Abstract
A model that provides reproducible, submaximal yet sufficient spinal cord injury is needed to allow experiments leading to development of therapeutic techniques and prediction of clinical outcome to be conducted. This study describes an experimental model for spinal cord injury that uses three different volumes of balloon inflation and durations of compression to create a controlled gradation outcome in adult dogs. Twenty-seven mongrel dogs were used for this study. A 3-french embolectomy catheter was inserted into the epidural space through a left hemilaminectomy hole at the L4 vertebral arch. Balloons were then inflated with 50, 100, or 150 µl of a contrast agent at the L1 level for 6, 12, or 24 h and spinal canal occlusion (SCO) measured using computed tomography. Olby score was used to evaluate the extent of spinal cord injury and a histopathologic examination was conducted 1 week after surgery. The SCO of the 50, 100, and 150 µl inflations was 22-46%, 51-70%, and 75-89%, respectively (p < 0.05). Olby scores were diminished significantly by a combination of the level of SCO and duration of inflation in all groups. Olby scores in the groups of 150 µl-12 h, 150 µl-24 h, and 100 µl-24 h were 0.5, 0, and 1.7, respectively. Based on these results, a SCO > 50% for 24 h, and > 75% for 12 h induces paraplegia up to a week after spinal cord injury.
Keywords: dog, balloon catheter, spinal cord compression injury, spinal cord occlusion
Introduction
Spinal cord injury models have been developed for studies related to development of therapeutics and surgical techniques, as well as for prediction of clinical outcome and prognosis [4,20]. These models are required to establish reliable, submaximal yet sufficient spinal cord injuries for study.
Several techniques, such as the NYU impactor [15], electromagnetic devices [16], and an aneurysm clip [21], have been developed for use as spinal cord injury models. A computer-controlled distraction [2] and a method of sustained cord injury [1] are regarded as good methods to provide critical data, however, these spinal cord injury models generally require custom-built lesion-making devices. Hemisection, transection, and bridge defect on the spinal cord have also been used to produce an injury for implant therapy [6,8], however these methods were different than those used to reproduce spontaneous injury. In addition, all of the models described above require laminectomy to expose the site of spinal cord injury, which could interfere with the delivery of therapeutics due to adhesion with surround tissues [13].
Since Tarlov first described the balloon-induced closed injury method in 1953, a number of modified balloon-induced methods have been developed over the past 50 years [3]. The balloon-induced method has been used because it is a simple method that does not cause any damage to the surrounding structures and dose-response based on volume of the balloon and degree of injury occurs in rats and dogs [20]. Although many studies have been performed using the balloon-induced method without direct exposure to the injury site, these studies were mainly volume dependent [9]. Therefore, the volume of balloon inflation must be measured again and used in combination with the size of the experimental animals when determining a sufficient amount of injury to inflict.
More recently, Purdy et al. [13] reported that both volume of inflation and duration of compression affected the degree of spinal cord injury. However, in this study, a percutaneous translumbar angioplasty balloon was placed in large dogs, resulting in an intradural compression of the spinal cord. Most of the injuries that occurred in this study, such as intervertebral disc extrusion and vertebral fracture, were affected extraduraly, therefore Purdy's model would not apply to naturally occurring animal spinal cord injuries. For this reason, we attempted to create a more detailed animal model of spinal cord compression injury that relied on 2 factors; volume of balloon inflation and duration of compression. We also compared functional outcomes to the percentage of spinal cord compression.
Materials and Methods
Animals
Twenty-seven healthy adult mongrel dogs comprised of 20 males and 7 females, aged 2 to 5 years old, with a body weight between 3 and 5 kg (3.8 ± 0.76 kg) were used in this study. Animals were assigned to one of the following nine groups: 50 µl-6 h, 100 µl-6 h, 150 µl-6 h, 50 µl-12 h, 100 µl-12 h, 150 µl-12 h, 50 µl-24 h, 100 µl-24 h, and 150 µl-24 h. The effects of the combinations of 3 different volumes (50, 100, 150 µl) of balloon inflation and three different durations (6, 12, 24 h) of balloon compression on each of the nine groups was then evaluated. All animal experiments were performed in accordance with the Seoul National University's Guidelines for Animal Experiments.
