Abstract
The degree of genetic diversity in 45 Bordetella (B.) bronchiseptica strains comprised of a vaccine strain (N = 1), reference strains (N = 3) and field isolates (N = 41) was evaluated using random amplified polymorphic DNA (RAPD) fingerprinting and pulsed-field gel electrophoresis (PFGE). Three candidate primers were selected for RAPD analysis after screening 20 random decamer oligonucleotides for their discriminatory abilities. The OPA-07, OPA-08 and OPA-18 primers yielded 10, 10, and 6 distinct fingerprint patterns, respectively. The most common identical RAPD pattern was produced by OPA-07 which was shared by 32 isolates (71.1%), the pattern produced by OPA-08 was shared by 26 isolates (57.8%), and the pattern produced by OPA-18 was shared by 40 isolates (88.9%). The RAPD patterns of the vaccine strain and the 3 reference strains did not match any of the patterns produced by the field isolates when primers OPA-07 and OPA-08 were used. PFGE using the restriction endonuclease XbaI produced a total of 15 patterns consisting of 4 PFGE types (A, B, B1 and C, differing by ≥ 4 bands) and 11 A subtypes (differing by ≤ 3 bands). Most of the field isolates exhibited identical type A and B patterns, suggesting that they were related. The vaccine strain and the three reference strains showed different PFGE patterns as compared to the identical type A strains.
Keywords: Bordetella bronchiseptica, genetic diversity, PFGE, RAPD
Introduction
Bordetella are Gram-negative bacteria that cause respiratory tract infections in humans and animals. Species in the genus Bordetella are close phenotypically, possess common antigens and share a high degree of DNA similarity [3]. Bordetella (B.) bronchiseptica infects many domestic and wild animal species. In pigs, for example, B. bronchiseptica is known to play a role in development of atrophic rhinitis (AR) and porcine respiratory disease complex [2]. AR is an infectious disease of pigs characterized by purulent nasal discharge, shortening or twisting of the snout, atrophy of the turbinate bones and reduced growth rate [13]. Many aspects of the biology of B. bronchiseptica have been studied, including colony morphology [20], hemolysin production [4], hemagglutination [5], and plasmid content [11], however reports regarding genetic typing of B. bronchiseptica are scarce. Serotyping has demonstrated that B. bronchiseptica isolates from pigs differ from those of other animal species, however, these results were based on only a few B. bronchiseptica isolates from each animal species tested [4,5,11,19,20]. Phenotypic typing based on expression of cellular characteristics may vary according to culture or experimental conditions, and is being gradually replaced by bacterial genomic analysis [6].
A number of molecular methods, including restriction enzyme analysis (REA) [24], Random amplified polymorphic DNA (RAPD) fingerprinting [15], ribotyping [12,21,27] and macro-restriction analysis by pulsed-field gel electrophoresis (PFGE) [6] have been used to study differences in epidemiology between different strains of B. bronchiseptica, and the results obtained using these methods suggest that considerable genomic diversity exists between strains. REA performed on 195 B. bronchiseptica isolates from 12 different host species worldwide showed 48 distinct fingerprint patterns after HinfI digestion and 39 fingerprint profiles after AluI digestion [24]. Ribotyping of B. bronchiseptica isolates obtained from several different animal species revealed that the isolates fell into distinct groups [21]. PFGE provides a highly reproducible restriction profile of large bacterial DNA fragments and therefore a means for discriminating between B. bronchiseptica isolates in epidemiologic studies [6,16]. Binns et al. [6] identified 17 PFGE types with numerous subtypes within a collection of 164 isolates, predominantly from cats. Keil and Fenwick [15] combined RAPD analysis and ribotyping to evaluate genetic diversity among 26 canine B. bronchiseptica isolates. Although many molecular methods have been used to study B. bronchiseptica isolates from different hosts, there are few reports on the typing of B. bronchiseptica isolates from swine [6,21,24]. To our knowledge, this study is the first to provide genotyping data obtained by both RAPD and PFGE analyses of a large number of Korean swine B. bronchiseptica field isolates and is also the first study to combine these methods to classify B. bronchiseptica isolates from swine. The purpose of this study was to evaluate the genetic diversity of B. bronchiseptica field isolates using RAPD and PFGE in comparison to a vaccine strain and 3 standard strains of B. bronchiseptica.
