131(199910)42:10<2123::AID-ANR12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, et al. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis & Rheumatism. 2002;46:1789–1803. doi: 10.1002/art.10356. [DOI] [PubMed] [Google Scholar]
- 27.Lemke AK, Lee J, Sandy J, Grodzinsky A, Mentlein R, Fay J, et al. IL-1 Induced Matrix Degradation and Gene Expression in the Meniscus Depends on the Anatomical Location; 52nd Annual Orthopaedic Research Society Meeting; Chicago, IL. 2006. p. 1043. [Google Scholar]
- 28.McNulty AL, Weinberg JB, Guilak F. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res. 2009;467:1557–1567. doi: 10.1007/s11999-008-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 30.Powell AJ, Little CB, Hughes CE. Low molecular weight isoforms of the aggrecanases are responsible for the cytokine-induced proteolysis of aggrecan in a porcine chondrocyte culture system. Arthritis Rheum. 2007;56:3010–3019. doi: 10.1002/art.22818. [DOI] [PubMed] [Google Scholar]
- 31.Bondeson J, Wainwright S, Hughes C, Caterson B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin Exp Rheumatol. 2008;26:139–145. [PubMed] [Google Scholar]
- 32.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of Integrative Repair of the Meniscus Following Acute Exposure to Interleukin-1 in vitro. Journal of Orthopaedic Research. 2008;26:504–512. doi: 10.1002/jor.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNulty AL, Moutos FT, Weinberg JB, Guilak F. Enhanced Integrative Repair of the Porcine Meniscus In Vitro by Inhibition of Interleukin-1 or Tumor Necrosis Factor-alpha. Arthritis Rheum. 2007;56:3033–3042. doi: 10.1002/art.22839. [DOI] [PubMed] [Google Scholar]
- 34.Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Interleukin-1 and tumor necrosis factor alpha inhibit repair of the porcine meniscus in vitro. Osteoarthritis & Cartilage. 2007;15:1053–1060. doi: 10.1016/j.joca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNulty AL, Guilak F. Integrative Repair of the Meniscus: Lessons from In Vitro Studies. Biorheology. 2008;45:487–500. [PMC free article] [PubMed] [Google Scholar]
- 36.Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. Philadelphia: Lippincott-Raven; 1997. pp. 179–207. [Google Scholar]
- 37.Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21:963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 38.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12:736–744. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Fink C, Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Guilak F. The effect of dynamic mechanical compression on nitric oxide production in the meniscus. Osteoarthritis Cartilage. 2001;9:481–487. doi: 10.1053/joca.2001.0415. [DOI] [PubMed] [Google Scholar]
- 40.Hennerbichler A, Fermor B, Hennerbichler D, Weinberg JB, Guilak F. Regional differences in prostaglandin E2 and nitric oxide production in the knee joint meniscus in response to dynamic mechanical compression. Biochemical and Biophysical Research Communications. 2007;358:1047–1053. doi: 10.1016/j.bbrc.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta T, Zielinska B, McHenry J, Kadmiel M, Haut Donahue TL. IL-1 and iNOS gene expression and NO synthesis in the superior region of meniscal explants are dependent on the magnitude of compressive strains. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Zielinska B, Killian M, Kadmiel M, Nelsen M, Haut Donahue TL. Meniscal tissue explants response depends on level of dynamic compressive strain. Osteoarthritis Cartilage. 2009;17:754–760. doi: 10.1016/j.joca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Deschner J, Wypasek E, Ferretti M, Rath B, Anghelina M, Agarwal S. Regulation of RANKL by biomechanical loading in fibrochondrocytes of meniscus. J Biomech. 2006;39:1796–1803. doi: 10.1016/j.jbiomech.2005.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. American Journal of Sports Medicine. 2007;35:754–762. doi: 10.1177/0363546506296416. [DOI] [PubMed] [Google Scholar]
- 45.Deng S-J, Bickett DM, Mitchell JL, Lambert MH, Blackburn RK, Carter IIIHLC, et al. Substrate Specificity of Human Collagenase 3 Assessed Using a Phage-displayed Peptide Library. The Journal of Biological Chemistry. 2000;275:31422–31427. doi: 10.1074/jbc.M004538200. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Xu J, Levin J, Hegen M, Li G, Robertshaw H, et al. Identification and characterization of 4-[[4-(2-butynyloxy)phenyl]sulfonyl]-N-hydroxy-2,2-dimethyl-(3S)thiomorpholinecarboxamide (TMI-1), a novel dual tumor necrosis factor-alpha-converting enzyme/matrix metalloprotease inhibitor for the treatment of rheumatoid arthritis. Journal of Pharmacology & Experimental Therapeutics. 2004;309:348–355. doi: 10.1124/jpet.103.059675. [DOI] [PubMed] [Google Scholar]
- 47.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 48.Granger DL, Anstey NM, Miller WC, Weinberg JB. Measuring nitric oxide production in human clinical studies. Methods Enzymol. 1999;301:49–61. doi: 10.1016/s0076-6879(99)01068-x. [DOI] [PubMed] [Google Scholar]