Induction of spinal cord Injury
The dogs were anesthetized by intravenous administration of diazepam (Melode; Dong Wha Pharm, Korea) at a dose of 0.3 mg/kg and propofol (Anepol; Ha Na Pharm, Korea) at 6 mg/kg with subcutaneous administration of atropine sulfate (Atropine; Je Il Pharm, Korea) at 0.05 mg/kg. Anesthesia was maintained by inhalation of 2% isoflurane (Aerane; Ilisung, Korea). Datex-Ohmeda (Microvtec Display, UK) was used to monitor physiologic conditions including rectal temperature, oxygen saturation, and pulse rate during anesthesia.
Dogs were laid in ventral recumbency and a left paramedian approach performed for left hemilaminectomy of L4. A three to five millimeter hole was made in the left vertebral arch of L4 using a high-speed pneumatic burr. A 3-french embolectomy occlusion catheter (SORIN Biomedica, Italy) was inserted into the hole drilled in the L4 vertebral arch (Fig. 1). Next, the balloon was advanced, under fluoroscopic guidance, until the tip of the catheter was under the cranial margin of the L1 vertebral body. The balloon was then inflated by injection of a contrast agent (Omnipaque; Amersham Health, Ireland) diluted 50 : 50 with saline. After soft tissues and skin were closed, computed tomography was performed to confirm the size and location of spinal cord occlusion. The inflated balloon was fixed using a Chinese finger type suture and then deflated after the scheduled duration of the injury (6, 12, or 24 h). After the operation, dogs were monitored in an intensive care unit (ICU), and if needed, manual bladder expression was performed at least three times a day until voluntary urination was established.
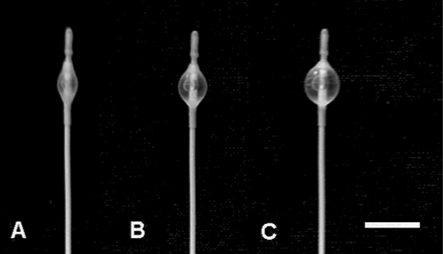
Fig. 1.
Three 3-french embolectomy occlusion catheters were inflated with 50 (A), 100 (B), and 150 (C) µl of contrast agent diluted 50 : 50 with saline. bar = 1 cm.
Evaluations
Spinal cord occlusion (SCO) assessment
Computed tomography was performed using a helical CT scanner (GE CT/e; General Electronic Medical System, Japan) under general anesthesia immediately after balloon inflation. Initially, scout lateral and ventrodorsal views were obtained. Thereafter, axial scanning was performed at the 1st lumbar vertebra level (slice thickness: 1 mm, interval: 1 mm, 120 kVp, 80 mA). The area of spinal canal was measured using a vertebra window (W/L; 4000/500) and the area of balloon inflation was measured with a spine window (W/L; 400/40). A-index, the ratio of the area of balloon to spinal canal, was calculated (Fig. 2) [19]. SCO was determined using the A-index and expressed as percentage of occlusion.
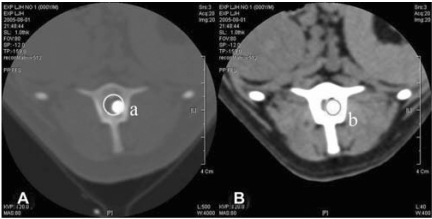
Fig. 2.
Transverse CT images of the epicenter of the lesion after balloon inflation. The 'a' represents the area of spinal canal in the vertebra window (A) and 'b' the area of balloon inflation in the spine window (B). The formula for the A-index calculation is b/a ×100 (%).
Behavioral assessment
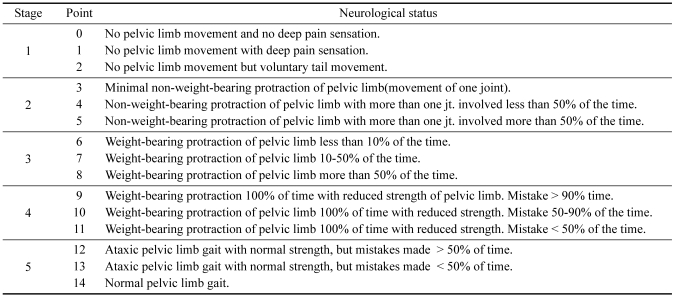
The first behavioral testing was performed 24 h after injury, then daily until 1 week after spinal cord injury. Each dog was videotaped walking on the floor for a minimum of 10 steps from both sides and behind. Dogs not able to bear weight on their hind limbs were also videotaped while supported by holding the base of their tail. Using the 15-point scoring system (Table 1), the dogs' gait was independently scored from the videotapes by 2 individuals unaware of experimental conditions [11]. The mean score was calculated for each dog at each time point.
