Abstract
Pressure overload diseases, such as valvular stenosis and systemic hypertension, manifest morphologically in patients as cardiac concentric hypertrophy. Prevention of cardiac remodeling due to increased pressure overload is important to reduce morbidity and mortality. Epigallocatechin-3 gallate (EGCG) is a major bioactive polyphenol present in green tea which has been found to be a nitric oxide-mediated vasorelaxant and to be cardioprotective in myocardial ischemia-reperfusion injury. Therefore, we investigated whether EGCG supplementation could reduce in vivo pressure overload-mediated cardiac hypertrophy. Cardiac hypertrophy was induced by suprarenal transverse abdominal aortic constriction (AC) in rats. Three weeks after AC surgery, heart to body weight ratio increased in the AC group by 34% compared to the sham group. EGCG administration suppressed the load-induced increase in heart weight by 69%. Attenuation of cardiac hypertrophy by EGCG was associated with attenuation of the increase in myocyte cell size and fibrosis induced by aortic constriction. Despite abolition of hypertrophy by EGCG, transstenotic pressure gradients did not change. Echocardiogram revealed that increased left ventricular systolic dimensions and deteriorated systolic function were relieved by EGCG. These results suggest that EGCG prevents the development of left ventricular concentric hypertrophy by pressure overload and may be a useful therapeutic modality to prevent cardiac remodeling in patients with pressure overload myocardial diseases.
Keywords: cardiac hypertrophy, EGCG, pressure overload
Introduction
Increased cardiovascular mortality is a serious problem in modern societies. Minimizing the risk of cardiac disease and alleviating the complications of cardiovascular dysfunction are the main therapeutic aims in modern medicine. Most heart diseases, regardless of etiology or pathogenesis, progress to congestive heart failure causing mortality [17]. The prognosis for patients with heart failure depends on the severity of cardiac dysfunction and the presence of complications and generally varies from guarded to poor. The single most powerful predictor for the development of heart failure is the presence of cardiac hypertrophy [18].
Cardiac concentric hypertrophy is a homeostatic response to elevated afterload that develops due to pressure overload (e.g. systemic hypertension or valvular stenosis). Although cardiac hypertrophy is the initial compensatory response to increased wall stress, it is followed by decompensating hypertrophy and ultimately leads to heart failure if the stimulus is sufficiently intense and prolonged. Cardiac concentric hypertrophy stimulated by an increase in blood pressure and wall stress is thought to be regulated by a number of intracellular signal transduction pathways including mitogen-activated protein kinase (MAPK), Janus kinase/signal transducers, activators of transcription (JAK/STAT), calcineurin, serine/threonine kinase, Akt and its downstream target, glycogen synthase kinase-3β [21]. Neurohormonal factors such as angiotensin II (Ang II) and endothelin-1 are activators of MAPK during conditions of pathological hypertrophy [27,29]. These observations have led to speculation that inhibition of these hypertrophic signals may provide effective clinical therapy for pathological hypertrophy and heart failure.
Based on considerable evidence accumulated during the last few years, much attention is focused on the use of naturally occurring botanicals for the prevention of many diseases. Epidemiological studies have shown a significant inverse association between dietary flavonoids and long-term mortality from coronary heart disease [5]. Epigallocatechin-3 gallate (EGCG), the major catechin derived from green tea, has been found to have protective effects on the cardiovascular system. These include anti-inflammatory effects [10], lowering of serum cholesterol levels, and reducing atherosclerosis [4]. The antioxidant properties of EGCG are well known [15] and have attracted considerable attention for the prevention of oxidative stress-related diseases such as ischemic heart diseases. The finding that reactive oxygen radicals significantly contribute to the genesis of reperfusion-induced dysrhythmias, contractile malfunction and vascular endothelium damage and that EGCG protects cardiac myocytes from ischemia/reperfusion injury also suggests that EGCG's cardioprotective effects may be mediated by free radical scavenging. However, there is little information about the effect of EGCG on cardiac hypertrophy. Recently, we demonstrated that EGCG inhibited platelet derived growth factor (PDGF)-BB-induced intracellular Ca2+ increase and extracellular signal-regulated kinase (ERK) activation in vascular smooth muscle cells (VSMCs) [1] and that EGCG could prevent Ang II-induced VSMCs hypertrophy through blocking c-Jun N-terminal kinases (JNKs) [29]. Moreover EGCG has been shown to inhibit PI3K/Akt and Erk1/2 pathways in several experimental models. These results suggest that EGCG could inhibit the signal pathways that regulate cardiac hypertrophy. Therefore, the purpose of this study is to investigate whether chronic administration of an oral daily dose of EGCG can attenuate cardiac hypertrophy induced by pressure overload in rats.