- 49.McHenry JA, Zielinska B, Donahue TL. Proteoglycan breakdown of meniscal explants following dynamic compression using a novel bioreactor. Ann Biomed Eng. 2006;34:1758–1766. doi: 10.1007/s10439-006-9178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal S, Long P, Gassner R, Piesco NP, Buckley MJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44:608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol. 2000;165:453–460. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blain EJ, Gilbert SJ, Wardale RJ, Capper SJ, Mason DJ, Duance VC. Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Arch Biochem Biophys. 2001;396:49–55. doi: 10.1006/abbi.2001.2575. [DOI] [PubMed] [Google Scholar]
- 53.Bevill SL, Briant PL, Levenston ME, Andriacchi TP. Central and peripheral region tibial plateau chondrocytes respond differently to in vitro dynamic compression. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Sun HB, Yokota H. Reduction of cytokine-induced expression and activity of MMP-1 and MMP-13 by mechanical strain in MH7A rheumatoid synovial cells. Matrix Biol. 2002;21:263–270. doi: 10.1016/s0945-053x(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 55.Wilson CG, Vanderploeg EJ, Zuo F, Sandy JD, Levenston ME. Aggrecanolysis and in vitro matrix degradation in the immature bovine meniscus: mechanisms and functional implications. Arthritis Res Ther. 2009;11:R173. doi: 10.1186/ar2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voigt H, Lemke AK, Mentlein R, Schunke M, Kurz B. Tumor necrosis factor alpha-dependent aggrecan cleavage and release of glycosaminoglycans in the meniscus is mediated by nitrous oxide-independent aggrecanase activity in vitro. Arthritis Res Ther. 2009;11:R141. doi: 10.1186/ar2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deschner J, Hofman CR, Piesco NP, Agarwal S. Signal transduction by mechanical strain in chondrocytes. Curr Opin Clin Nutr Metab Care. 2003;6:289–293. doi: 10.1097/01.mco.0000068964.34812.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lima EG, Tan AR, Tai T, Bian L, Ateshian GA, Cook JL, et al. Physiologic deformational loading does not counteract the catabolic effects of interleukin-1 in long-term culture of chondrocyte-seeded agarose constructs. J Biomech. 2008;41:3253–3259. doi: 10.1016/j.jbiomech.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chowdhury TT, Arghandawi S, Brand J, Akanji OO, Bader DL, Salter DM, et al. Dynamic compression counteracts IL-1beta induced inducible nitric oxide synthase and cyclo-oxygenase-2 expression in chondrocyte/agarose constructs. Arthritis Res Ther. 2008;10:R35. doi: 10.1186/ar2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chowdhury TT, Bader DL, Lee DA. Dynamic compression inhibits the synthesis of nitric oxide and PGE(2) by IL-1beta-stimulated chondrocytes cultured in agarose constructs. Biochem Biophys Res Commun. 2001;285:1168–1174. doi: 10.1006/bbrc.2001.5311. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi K, Fujimoto E, Deie M, Sumen Y, Ikuta Y, Ochi M. Regional differences in the healing potential of the meniscus-an organ culture model to eliminate the influence of microvasculature and the synovium. Knee. 2004;11:271–278. doi: 10.1016/j.knee.2002.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Reindel ES, Ayroso AM, Chen AC, Chun DM, Schinagl RM, Sah RL. Integrative Repair of Articular Cartilage In Vitro: Adhesive Strength of the Interface Region. Journal of Orthopaedic Research. 1995;13:751–760. doi: 10.1002/jor.1100130515. [DOI] [PubMed] [Google Scholar]
- 63.Ahsan T, Lottman LM, Harwood F, Amiel D, Sah RL. Integrative Cartilage Repair: Inhibition of B-Aminopropionitrile. Journal of Orthopaedic Research. 1999;17:850–857. doi: 10.1002/jor.1100170610. [DOI] [PubMed] [Google Scholar]
- 64.DiMicco MA, Sah RL. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. Journal of Orthopaedic Research. 2001;19:1105–1112. doi: 10.1016/S0736-0266(01)00037-7. [DOI] [PubMed] [Google Scholar]
- 65.DiMicco MA, Waters SN, Akeson WH, Sah RL. Integrative articular cartilage repair: dependence on developmental stage and collagen metabolism. Osteoarthritis & Cartilage. 2002;10:218–225. doi: 10.1053/joca.2001.0502. [DOI] [PubMed] [Google Scholar]
- 66.Chowdhury TT, Salter DM, Bader DL, Lee DA. Signal transduction pathways involving p38 MAPK, JNK, NFkappaB and AP-1 influences the response of chondrocytes cultured in agarose constructs to IL-1beta and dynamic compression. Inflamm Res. 2008;57:306–313. doi: 10.1007/s00011-007-7126-y. [DOI] [PubMed] [Google Scholar]
- 67.Torzilli PA, Bhargava M, Park S, Chen CT. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthritis Cartilage. 2009 doi: 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Natsu-Ume T, Majima T, Reno C, Shrive NG, Frank CB, Hart DA. Menisci of the rabbit knee require mechanical loading to maintain homeostasis: cyclic hydrostatic compression in vitro prevents derepression of catabolic genes. J Orthop Sci. 2005;10:396–405. doi: 10.1007/s00776-005-0912-x. [DOI] [PubMed] [Google Scholar]
- 69.Attur MG, Dave M, Cipolletta C, Kang P, Goldring MB, Patel IR, et al. Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J Biol Chem. 2000;275:40307–40315. doi: 10.1074/jbc.M002721200. [DOI] [PubMed] [Google Scholar]
- 70.Murata M, Bonassar LJ, Wright M, Mankin HJ, Towle CA. A role for the interleukin-1 receptor in the pathway linking static mechanical compression to decreased proteoglycan synthesis in surface articular cartilage. Arch Biochem Biophys. 2003;413:229–235. doi: 10.1016/s0003-9861(03)00129-2. [DOI] [PubMed] [Google Scholar]