Materials and Methods
Bacterial strains
Forty-five B. bronchiseptica strains comprised of 1 vaccine strain, 3 reference strains and 41 field isolates were evaluated. The field isolates were obtained from 6-month-old slaughtered pigs from the provinces of Gangwon and Gyeonggi Province in Korea between October 2001 and October 2002. All isolates were identified as B. bronchiseptica using Smith-Baskerville medium [26] and standard methods [8,14]. Three reference strains (ATCC 19395, 10580, and 4617) and the B. bronchiseptica vaccine P4 strain (HAPVAC; Choongang Vaccine Laboratory, Korea) were minimally passed and stored in a Microbank (KOMED, Korea) at -70℃ until used.
DNA preparation for RAPD
Bacterial isolates were inoculated into 2 ml fresh brain heart infusion broth (Difco, USA) and incubated at 37℃ for 24 h. Genomic DNA from each strain was obtained using a DNeasy Tissue Kit (QIAGEN, Germany) according to the manufacturer's instructions. A set of 20 commercially available primers (Oligo 10-mer kit A; QIAGEN, Germany) was screened to identify suitable primers for RAPD analysis of swine B. bronchiseptica isolates. Primers OPA-07 (5'-GAAACGGGTG-3'), OPA-08 (5'-GTGACGTAGG-3') and OPA-18 (5'-AGGTGACCGT-3') resulted in informative fingerprints and were used to evaluate the remaining strains.
RAPD
PCR consisted of 50 ng of total B. bronchiseptica DNA, 5 mM MgCl2 (Promega, USA), 12 pmole primer, 2.5 units of GoTaq DNA polymerase (Promega, USA), and 500 mM dNTPs (Takara, Japan) in 25 mM Tris-HCl (pH9.0)-25 mM NaCl in a volume of 25 µl was subjected to the following conditions: 2 min of initial denaturation at 95℃ followed by 45 cycles of 1 min of denaturation at 94℃, 1 min of annealing at 33℃, 2 min of extension at 72℃. Reactions were performed using a UNO-II thermalcycler (Biometra, Germany). Following PCR, 10 µl of the reaction mixture was analyzed by gel electrophoresis in a 2.0% agarose gel containing 500 ng/ml ethidium bromide. A 100-bp DNA ladder (Jeil Biotechservice, Korea) was used to determine molecular size. The agarose gels were photographed under UV light using a Biocapt (Vilber Lourmat, France) and the DNA bands were analyzed using Bio-Profil Bio 2D software (Vilber Lourmat, France), which was also used to construct dendrograms of the isolates.
Preparation of DNA for PFGE
The methods used to conduct PFGE essentially followed the 'pulse Net' system protocol described by the Centers for Disease Control [7,9]. Briefly, B. bronchiseptica were cultured in Luria Bertani agar (Difco, USA) plates and incubated at 37℃ overnight. Colonies were then harvested and suspended in TE suspension buffer (100 mM Tris-HCl and 100 mM EDTA, pH 7.5). The turbidity of the bacterial cell suspension was set to 20% transmittance using a colorimeter (BioMeieux, France). Proteinase K and 1.2% Seakem Gold agarose (FMC Bioproducts, USA) were then mixed with the cell suspension and dispensed into disposable plug molds (Bio-Rad, USA). ES buffer (0.5M EDTA, pH 9.0, 1% sodium-lauroyl-sarcosine) and proteinase K were added to the plugs, which were then incubated in a 55℃ water bath for 1 h. After proteolysis, the plugs were washed once for 15 min in sterile distilled water then 4 times for 30 min in TE buffer (10 mM Tris-HCl and 1 mM EDTA, pH 7.5) preheated to 50℃. Washed plugs were stored in TE buffer (10 mM Tris-HCl and 1 mM EDTA, pH 7.5) at 4℃ until ready for restriction enzyme digestion. The stored plugs were cut into 2-1 mm wide slices with a razor blade and the 2 halves transferred to a tube containing the restriction enzyme XbaI (30 U; Promega, USA) and digested at 37℃ for 3 h. After incubation, the enzyme mix was aspirated from the tube and replaced with 500 µl of TE washing buffer.