Materials and Methods
In vivo hypertrophy model
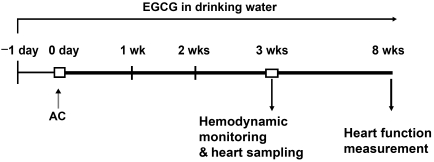
Male Sprague Dawley rats (7 weeks old, 200-220 g) were purchased from Koatech (Korea). The experimental protocol was approved by the Chungbuk National University Medical School Research Institutional Animal Care and Use Committee. All surgical procedures were performed on animals anesthetized with ketamine (80 mg/kg IP) and xylazine (5 mg/kg IP). Abdominal aortic constriction (AC) was performed using a 4-0 suture tied twice around the suprarenal aorta and a 21-gauge needle. The needle was then removed yielding a 0.8 mm internal diameter. Rats were randomly assigned to AC or sham-operated groups and the sham-operated rats underwent the same procedure, with the exception that the aorta was not constricted. A freshly prepared solution with different doses of EGCG (Sigma, USA) was supplied every day to aortic banded or sham-operated rats as the sole source of drinking water over a period of 21 consecutive days, whereas control animals were supplied with water from the same source, lacking EGCG (Fig. 1). Dietary administration was chosen to establish clinical relevance to human dietary habits. Establishment of cardiac hypertrophy was confirmed by echocardiography by measuring left ventricular (LV) wall thickness and dimensions, heart weight, and by histological analysis. The rats grew and gained weight during the course of the study, and as a control, we measured heart weight (HW) as a function of body weight (BW).
Fig. 1.
Experimental design. One day before the operation, rats were randomly treated with EGCG or no drug. EGCG was dissolved in drinking water and the administered solutions were replaced every day for 3 weeks. Hemodynamic and morphologic measurements were performed at for various time intervals after abdominal aortic constriction (AC). Cardiac function was measured after 8 weeks of AC.
Hemodynamics measurements
Rat blood pressure was evaluated by direct cannulation of the right carotid and left femoral artery. Mean arterial blood pressure, heart rate and pressure gradient between carotid and femoral arterial pressure were obtained from a pressure transducer attached to each cannula, which was inserted through a fluid-filled catheter. The values were recorded using a computer data acquisition system (ML870; AD Instrument, Australia) after blood pressure was stable for 10 min.
Histological analysis and cardiomyocyte size measurement
All hearts were arrested in diastole with KCl (30 mM), followed by perfusion fixation with 10% paraformaldehyde. Fixed hearts were embedded in paraffin, and 4 mm thick sections were stained with hematoxylin and eosin for assessing overall morphology.
The surface area of a 2D silhouette of the myocyte was estimated by measurement of the length and width at 20 different randomly chosen points from a cross section of the LV free wall. Morphometric analysis was performed with isolution software (IMT, Korea). Our 2D surface area (length × width, µm2) is directly proportional to the surface area of a cylinder (2π × radius × length). The extent of LV fibrosis was measured using Cason's trichrome staining. Five sections of each heart were measured.