Table 1.
Assessment of pelvic limb function by Olby score*
*Olby et al., 2001 [12].
Histopathological assessment
To assess histopathological changes, all dogs were euthanized 1 week after surgery. The spinal cords of T10 to L4 from all the dogs were harvested without perfusion and fixed in 10% formaldehyde. The specimens were cut into 5-millimeter-length transverse sections and 4-micrometer-thick slices made from each of these sections were mounted on silane-coated glass slides. The slides were then stained with hematoxylin-eosin (H&E) to detect hemorrhage and vacuolar formation.
Statistical evaluation
One-way analysis of variance was performed using SCO as a dependant variable for volume of balloon inflations. Olby score was used as a dependant variable for analysis with a univariate general linear model with 2 fixed factors; volume and duration of balloon inflation. A p value of < 0.05 was considered statistically significant for all analyses.
Results
Spinal canal occlusion
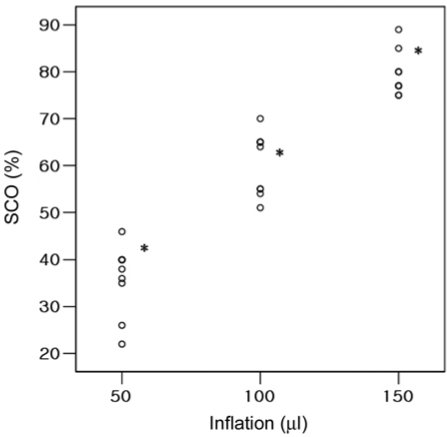
SCO with 50, 100, and 150 µl of balloon inflation were 22-46, 51-70, and 75-89%, respectively. Increasing the volume of inflation resulted in a statistically significant increase of the percentage of SCO (p < 0.05) (Fig. 3).
Fig. 3.
Percentage of spinal cord occlusion (SCO) in relation to volumes of inflation. *Indicates significant differences (p < 0.05).
Behavioral outcomes
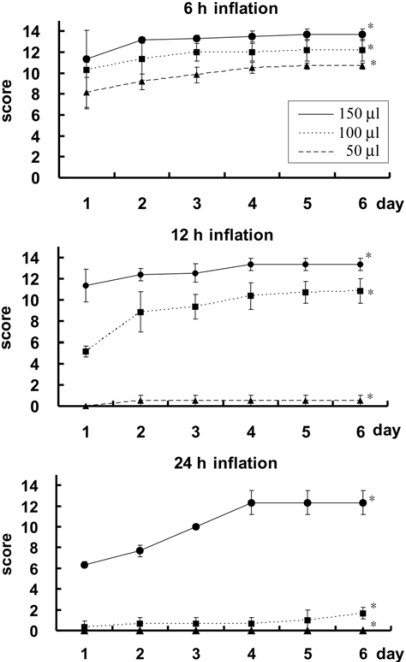
The 150 µl-12 h, 100 µl-24 h, and 150 µl-24 h groups showed paraplegia on the first day of injury with Olby scores of 0, 0.3, and 0, respectively (Fig. 4). The remaining groups showed various degrees of paraparesis. Most of the paraplegic dogs showed involuntary urination on the first day following spinal cord injury. Manual bladder expression was necessary in 2 dogs from the 150 µl-12 h group, two from the 100 µl-24 h group and all of the dogs in the 150 µl-24 h group. The gait of all dogs in groups with a 50 µl volume recovered to near normal (stage 5 of the Olby scoring system) by the fourth day. The groups of 50 µl-6 h, 100 µl-6 h, 150 µl-6 h, 50 µl-12 h, 100 µl-12 h, and 100 µl-24 h showed weight-bearing protraction of the pelvic limb a week after surgery with Olby scores of 13.7, 12.2, 10.7, 13.3, 10.8 and 12.3, respectively. The groups of 150 µl-12 h, 100 µl-24 h, and 150 µl-24 h remained paraplegic for a week after the surgery with Olby scores of 0.5, 1.7, and 0, respectively. Olby score between groups at each day after operation was very significant differences (p < 0.01).