PFGE
DNA obtained from bacteria was electrophoresed using a contour homogeneous field electrophoresis system (DR II; Bio-Rad, USA). Digested plugs were electrophoresed in 1% Seakem gold agarose gel (FMC Bioproducts, USA) with 0.5X TBE buffer. The electrophoresis conditions were as follows: initial switch time, 2.2 s; final switch time, 55.0 s; run time, 15.5 h; gradient, 6.0 V/cm; buffer temperature, 14℃. A standard lambda DNA ladder (Bio-Rad, USA) was used as a size marker. After electrophoresis, the gel was stained with ethidium bromide staining solution for 30 min, then destained in water for 20 min. The stained gel was viewed on an UV transilluminator and photographed with Polaroid film and scanned using a Bio-Pn 05 System (Vilber Lourmat, France).
Data analysis of PFGE
PFGE DNA patterns were compared using Tenover's criteria [28]. The most frequently repeated PFGE pattern was designated type "A". Because all type A patterns were identical it was used as the standard to differentiate the other banding patterns. Subtypes A1 to A11 differed from the major type A pattern by less than three bands; the major type B pattern differed by 5-6 bands; and the type C pattern differed by 7 bands. After manual inspection, a dendrogram was constructed using Bio-Profil Bio 2D (Vilber Lourmat, France) software to depict the relatedness of each type.
Results
RAPD
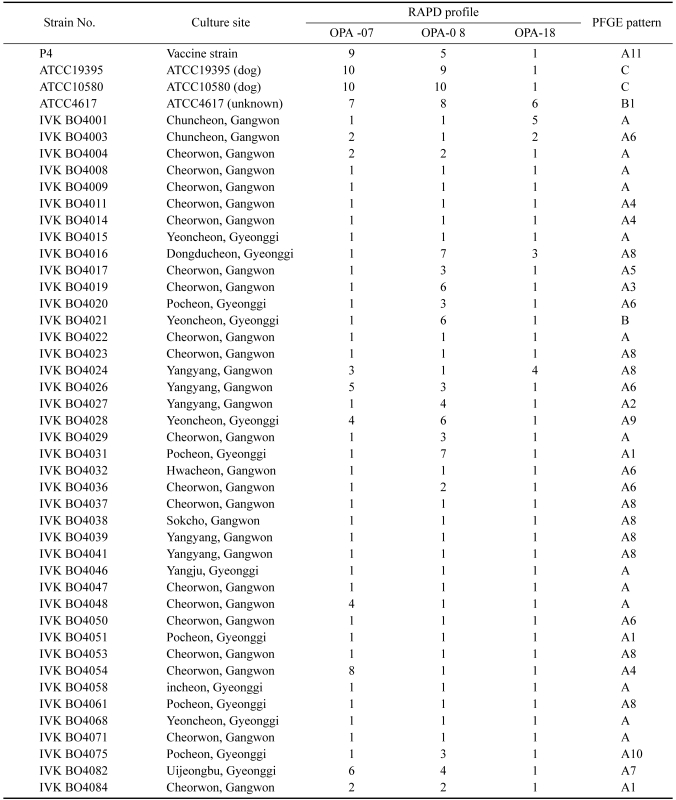
The 3 different primers, OPA-07, OPA-08 and OPA-18 produced different numbers of patterns in the RAPD analyses of 45 B. bronchiseptica strains (Table 1). OPA-07, OPA-08, and OPA-18 yielded 10, 10, and 6 patterns, respectively (Table 2).
Table 1.
Summary of the properties of B. bronchiseptica: culture site, RAPD and PFGE type of the tested strains
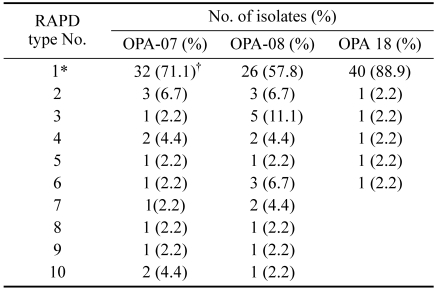
Table 2.