Echocardiography
After 21 days of aortic constriction, rats were anesthetized with intraperitoneal pentobarbital (50 mg/kg), and cardiac dimension and function were analyzed by 10-MHz pulsewave Doppler echocardiography (SONOACE 8800; Medison, Korea). Two dimensionally guided M-mode of LV at the papillary level was obtained from the parasternal long-axis view. For each rat, measurements were made from at least 4 beats. LV cavity dimension and wall thickness were measured, and percent change in LV dimension (fractional shortening; FS) and relative wall thickness (RWT) were calculated as follows: FS = [(LVDd-LVSd)/LVDd] × 100, where LVDd is LV dimension at end-diastole and LVSd is LV dimension at end-systole. RWT = (posterior wall thickness at end diastole)/ LVDd × 2.
Statistical analysis
Results are presented as mean ± SE. Data obtained were compared using the unpaired Students t-test or one-way ANOVA. Statistical significance was defined as a value of P < 0.05.
Results
Effects of EGCG on load-induced increase in heart weight
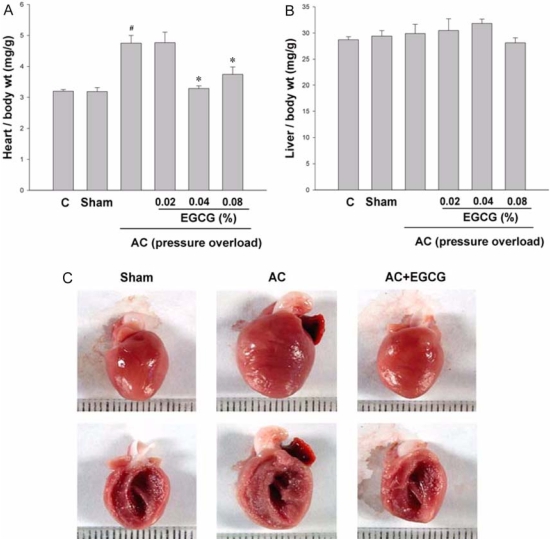
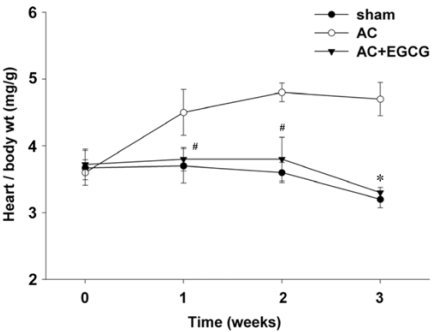
The amount of water intake was measured every day. Administration of EGCG in the drinking water at 0.02%, 0.04% and 0.08% did not alter water intake in either the sham-operated or aortic constricted rats. AC induced a 48% increase in HW/BW ratio compared to sham-operated rats. EGCG administration completely attenuated this increase (Fig. 2A). We found no significant difference between that the effect of 0.04% EGCG on pressure overload induced cardiac hypertrophy and that of 0.08% EGCG. The estimated EGCG intake of rats fed with 0.04% EGCG solution was 30 mg/kg. This estimate is based on an average consumption of 200 ml/day. These results are comparable with those in previous studies in which EGCG was non toxic to the liver and other organs after rats were injected intraperitoneally with 70-92 mg/kg daily [11]. Therefore, we selected 0.04% as the experimental dose. EGCG did not affect either body weight or liver weight (Fig. 2B). Next, we examined the effect of EGCG on HW/BW at various time intervals after AC surgery. During the 3 weeks after constriction, HW/BW ratio was significantly increased. The anti-hypertrophic effect of EGCG appeared as early as 1 week after surgery and was sustained during the course of the study (Fig. 4).
Fig. 2.
Inhibition of pressure overload induced cardiac hypertrophy in vivo by EGCG. A, Heart wet weight was normalized to body weight in each rat as an index of cardiac hypertrophy. Heart weight to body weight ratio was attenuated in a dose-dependent manner by EGCG after 3 weeks of aortic constriction (AC). B, Liver weight to body weight ratio. #P < 0.001 compared with sham, n = 8; *P < 0.001 compared with AC, n ≥ 6. C, Representative hearts of rats treated with AC or AC + 0.04% EGCG for 3 weeks. scale = 1 mm.