Fig. 4.
Change of Olby scores after spinal cord injury. Data points represent the group means ± SD. Significant differences in Olby score between groups at each day after operation are shown. *Indicates significant differences (p < 0.01).
Histopathology
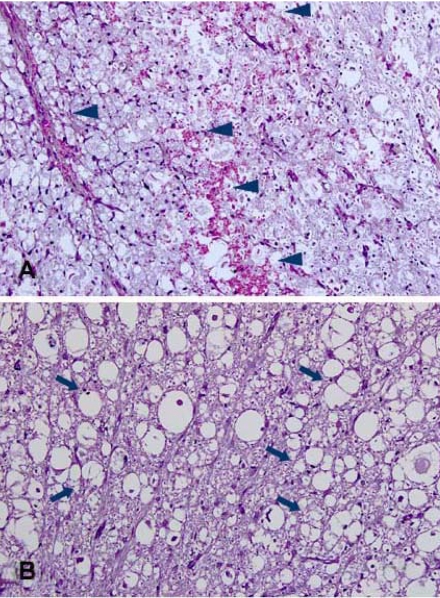
Upon gross observation, dura and surrounding tissues were clear in the 50 µl-6 h group, and relatively clear in the 100 µl-6 h and 150 µl-6 h groups. Thinning of the dura and paleness of the spinal cord was observed in one dog in the 100 µl-12 h group and one in the 50 µl-24 h group as well as in all of the dogs in the 100 µl-24 h, 150 µl-12 h and 150 µl-24 h groups. Varying adhesions of dura were seen in the 50 µl-24 h, 100 µl-12 h, 100 µl-24 h, 150 µl-12 h and 150 µl -24 h groups. Histopathologic examination did not show hemorrhage or vacuolar formations in the 50 µl-6 h group. Hemorrhage and vacuolar formations were detected in one dog in the 100 µl-6 h group. Evidence of hemorrhage was seen in all animals in the 150 µl-6 h group and vacuolar formation was detected in one dog from this group as well. All remaining groups showed evidence of hemorrhage, but vacuolar formations were not seen in the 50 µl-12 h, 100 µl-12 h and 50 µl-24 h groups. There was severe hemorrhage and vacuolar formation in the 150 µl-12 h, 100 µl-24 h, and 150 µl-24 h groups (Fig. 5).
Fig. 5.
Histopathological findings at injured epicenter in the 150 µl-12 h (A) and 150 µl-24 h (B) groups 1 week after spinal cord injury. Severe hemorrhages (arrowheads) (A) and vacuolar formations (arrows) (B) were observed. H&E stain. ×200.
Discussion
The results of this study indicate that using a combination of three different volumes and 3 different durations of balloon-compression could produce a gradable model of canine spinal cord injury. The three different volumes used in this study produced SCOs less than 50%, between 50% and 75%, and greater than 75%.
Severe spinal cord injuries generally did not heal, regardless of the type of intervention. On the other hand, minor injuries showed improvement even when little or no treatment was provided. Therefore, in order to make a valid model of a severe injury, it is important to establish a model of irreversible injury by applying minimal but sufficient compression to the spinal cord [3,18]. In addition, useful experimental models should fulfill several criteria, including production of reproducible, quantifiable pathologic changes comparable to clinical lesions and production of measurable neurological deficits [10].
This study showed that the duration of compression affected the outcome of the experiment. Among the groups that demonstrated SCO > 50% for 24 h, and > 75% for 12 h groups remained paraplegic for 1 week. When greater than 75% compression was applied, the histopathology of the 150 µl-12 h and 150 µl-24 h groups showed severe hemorrhages and vacuolar formations and no functional improvement was observed during the week following surgery.
In a recent canine study, Purdy et al. [13] demonstrated that the severity of compression injuries depended on the level of SCO, and that three different durations of compression (30 min, 2 h, and 4 h) had less effect than the degrees of compression. However, these durations may not have been long enough to contribute the severity of injury. In addition, considering that surgeons cannot see most canine patients with spinal cord injury within these periods, these periods of time do not realistically mimic clinical situations. However, Carlson et al. [1] reported that longer periods of displacement allow propagation of the secondary injury process, resulting in limited functional recovery and more extensive tissue damage. In our study, we extended the durations of compression up to 24 h and demonstrated that both the degree and duration of compression could modulate the functional outcomes 1 week after a severe spinal cord injury.