RAPD patterns of B. bronchiseptica
*The most common identical RAPD pattern.
†Data are number of isolates.
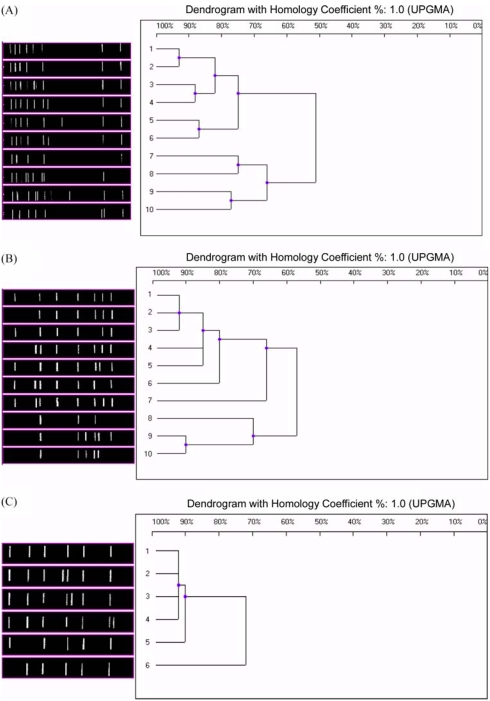
The 10 distinct DNA patterns produced by OPA-07 fingerprinting were designated as OPA-07(1) through OPA-07(10) (Fig. 1A). Fingerprint OPA-07(1) was the most common RAPD pattern, shared by 32 of the 45 isolates (71.1%) although the vaccine strain and the 3 reference strains did not produce this pattern. The P4 vaccine strain was defined by fingerprint OPA-07(9), the ATCC 19395 and 10580 strains by OPA-07(10), and ATCC 4617 by OPA-07(7).
Fig. 1.
RAPD patterns and associated dendrograms of B. bronchiseptica strains. (A) RAPD patterns and associated dendrograms of B. bronchiseptica strains generated using primer OPA-07. (B) RAPD patterns and associated dendrograms of B. bronchiseptica strains generated using primer OPA-08. (C) RAPD patterns and associated dendrograms of B. bronchiseptica strains generated using primer OPA-18.
The 10 distinct DNA patterns produced by OPA-08 fingerprinting were designated as OPA-08(1) through OPA-08(10) (Fig. 1B). Fingerprint OPA-08(1) was the most common RAPD pattern, shared by 26 isolates (57.8%). The P4 vaccine strain was defined by fingerprint OPA-08(5), the ATCC 19395 and 10580 strains by OPA-08(9) and OPA-08(10), respectively, and ATCC 4617 by OPA-08(8).
The 6 distinct RAPD patterns produced by OPA-18 fingerprinting were designated as OPA-18(1) through OPA-18(6) (Fig. 1C). Fingerprint OPA-18(1) was the most common RAPD pattern, shared by 40 of the 45 isolates (88.9%), including the vaccine strain and 2 of the reference strains, ATCC 19395 and ATCC 10580. The third reference strain, ATCC 4617, was defined by fingerprint OPA-18(6).
These results indicate there is considerable heterogeneity among B. bronchiseptica strains based on RAPD analysis. Although common fingerprints exist, most notably OPA-07(1), OPA-08(1), and OPA-18(1), overall classification of the isolates is dependant upon the primer used. Moreover, the field isolates appear to be genetically distinct from the vaccine and reference strains.