Fig. 4.
Inhibition of cardiac hypertrophy in vivo by EGCG at various time intervals with pressure overload. #P < 0.05 compared with sham, *P < 0.001 compared with AC (n ≥ 11).
Effects of ECGC on cardiac and myocyte morphometric changes
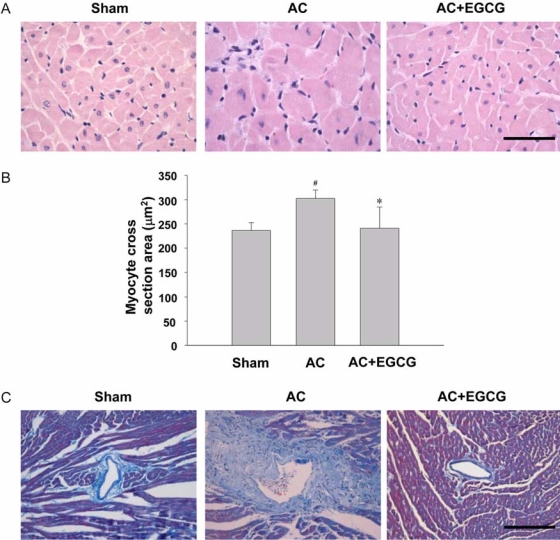
Aortic constriction caused a significant increase in both heart mass and LV wall thickness in comparison to sham-operated rats. AC-induced cardiac enlargement was almost completely prevented by EGCG, as shown in Fig. 2C. To examine whether EGCG changed heart size through the regulation of myocyte cell size, the myocyte cross-sectional area of the LV myocardium was measured in hearts from rats subjected to AC for 3 weeks (Fig. 3A & B). The cell area of aortic constricted rats was increased by 28% compared to sham-operated rats. This increase was almost completely abrogated by EGCG administration. AC also caused significant interstitial collagen deposition, as demonstrated by Masson's trichrome staining. This fibrosis was reduced in AC rats treated with EGCG for 3 weeks (Fig. 3C).
Fig. 3.
Effect of ECGC on myocyte cell area and cardiac fibrosis. A, heart tissues. H&E stain. bar = 50 µm. B, Cardiomyocyte cross sectional area was decreased in the EGCG-treated group after 3 weeks of aortic constriction (AC). #P < 0.001 compared with sham, *P < 0.001 compared with AC. C, Inhibition of perivascular fibrosis in vivo by EGCG. Cason's stain. bar = 200 µm.
Effects of EGCG on hemodynamic loading conditions
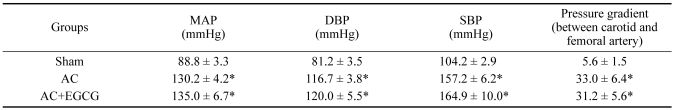
Blockade of cardiac hypertrophy by EGCG in pressure-overloaded rats raised the possibility that EGCG might ameliorate the hypertension induced by AC, and thus the blockade of the hypertrophy might have been caused secondarily by reduction of afterload rather than by a direct cardiac effect. To rule out this possibility, blood pressure was measured. As expected, aortic constriction markedly increased the blood pressure and produced a large pressure gradient between the carotid and femoral arteries. However, EGCG did not affect the blood pressure, so that there was no significant difference in pressure gradient between aortic-banded rats and EGCG-treated aortic-banded rats (Table 1). Thus, EGCG attenuated load-induced cardiac hypertrophy in the presence of comparable hemodynamic loading conditions.
Table 1.
Hemodynamic measurements at 3 weeks of pressure overload
Data are mean ± SE; n ≥ 8. AC, aortic constriction; MAP, mean arterial pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
*P < 0.001 compared with sham.