To date, dogs have commonly been used to study spinal cord injuries because neurological examinations could be carried out easily, and more detailed pathophysiological studies could be conducted [3]. In addition, the balloon compression technique provides a scale of animal models that mimic human spinal injury with quantifiable trauma stimulus that could be correlated with functional recovery and morphology of the cord lesion [20]. This experimental traumatic spinal cord injury was also suitable for research on intervertebral disc herniation and vertebral fractures that were seen commonly in canine spinal cord injury [12]. Naturally, the method using the subarachnoid approach had an advantage for studying the spinal cord itself, as opposed to simple removal of the mass. However, when this approach was used, the introduced catheter was likely to damage the blood-brain barrier of the spinal cord and disturb maintenance of spinal cord homeostasis. [3].
More recently, a study by Fukuda et al. [3] introduced a new balloon method without laminectomy that was simple and took only 2 to 3 h to implement. The study by Fukuda also had only a few complications, including hemorrhage from a segmental artery and vein running along the spinal nerve root [3]. Compared to the study by Fukuda, our method of drilling a hemilaminectomy hole for insertion of a balloon catheter provided easy exposure of the spinal cord with no risk of hemorrhage in a relatively short time (30 min to 1 h). Embolectomy catheters used in this study, commonly used as arterial catheters, were more rigid and less irritable than other forgaty catheters [5]. The catheter used in the present study also had the advantage of being detectable in fluoroscopy without contrast.
The Olby scoring system was used for quantitative evaluation of functional outcomes in this study and facilitated behavioral analysis of canine models. Classically, the Tarlov scale has been used for quantitative evaluation of neurological status resulting from spinal cord injury in dogs [14,17]. BBB score for rodents or modification of the Tarlov scale also have been used [7], however, those scoring systems were not sensitive enough to describe the details of functional status due to the large variation resulting from the wide category of each level. Olby et al. [11] modified the BBB open field scoring system for dogs based on pelvic limb gait of dogs with acute spinal cord injury resulting in thoracolumbar vertebral disc herniations. The reliability of Olby score has been verified by several studies [12,22].
We did not utilize the somatosensory evoked potential (SEP) measurements of each group to compare the severity of injury. Generally, SEP had been used as an objective standard that showed a flat waveform when the spinal cord was injured irreversibly [17]. However, in dogs, although SEP measures of the severity of spinal cord injury were somewhat correlated with the functional outcomes of the animal, SEP measurements were less sensitive than even crude functional scoring [11].
It is suggested that a SCO > 50% for 24 h, and > 75% for 12 h would be appropriate for use as a severe spinal cord injury model. Further investigation, including an extended period of follow up and imaging study for identifying the lesions with parenchymal abnormalities using magnetic resonance image, should be conducted.
Acknowledgments
This work was supported by the BK21 Program for Veterinary Science and Seoul R&BD Program (10548).