PFGE
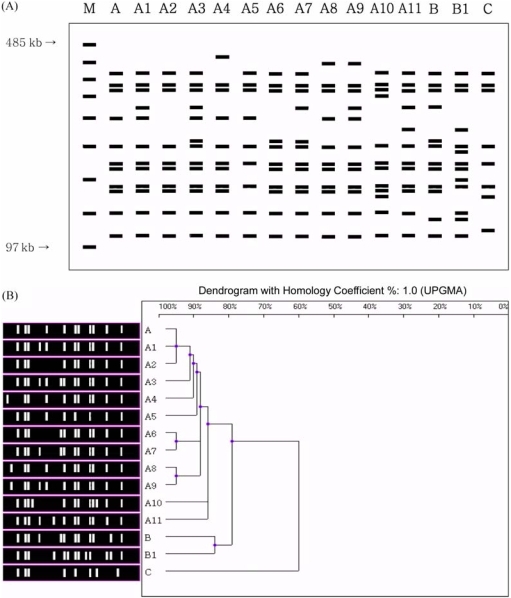
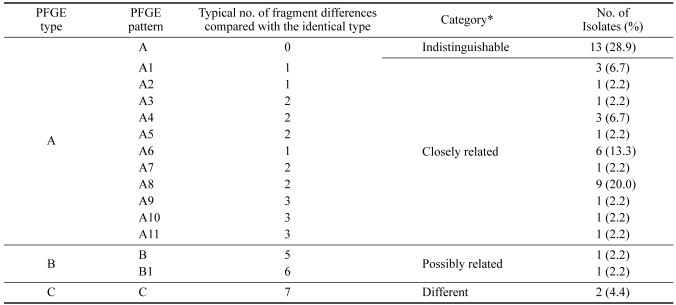
The same 45 B. bronchiseptica strains were also tested by PFGE (Table 1). PFGE of XbaI-digested genomic DNA produced patterns of well-resolved bands ranging in size from 100 to 390 kb (Fig. 2A). The majority of isolates produced between 8 and 13 bands, yielding a diverse array of DNA profiles. A total of 15 distinct PFGE patterns were observed, including 4 major types (differing by > 4 bands; A, B, B1 and C) and 11 A subtypes (differing by ≤ 3 bands; A1 to A11) (Fig. 2A). The most common PFGE pattern, which includes 13 isolates (28.9%), was named 'identical type A' (Table 3). Subtypes A1 to A11 were closely related to identical type A and differed from identical type A by only 1 to 3 bands. Types B and B1 differed from identical type A by 5 and 6 bands, respectively, however, they may still be classified as being related to type A strains based on Tenover's criteria [28]. Type C and identical type A differed by seven bands and can therefore be considered different isolates.
Fig. 2.
Schematic magnification of PFGE patterns and dendrogram of XbaI PFGE patterns of B. bronchiseptica performed according to UPGMA. (A) Lanes: M, lambda DNA standard marker; A~C, B. bronchiseptica PFGE patterns (B) PFGE patterns and associated dendrograms of B. bronchiseptica strains generated by digestion with XbaI.
Table 3.
PFGE patterns of B. bronchiseptica digested with XbaI
*Tenover et al., 1995 [28].
The P4 vaccine strain used in Korea has a type A11 PFGE pattern (it differs by 3 bands from identical type A), both ATCC 19395 and ATCC 10580 have type C patterns (they differ by 7 bands) and ATCC 4617 strain has a type B1 pattern (it differs by 6 bands).
Dendrogram analysis of B. bronchiseptica DNA digested with XbaI showed the relationship of each strain to one another in comparison to the PFGE profile (Fig. 2B). A PFGE profile relatedness diagram was constructed using the unweighted pair group method of average linkage (UPGMA). Three major relatedness clusters can be recognized. Subtypes A1 to A11 are closely related to identical type A (86% homology), and these subtypes include most of the field isolates. Types B and B1 are less closely related to identical type A (79% homology). Type C is different from identical type A (60% homology).
Discussion
The molecular epidemiology of B. bronchiseptica was investigated using a variety of techniques, including electromorphotyping [18] and ribotyping [21], which have shown a lack of genetic diversity among field isolates. Genomic analysis by RAPD and PFGE has been successful for many bacteria, including various Bordetella species [15,17,31,32].
We analyzed 45 B. bronchiseptica isolates by RAPD and PFGE of XbaI-digested genomic DNA and compared the results from these 2 methods. RAPD analysis using the OPA-07, OPA-08, and OPA-18 primers yielded 10, 10, and 6 distinct fingerprint patterns, respectively. In contrast, PFGE showed 15 patterns, which demonstrates that PFGE is a discriminating and reproducible method for genotyping swine B. bronchiseptica isolates. PFGE has a higher discriminatory power than RAPD because it produces a greater variety of fingerprints. Moreover, RAPD is less reliable than PFGE because the interpretation of some of the RAPD patterns is complicated by inconsistent band intensity (data not shown). Previous studies also have reported that PFGE is more discriminating than RAPD for microorganisms [1,25,29].