Effect of EGCG on cardiac function of aortic constricted rats
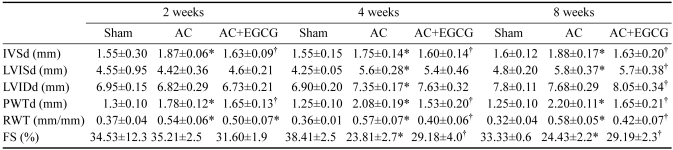
Eight weeks after constriction we examined the effect of EGCG on cardiac function using echocardiography (Fig. 5). Compared with sham-operated rats, aortic-banded rats displayed a substantial increase in interventricular septum thickness (IVSd) and LV posterior wall thickness in diastole (PWT). RWT was also markedly increased from 0.32 ± 0.04 in the sham group to 0.58 ± 0.05 in the AC group, which reveals evidence of concentric hypertrophy. Importantly, EGCG treatment significantly decreased PWT and RWT. EGCG-treated aortic-banded rats had a 20% increase in cardiac contractility, assessed by fractional shortening, compared to the AC group that did not receive EGCG (Table 2).
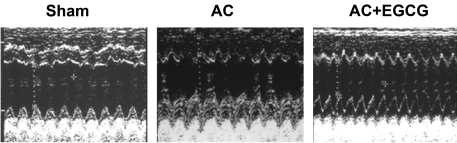
Fig. 5.
Representative M-mode echocardiograms after 8 weeks of aortic constriction (AC). Quantitative data are shown in Table 2. Concentric cardiac remodeling was observed in the AC group.
Table 2.
Echocardiographic changes after pressure overload
Data are mean ± SE; n ≥ 6. AC, aortic constriction; IVSd, interventricular septum thickness; LVISd, Left ventricular systolic diameter; LVIDd, Left ventricular diastolic diameter; PWTd, posterior wall thickness of the left ventricle in diastole, respectively; RWT, relative wall thickness; FS, fractional shortening. *P < 0.05 compared with the sham; †P < 0.05 compared to the AC.
Discussion
In the present study, we found that a daily oral dose of the bioflavonoid EGCG can attenuate the cardiac hypertrophic changes induced by pressure overload. In addition, we showed that EGCG not only prevented cardiac hypertrophy resulting from pressure overload, but also improved cardiac performance mostly by reducing LV end diastolic and systolic dimensions.
Sustained high blood pressure is one of the strongest causes of the development of cardiac hypertrophy. EGCG and related bioflavonoids have shown to have vasodilatory effects probably induced both by facilitating diuretic vasorelaxation and by evoking endothelial-dependent vasorelaxation [14]. Likewise, it has been demonstrated that EGCG has an anti-hypertensive effect in the 5/6 nephrectomy rat model [20]. Therefore, blockage of pressure overload induced cardiac hypertrophy by EGCG raised the possibility that EGCG might ameliorate the hypertension induced by AC surgery and that the blockage of hypertrophy might have been secondary to afterload reduction rather than a direct cardiac effect. In this study, AC produced a large pressure gradient between carotid and femoral arteries; this gradient was not affected by EGCG treatment (Table 1). Hemodynamic measurements 3 weeks after surgery revealed that EGCG had no significant effect on the transstenotic aortic pressure gradients over time. By contrast, it has been recently reported that EGCG administration attenuated the increased blood pressure induced by aortic banding and essentially blocked the development of cardiac hypertrophy in this model [13]. The conflicting results from this study of Li et al. [13] are probably due to the difference in the dose of ECGC administered and the more severe banding conditions. Although the beneficial effect of EGCG on cardiovascular structure seems to be related to its blood pressure lowering effect, it is unlikely that the reduction in cardiac hypertrophy seen with this lower dose of EGCG was caused by relief of pressure overload. Our data suggests that the primary target organ of EGCG is the heart rather than blood vessels.
Previous reports that EGCG induced apoptosis of tumor cells raise the possibility that apoptosis in cardiomyocytes may occur in our animal model, leading to a reduction in cardiac mass. We did not perform the TUNEL assay in this study but it has been previously reported that pressure overload itself leads to an increase in TUNEL-positive cardiomyocytes [26]. Moreover, EGCG does not significantly affect the survival of cultured rat cardiomyocytes and has even been shown to have a protective effect against apoptosis induced by ischemic reperfusion injury [23]. Although EGCG has proapoptotic effects in transformed cells, apoptosis of cardiomyocytes does not appear to be responsible for the reduction of heart mass.