References
- 1.Carlson GD, Gorden CD, Oliff HS, Pillai JJ, LaManna JC. Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J Bone Joint Surg Am. 2003;85-A:86–94. [PubMed] [Google Scholar]
- 2.Dabney KW, Ehrenshteyn M, Agresta CA, Twiss JL, Stern G, Tice L, Salzman SK. A model of experimental spinal cord trauma based on computer-controlled intervertebral distraction: characterization of graded injury. Spine. 2004;29:2357–2364. doi: 10.1097/01.brs.0000143108.65385.74. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda S, Nakamura T, Kishigami Y, Endo K, Azuma T, Fujikawa T, Tsutsumi S, Shimizu Y. New canine spinal cord injury model free from laminectomy. Brain Res Brain Res Protoc. 2005;14:171–180. doi: 10.1016/j.brainresprot.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Grill RJ. User-defined variables that affect outcome in spinal cord contusion/compression models. Exp Neurol. 2005;196:1–5. doi: 10.1016/j.expneurol.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Haldipur N, Tan P, Katory M, Singh S. A safe method of retrograde passage of fogarty embolectomy catheter through difficult iliac arteries. Eur J Vasc Endovasc Surg. 2002;23:559–561. doi: 10.1053/ejvs.2002.1627. [DOI] [PubMed] [Google Scholar]
- 6.Himes BT, Liu Y, Solowska JM, Snyder EY, Fischer I, Tessler A. Transplants of cells genetically modified to express neurotrophin-3 rescue axotomized Clarke's nucleus neurons after spinal cord hemisection in adult rats. J Neurosci Res. 2001;65:549–564. doi: 10.1002/jnr.1185. [DOI] [PubMed] [Google Scholar]
- 7.Kuh SU, Cho YE, Yoon DH, Kim KN, Ha Y. Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir. 2005;147:985–992. doi: 10.1007/s00701-005-0538-y. [DOI] [PubMed] [Google Scholar]
- 8.Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Martin D, Schoenen J, Delree P, Gilson V, Rogister B, Leprince P, Stevenaert A, Moonen G. Experimental acute traumatic injury of the adult rat spinal cord by a subdural inflatable balloon: methodology, behavioral analysis, and histopathology. J Neurosci Res. 1992;32:539–550. doi: 10.1002/jnr.490320409. [DOI] [PubMed] [Google Scholar]
- 10.Olby N. Current concepts in the management of acute spinal cord injury. J Vet Intern Med. 1999;13:399–407. doi: 10.1892/0891-6640(1999)013<0399:ccitmo>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Olby N, Harris T, Burr J, Munana K, Sharp N, Keene B. Recovery of pelvic limb function in dogs following acute intervertebral disc herniations. J Neurotrauma. 2004;21:49–59. doi: 10.1089/089771504772695940. [DOI] [PubMed] [Google Scholar]
- 12.Olby NJ, De Risio L, Munana KR, Wosar MA, Skeen TM, Sharp NJ, Keene BW. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res. 2001;62:1624–1628. doi: 10.2460/ajvr.2001.62.1624. [DOI] [PubMed] [Google Scholar]
- 13.Purdy PD, White CL, 3rd, Baer DL, Frawley WH, Reichard RR, Pride GL, Jr, Adams C, Miller S, Hladik CL, Yetkin Z. Percutaneous translumbar spinal cord compression injury in dogs from an angioplasty balloon: MR and histopathologic changes with balloon sizes and compression times. AJNR Am J Neuroradiol. 2004;25:1435–1442. [PMC free article] [PubMed] [Google Scholar]
- 14.Rucker N, Lumb W, Scott R. Combined pharmacologic and surgical treatments for acute spinal cord trauma. Am J Vet Res. 1981;42:1138–1142. [PubMed] [Google Scholar]
- 15.Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine. 2004;29:1971–1979. doi: 10.1097/01.brs.0000138273.02820.0a. [DOI] [PubMed] [Google Scholar]
- 16.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 17.Shores A, Redding RW, Knecht CD. Spinal-evoked potentials in dogs with acute compressive thoracolumbar spinal cord disease. Am J Vet Res. 1987;48:1525–1530. [PubMed] [Google Scholar]
- 18.Sykova E, Jendelova P, Urdzikova L, Lesny P, Hejcl A. Bone Marrow Stem Cells and Polymer Hydrogels-Two Strategies for Spinal Cord Injury Repair. Cell Mol Neurobiol. 2006;26:1113–1129. doi: 10.1007/s10571-006-9007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thelander U, Fagerlund M, Friberg S, Larsson S. Describing the size of lumbar disc herniations using computed tomography. A comparison of different size index calculations and their relation to sciatica. Spine. 1994;19:1979–1984. doi: 10.1097/00007632-199409000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Vanicky I, Urdzikova L, Saganova K, Cizkova D, Galik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma. 2001;18:1399–1407. doi: 10.1089/08977150152725687. [DOI] [PubMed] [Google Scholar]
- 21.von Euler M, Seiger A, Sundstrom E. Clip compression injury in the spinal cord: a correlative study of neurological and morphological alterations. Exp Neurol. 1997;145:502–510. doi: 10.1006/exnr.1997.6481. [DOI] [PubMed] [Google Scholar]
- 22.Webb AA, Jeffery ND, Olby NJ, Muir GD. Behavioural analysis of the efficacy of treatments for injuries to the spinal cord in animals. Vet Rec. 2004;155:225–230. doi: 10.1136/vr.155.8.225. [DOI] [PubMed] [Google Scholar]