To select suitable candidate primers for genotyping B. bronchiseptica isolates, 20 commercially available arbitrary primers were screened against swine B. bronchiseptica. Three primers (OPA-07, OPA-08 and OPA-18) resulted in informative fingerprints, and were evaluated with the remaining strains. In contrast, Keil and Frenwick [15] found that the OPA-02 and OPA-04 primers were suitable primers for RAPD with canine B. bronchiseptica. This difference may be explained by the existence of host-species-specific B. bronchiseptica. In our results, RAPD with OPA-02 and OPA-04 produced 5 and 7 patterns, respectively, with 41 swine B. bronchiseptica. Previous studies have indicated that RAPD fingerprinting with the OPA-04 primer resulted in 4 distinct fingerprint patterns among 26 canine B. bronchiseptica isolates obtained between 1970 and 1997 [15].
Zhang et al. [33] proposed that the same RAPD patterns or patterns with only a single major band difference should be classified as "indistinguishable", and patterns having 2 or more major band differences could be classified as "different". Based on these criteria, our RAPD results of 41 B. bronchiseptica field isolates using the OPA-07 primer included 6 groups of indistinguishable fingerprints and 1 different pattern; using the OPA-08 and OPA-18 primers, all the patterns can be classified as indistinguishable. Of the 15 PFGE patterns we detected, most of the field isolates (97.6%) fell into 2 types (A and B) that are likely closely related. Binns et al. [6] showed that 12 B. bronchiseptica swine isolates were distributed among 3 PFGE types, and 9 (75%) of the isolates were of 1 type, despite being obtained from pigs in different parts of UK. These findings indicated that the swine B. bronchiseptica field isolates had minor genetic variation but were still closely related.
In this study, we also evaluated three ATCC reference strains (isolated from canine and unknown hosts) by RAPD and PFGE. Except for the RAPD results obtained using the OPA-18 primer, the RAPD and PFGE patterns of the 3 reference strains did not match those of field isolates. This is strong evidence of the existence of specific strains of B. bronchiseptica. Sources of infection other than pigs have also been considered important, since B. bronchiseptica has been recovered form cats, dogs, rats, rabbits, and other wildlife that may gain access to pig farms, but their significance remains doubtful [10]. Ross et al. [22] showed that some non-porcine isolates produced a degree of turbinate hypoplasia in young pigs. However, Rutter and Collings [23] concluded that infection of pigs with B. bronchiseptica strains from other species is not likely to be hazardous. Our results suggest it should be possible to study the molecular epidemiology of atrophic rhinitis using RAPD and PFGE to trace cross-species transmission of B. bronchiseptica.
We analyzed strains of B. bronchiseptica from 2 different areas. All 28 isolates from Gangwon province were found to be A type by PFGE. In contrast, 13 isolates from Gyeonggi province included both A and B types. It is necessary to analyze a large number of isolates from different areas in order to resolve the relation of PFGE types with respect to isolation areas.
When the results from RAPD and PFGE were compared, there was no direct correlation observed between the RAPD type and PFGE group. These methods exploit different types of DNA polymorphism. PFGE is based on restriction enzyme polymorphism and analyzes the whole chromosomal DNA. In contrast, RAPD analyzes dispersed chromosomal loci, sequence polymorphisms of the regions complementary to the primers, and length polymorphisms of the regions that are amplified [1]. Previous studies indicated that RAPD could be used for genetic comparison of Mycobacterium abscessus strains, including strains that cannot be distinguished by PFGE [33]. In contrast, both techniques (RAPD with primer P2 and PFGE with NotI) produced the same results when used for typing Moraxella catarrhalis strains [30].
In conclusion, RAPD and PFGE analyses of B. bronchiseptica indicate genetic variability both within swine field isolates and between field isolates and the P4 vaccine strain. Even though there is genetic variation, most types could be classified as "indistinguishable" by RAPD and as type A by PFGE. These results reveal high genetic homogeneity among swine B. bronchiseptica isolates form Korea. Further studies are needed to expand the investigation areas of swine B. bronchiseptica isolates, ultimately to a national scale. In addition, studies of cross-species transmission of field B. bronchiseptica between swine and other animals are needed.