Pressure overload induces LV hypertrophy as a compensatory response to increased wall stress. This has been considered the central mechanism by which cardiac function is maintained within normal ranges in chronically overloaded hearts. Accordingly, suppression of myocardial hypertrophy is expected to cause heart failure. However, it has been suggested that under conditions of pressure overload, the development of cardiac hypertrophy and normalization of wall stress may not be a required compensatory response to pressure overload and may not be necessary to preserve cardiac function. Recently, it has been demonstrated that using genetically engineered mice that have markedly blunted growth responses to pressure overload, cardiac function was well maintained after loading (using a partial aortic constriction), despite the failure to correct wall stress. Indeed, function was in fact better maintained than in wild-type mice, in which hypertrophy ensued [6]. Moreover, Hill et al. [8] reported preserved cardiac output without hypertrophic compensation in the setting of pressure overload in thoracic aortic banding mice treated with cyclosporine A.
In this study, we examined the effect of EGCG on cardiac function using echocardiography. Treatment with EGCG greatly attenuated cardiac hypertrophy and there was no detectable impairment of cardiac function for 3 weeks. This result suggests that treatment with EGCG allowed the heart to adapt to pressure overload, even in the absence of LV hypertrophy.
Maintenance of cardiac output in the face of increased afterload without sufficient LV hypertrophy implies a positive inotropic effect. It has been known that under some experimental conditions, an increase in afterload causes an increase in ventricular inotropy. This homeometric autoregulation is called the Anrep effect [25]. Increased inotropy partially compensates for the increased end-systolic volume and decreased stroke volume caused by an increase in afterload. In this study, EGCG-treated aortic-banded rats had increased LV diastolic dimensions after 4 weeks. We presume that the Anrep effect in the EGCG-treated aortic-banded rat heart, which is under sustained pressure overload, was gradually exhausted, whereas cardiac function is still maintained. Additional work will be necessary to test this hypothesis and to evaluate the long-term effects of EGCG on cardiac function and survival in aortic constricted rats.
Reactive oxygen species (ROS) have been shown in a variety of cell types to act as intracellular signaling molecules in the stress response, leading to apoptosis, proliferation, and transformation. In cardiomyocytes, ROS have been found to be involved in cardiac hypertrophy in vitro [22] and to mediate hypertrophy induced by several stimuli, such as mechanical stretch [19], Ang II [16], and tumor necrosis factor-α [7]. EGCG has been known to have potent antioxidant activity [15]. We found that the levels of malondialdehyde (MDA) were markedly increased in the AC group and EGCG treatment resulted in an almost complete elimination of the increase in MDA (data not shown). Therefore, we propose that the antioxidant effect might also play a role in the prevention of cardiac hypertrophy in AC rats treated with EGCG.
A different location of the aortic constriction may differently activate the signal transduction pathway. When the aortic arch was constricted, mechanical force may have been the major cause of cardiac hypertrophy. In contrast, the renin-angiotensin system (RAS) may be involved predominantly in suprarenal abdominal aorta constriction and contribute to the initial development of cardiac hypertrophy and sympathetic activation in the compensated heart. It has been shown that losartan, an Ang II AT1 receptor antagonist, attenuates cardiac hypertrophy in suprarenal abdominal aorta constricted rat [12], but has no effect on the weight gain of the ventricle during aortic arch constriction [2]. Given that EGCG can inhibit Ang II mediated signal transduction in cultured cardiomyocytes [28], the antihypertrophic effect of EGCG in the present study could have been mediated by RAS blockage. However, EGCG had no significant effect on systemic blood pressure, which sensitive to Ang II. These findings suggest that inhibition of RAS by EGCG does not play a major role in the EGCG-mediated antihypertrophic effect. Calcineurin inhibitors, such as cyclosporine A and FK506 have been well known to inhibit cardiac hypertrophy. However, nephrotoxicity and the immunosuppressive effect of calcineurin inhibitors limit their therapeutic benefit [24]. In our experiment, EGCG completely inhibited cardiac hypertrophy induced by pressure overload without significant effects on normal heart and liver. There was no evidence of nonspecific toxicity since no effects on normal growth, weight gain, or physical activity were found. Therefore, EGCG, or its derivatives, might be a useful modality to suppress cardiac hypertrophy.
In congestive heart failure by concentric hypertrophy, positive inotropic agents may significantly reduce intraventricular volume and cardiac output and thus contribute to a increase in mortality, so positive inotropes are prohibited in the treatment of concentric hypertrophy. Hotta et al. [9] showed that EGCG (10-6, 10-5 M) increased LV developed pressure and claimed that EGCG represents a positive inotropic effect via the nitric oxide pathway [9]. Given the low bioavailability of EGCG, it is unlikely that EGCG had an inotropic effect in our experimental system.
In this study, we demonstrated that EGCG (approximately 30 mg EGCG/kg body weight) prevents the development of cardiac hypertrophy induced by pressure overload. It has been reported that most of the EGCG does not get into the blood, but is excreted through the bile to the colon, so that serum concentration of total EGCG was 43 nM in male rats treated with 75 mg EGCG/kg body weight by gavage [3]. Even if such a low dose of EGCG had a positive inotropic effect, this effect partially compensated for the increase in afterload and helped the heart to adapt to pressure overload at the development of hypertrophy stage. However, we can not exclude the possibility that EGCG would have a harmful effect on advanced chronic heart failure and a severely dilated ventricle. Future studies are necessary to examine whether EGCG can regress established cardiac hypertrophy, which might be of greater clinical significance because the patient would most likely already have hypertrophy at the initiation of treatment.
In conclusion, this study demonstrates that EGCG completely inhibited cardiac hypertrophy and improved cardiac performance in pressure overloaded hearts. These findings may have important clinical implications in developing new therapeutic strategies to prevent the transition from cardiac hypertrophy to heart failure.
Acknowledgments
This study was supported by grants from Chungbuk National University (2005) and the Research Center for Bioresource and Health and Sama Pharmaceutical Co., Korea.
References
- 1.Ahn HY, Hadizadeh KR, Seul C, Yun YP, Vetter H, Sachinidis A. Epigallocathechin-3 gallate selectively inhibits the PDGF-BB-induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation of sis-transfected NIH 3T3 fibroblasts and human glioblastoma cells (A172) Mol Biol Cell. 1999;10:1093–1104. doi: 10.1091/mbc.10.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba HA, Iwai T, Bauer M, Irlbeck M, Schmid KW, Zimmer HG. Differential effects of angiotensin II receptor blockade on pressure-induced left ventricular hypertrophy and fibrosis in rats. J Mol Cell Cardiol. 1999;31:445–455. doi: 10.1006/jmcc.1998.0879. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- 4.Chyu KY, Babbidge SM, Zhao X, Dandillaya R, Rietveld AG, Yano J, Dimayuga P, Cercek B, Shah PK. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;109:2448–2453. doi: 10.1161/01.CIR.0000128034.70732.C2. [DOI] [PubMed] [Google Scholar]
- 5.Duarte J, Perez-Vizcaino F, Utrilla P, Jimenez J, Tamargo J, Zarzuelo A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen Pharmacol. 1993;24:857–862. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- 6.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi Y, Otsu K, Nishida K, Hirotani S, Nakayama H, Yamaguchi O, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of reactive oxygen species-mediated NF-κB activation in TNF-α-induced cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2002;34:233–240. doi: 10.1006/jmcc.2001.1505. [DOI] [PubMed] [Google Scholar]