Acknowledgments
This work supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD) [R05-2003-000-11001-0].
References
- 1.Barbier N, Saulnier P, Chachaty E, Dumontier S, Andremont A. Random amplified polymorphic DNA typing versus pulsed-field gel electrophoresis for epidemiological typing of vancomycin-resistant enterococci. J Clin Microbiol. 1996;34:1096–1099. doi: 10.1128/jcm.34.5.1096-1099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baysinger A. PRDC: is it new or déjá vu? Pork '99. 1999;19:64. [Google Scholar]
- 3.Bemis DA, Burns EH. Bordetella. In: Gyles CL, Thoen CO, editors. Pathogenesis of Bacterial Infections in Animals. 2nd ed. Ames: Iowa State University Press; 1993. pp. 201–215. [Google Scholar]
- 4.Bemis DA, Greisen HA, Appel MJ. Bacteriological variation among Bordetella bronchiseptica isolates from dogs and other species. J Clin Microbiol. 1977;5:471–480. doi: 10.1128/jcm.5.4.471-480.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bemis DA, Plotkin BJ. Hemagglutination by Bordetella bronchiseptica. J Clin Microbiol. 1982;15:1120–1127. doi: 10.1128/jcm.15.6.1120-1127.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binns SH, Speakman AJ, Dawson S, Bennett M, Gaskell RM, Hart CA. The use of pulsed-field gel electrophoresis to examine the epidemiology of Bordetella bronchiseptica isolated from cats and other species. Epidemiol Infect. 1998;120:201–208. doi: 10.1017/s0950268897008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang N, Chui L. A standardized protocol for the rapid preparation of bacterial DNA for pulsed-field gel electrophoresis. Diagn Microbiol Infect Dis. 1998;31:275–279. doi: 10.1016/s0732-8893(98)00007-8. [DOI] [PubMed] [Google Scholar]
- 8.Cowan ST, Steel KJ. Manual for the Identification of Medical Bacterial. 2nd ed. London: Cambridge University Press; 1974. pp. 89–90. [Google Scholar]
- 9.Gautom RK. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gramnegative organisms in 1 day. J Clin Microbiol. 1997;35:2977–2980. doi: 10.1128/jcm.35.11.2977-2980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles CJ. Bordetellosis. In: Leman AD, Straw BE, Mengeling WL, D'Allaire S, Taylor DJ, editors. Disease of Swine. 7th ed. Ames: Iowa State University Press; 1992. pp. 436–445. [Google Scholar]
- 11.Graham AC, Abruzzo GK. Occurrence and characterization of plasmids in field isolates of Bordetella bronchiseptica. Am J Vet Res. 1982;43:1852–1855. [PubMed] [Google Scholar]
- 12.Grimont F, Grimont PA. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986;137B:165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- 13.Harris DL, Switzer WP. Turbinate atrophy in young pigs exposed to Bordetella bronchiseptica, Pasteurella multocida, and combined inoculum. Am J Vet Res. 1968;29:777–785. [PubMed] [Google Scholar]
- 14.Johnson R, Sneath PHA. Taxonomy of Bordetella and related organisms of the families Achromobacteraceae, Brucellaeae and Neisseriaceae. Int J Syst Bacteriol. 1973;23:381–404. [Google Scholar]
- 15.Keil DJ, Fenwick B. Evaluation of canine Bordetella bronchiseptica isolates using randomly amplified polymorphic DNA fingerprinting and ribotyping. Vet Microbiol. 1999;66:41–51. doi: 10.1016/s0378-1135(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 16.Khattak MN, Matthews RC. Genetic relatedness of Bordetella species as determined by macrorestriction digests resolved by pulsed-field gel electrophoresis. Int J Syst Bacteriol. 1993;43:659–664. doi: 10.1099/00207713-43-4-659. [DOI] [PubMed] [Google Scholar]
- 17.Moissenet D, Valcin M, Marchand V, Grimprel E, Begue P, Garbarg-Chenon A, Vu-Thien H. Comparative DNA analysis of Bordetella pertussis clinical isolates by pulsed-field gel electrophoresis, randomly amplified polymorphism DNA, and ERIC polymerase chain reaction. FEMS Microbiol Lett. 1996;143:127–132. doi: 10.1111/j.1574-6968.1996.tb08471.x. [DOI] [PubMed] [Google Scholar]
- 18.Musser JM, Hewlett EL, Peppler MS, Selander RK. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986;166:230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen KB. The serology of Bordetella bronchiseptica isolated from pigs compared with strains from other animal species. Acta Pathol Microbiol Scand Suppl. 1975;83:590–594. doi: 10.1111/j.1699-0463.1975.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 20.Peppler MS, Schrumpf ME. Phenotypic variation and modulation in Bordetella bronchiseptica. Infect Immun. 1984;44:681–687. doi: 10.1128/iai.44.3.681-687.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Register KB, Boisvert A, Ackermann MR. Use of ribotyping to distinguish Bordetella bronchiseptica isolates. Int J Syst Bacteriol. 1997;47:678–683. doi: 10.1099/00207713-47-3-678. [DOI] [PubMed] [Google Scholar]
- 22.Ross RF, Switzer WP, Duncan JR. Comparison of pathogenicity of various isolates of Bordetella bronchiseptica in young pigs. Can J Comp Med Vet Sci. 1967;31:53–57. [PMC free article] [PubMed] [Google Scholar]
- 23.Rutter JM, Collings LA. The virulence of Bordetella bronchiseptica in atrophic rhinitis of pigs. In: Pedersen KB, Nielsen NC, editors. Atrophic Rhinitis in Pigs. Luxembourg: Commission of the European Communities; 1983. pp. 77–83. [Google Scholar]
- 24.Sacco RE, Register KB, Nordholm GE. Restriction endonuclease analysis discriminates Bordetella bronchiseptica isolates. J Clin Microbiol. 2000;38:4387–4393. doi: 10.1128/jcm.38.12.4387-4393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo YS, Lee SH, Shin EK, Kim SJ, Jung R, Hahn TW. Pulsed-field gel electrophoresis genotyping of Salmonella gallinarum and comparison with random amplified polymorphic DNA. Vet Microbiol. 2006;115:349–357. doi: 10.1016/j.vetmic.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Smith IM, Baskerville AJ. A selective medium facilitating the isolation and recognition the Bordetella bronchiseptica in pigs. Res Vet Sci. 1979;27:187–192. [PubMed] [Google Scholar]
- 27.Stull TL, LiPuma JJ, Edlind TD. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988;157:280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- 28.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tynkkynen S, Satokari R, Saarela M, Mattila-Sandholm T, Saxelin M. Comparison of ribotyping, randomly amplified polymorphic DNA analysis, and pulsed-field gel electrophoresis in typing of Lactobacillus rhamnosus and L. casei strains. Appl Environ Microbiol. 1999;65:3908–3914. doi: 10.1128/aem.65.9.3908-3914.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vu-Thien H, Dulot C, Moissenet D, Fauroux B, Garbarg-Chenon A. Comparison of randomly amplified polymorphic DNA analysis and pulsed-field gel electrophoresis for typing of Moraxella catarrhalis strains. J Clin Microbiol. 1999;37:450–452. doi: 10.1128/jcm.37.2.450-452.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winstanley C, Shina A, Dawson S, Gaskell RM, Hart CA. Variation in Bordetella bronchiseptica flaA does not correlate with typing by macro-restriction analysis by pulsed-field gel electrophoresis. J Med Microbiol. 2001;50:255–260. doi: 10.1099/0022-1317-50-3-255. [DOI] [PubMed] [Google Scholar]
- 32.Yuk MH, Heininger U, Martinez de Tejada G, Miller JF. Human but not ovine isolates of Bordetella parapertussis are highly clonal as determined by PCR-based RAPD fingerprinting. Infection. 1998;26:270–273. doi: 10.1007/BF02962245. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Rajagopalan M, Brown BA, Wallace RJ., Jr Randomly amplified polymorphic DNA PCR for comparison of Mycobacterium abscessus strains from nosocomial outbreaks. J Clin Microbiol. 1997;35:3132–3139. doi: 10.1128/jcm.35.12.3132-3139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]