- 8.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 9.Hotta Y, Huang L, Muto T, Yajima M, Miyazeki K, Ishikawa N, Fukuzawa Y, Wakida Y, Tushima H, Ando H, Nonogaki T. Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: the measurements of cellular Ca2+ and nitric oxide release. Eur J Pharmacol. 2006;552:123–130. doi: 10.1016/j.ejphar.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-κB signaling pathway. Pharm Res. 2004;21:661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 11.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Li P, Feng X, Li Z, Hou R, Han C, Zhang Y. Effects of losartan on pressure overload-induced cardiac gene expression profiling in rats. Clin Exp Pharmacol Physiol. 2003;30:827–832. doi: 10.1046/j.1440-1681.2003.03917.x. [DOI] [PubMed] [Google Scholar]
- 13.Li HL, Huang Y, Zhang CN, Liu G, Wei YS, Wang AB, Liu YQ, Hui RT, Wei C, Williams GM, Liu DP, Liang CC. Epigallocatechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic Biol Med. 2006;40:1756–1775. doi: 10.1016/j.freeradbiomed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Lorenz M, Wessler S, Follmann E, Michaelis W, Dusterhoft T, Baumann G, Stangl K, Stangl V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 15.Nagai K, Jiang MH, Hada J, Nagata T, Yajima Y, Yamamoto S, Nishizaki T. (-)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 2002;956:319–322. doi: 10.1016/s0006-8993(02)03564-3. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-α and angiotensin II. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- 17.Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, Grobbee DE. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999;20:447–455. [PubMed] [Google Scholar]
- 18.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 19.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res. 2001;89:453–460. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 20.Priyadarshi S, Valentine B, Han C, Fedorova OV, Bagrov AY, Liu J, Periyasamy SM, Kennedy D, Malhotra D, Xie Z, Shapiro JI. Effect of green tea extract on cardiac hypertrophy following 5/6 nephrectomy in the rat. Kidney Int. 2003;63:1785–1790. doi: 10.1046/j.1523-1755.2003.00914.x. [DOI] [PubMed] [Google Scholar]
- 21.Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res. 2000;47:23–37. doi: 10.1016/s0008-6363(00)00076-6. [DOI] [PubMed] [Google Scholar]
- 22.Siwik DA, Tzortzis JD, Pimental DR, Chang DL, Pagano PJ, Singh K, Sawyer DB, Colucci WS. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res. 1999;85:147–153. doi: 10.1161/01.res.85.2.147. [DOI] [PubMed] [Google Scholar]
- 23.Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004;18:1621–1623. doi: 10.1096/fj.04-1716fje. [DOI] [PubMed] [Google Scholar]
- 24.Ventura HO, Malik FS, Mehra MR, Stapleton DD, Smart FW. Mechanisms of hypertension in cardiac transplantation and the role of cyclosporine. Curr Opin Cardiol. 1997;12:375–381. [PubMed] [Google Scholar]
- 25.von Anrep G. On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol. 1912;45:307–317. doi: 10.1113/jphysiol.1912.sp001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA. 2003;100:15883–15888. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue TL, Gu JL, Wang C, Reith AD, Lee JC, Mirabile RC, Kreutz R, Wang Y, Maleeff B, Parsons AA, Ohlstein EH. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:37895–37901. doi: 10.1074/jbc.M007037200. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Song HJ, Kim CH, Kim HS, Kim EG, Sachinidis A, Ahn HY. Inhibitory effect of epigallocatechin 3-O-gallate on vascular smooth muscle cell hypertrophy induced by angiotensin II. J Cardiovasc Pharmacol. 2004;43:200–208. doi: 10.1097/00005344-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Zou Y, Komuro I, Yamazaki T, Kudoh S, Aikawa R, Zhu W, Shiojima I, Hiroi Y, Tobe K, Kadowaki T, Yazaki Y. Cell type-specific angiotensin II-evoked signal transduction pathways: critical roles of Gβγ subunit, Src family, and Ras in cardiac fibroblasts. Circ Res. 1998;82:337–345. doi: 10.1161/01.res.82.3.337. [DOI] [PubMed] [Google Scholar]