Abstract
Controlling Salmonella in integrated broiler operation is complicated because there are numerous potential sources of Salmonella contamination, including chicks, feed, rodents, wild poultry operations, and the processing plant. The objective of this study was to investigate the distribution of Salmonella through all phases of two integrated broiler operations and to determine the key areas related to the control of all known sources of infection. Two different Salmonella serotypes were observed at integrated broiler chicken company A. S. enteritidis, the predominant company A isolate, was consistently found in the breeder farm, hatcheries, broiler farms, and chicken slaughterhouse. At company B, a total of six different serotypes, S. heidelberg, S. senftenberg, S. enteritidis, S. blockley, S. gallinarum, and S. virchow, were detected. Although S. heidelberg was not found in the broiler farms, it was consistently found in the breeder farm, hatcheries, and chicken slaughterhouse. In addition, S. enteritidis was found in the hatcheries, broiler farm, and chicken slaughterhouse. In order to obtain the genetic clonality, 22 S. enteritidis isolates were digested with XbaI and analyzed by pulsed-field gel electrohporesis (PFGE). A difference in the PFGE pattern was found to be related to the origin of the integrated broiler operation. These data support the critical need to control Salmonella in breeder farms and hatcheries, and demonstrate important points related to the control of infection in large-scale poultry operations of Korea.
Keywords: broiler, operation, Salmonella spp. slaughterhouse
Introduction
Although many other pathogens have recently received considerable attention, salmonellae remain among the leading sources of food-borne illness throughout much of the world. In the last 10 to 15 years, a great increase in human food-borne infections caused by Salmonella, including Salmonella enterica subsp. enterica serovar Enteritidis, has been noted in the United States, Europe, Japan, and Korea.
Poultry products have consistently been identified as important sources of salmonellae that cause illness in humans. Ovarian or vertical transfer of infection from breeding hens to progeny is an important aspect of the epidemiology of Salmonella spp. infection within the poultry industry [12,14]. Salmonella control in integrated broiler operation is complicated because there are many opportunities for Salmonella to gain entry to these extensive, integrated operations and to be amplified by the mass production of feed, and the hatching, handling, and processing facilities [18,20].
The statutory monitoring and control of S. enteritidis in the UK has resulted in improved hygiene and biosecurity measures that have helped to control all Salmonella serovars. These control methods, together with the vaccination of breeders and layers, have considerably reduced the egg-borne transmission of S. enteritidis, and as a result, horizontal transmission from the farm, hatchery environment, or feed has gained importance in recent years [1].
The objective of this study was to investigate the distribution of Salmonella through all phases of two integrated broiler operations and to determine the key areas related to the control of infection at all known sources.
Materials and Methods
Sample collection: sample sites
Samples were obtained from five breeder farms, from four hatcheries, from ten broiler farms, and from two chicken slaughterhouses of two integrated broiler chicken companies.
Sample collection: breeder farms
Cloacal swabs, cecal droppings, nest box swabs, egg sorting, dispatch area swabs, and dust on the wall were collected for investigation. The swabs of nest box areas, and those taken from egg sorting and dispatch areas were collected using four premoistened 10 by 10 cm gauze pads with sterile buffered peptone water (BPW; Difco, USA) and then swabbing approximately 10 to 20 nest boxes and a 25 m2 egg sorting area. Cloacal swabs and cecal droppings were collected by swabbing or dipping with 50 sterile, cotton-tipped applicators into the cloaca or cecal dropping. Dust on the wall was collected by placing approximately 50 g in sterile Whirlpac bags. Each of the samples were taken directly and divided into two 225 ml BPW solutions.
Sample collection: hatcheries
Hatchery samples were collected on the day of hatching, and samples were obtained from hatcher interiors, chick sorting and dispatch areas, chick boxes with meconium, ventilation outlets, and waste areas. Eggshell fragments and fluff from hatching trays (from the top, middle, and bottom of the stack) of the hatcher interior and macerator of the waste area were collected by placing approximately 50 g samples in sterile Whirlpac bags, respectively. Samples from chick sorting areas, chick boxes, and ventilation outlets were collected by swabbing using four premoistened gauze pads with sterile BPW. All samples were taken directly and divided into two 225 ml BPW solutions, respectively, as described above.
Sample collection: broiler farms
Cloacal swabs, cecal droppings, and dust on the wall were taken for investigation. Samples were collected by the same method as that described at breeder farms.
Sample collection: chicken slaughterhouses
The first chilling water, the third chilling water, and five carcasses were taken for investigation. Chilling water was collected by placing approximately 50 ml into a sterile specimen cup. A carcass rinse was collected from the rehang belt prior to the rehanging of carcasses on the drip line. Each carcass was aseptically placed into a vacuum bag (Cryovag; Sealed Air, USA), and 400 ml of sterile BPW was added to the bag. The bag was shaken 50 times, the carcass was replaced on the line, and approximately 50 ml of rinse water were poured into a sterile specimen cup. All samples were taken directly and divided into two 225 ml BPW solutions, respectively, as described above.
Isolation and identification of Salmonella
Samples that were collected in 225 ml BPW were taken to the laboratory under ambient conditions on the day of collection and incubated at 37℃ for 18 h. After preenrichment, 0.1 ml of the broth was transferred into a 10 ml Rappaport-Vassiliadis broth (RV broth; Difco, USA), which was prepared according to the instructions on the package. The RV broth was incubated overnight at 41.5. The RV broth samples were streaked onto Ramback agar (Difco, USA) and incubated overnight at 37℃.
Two typical colonies were picked and transferred to MacConkey agar (Difco, USA) for pure culturing and incubated overnight at 37℃. Samples on the MacConkey agar reacted with Salmonella O antiserum (Difco, USA). Colonies showing typical agglutination by O antiserum were serotyped with Salmonella H antiserum (Difco, USA).
Pulsed-field gel electrophoresis (PFGE)
A total of 22 S. enteritidis isolates from different sources at two integrated broiler chicken companies were used. PFGE was performed according to the 'One-Day (24-28 h) Standardized Laboratory Protocol for Molecular Subtyping of Non-typhoidal Salmonella by PFGE' [6] on a CHEF Mapper XA system (Bio-Rad Laboratories, USA). PFGE patterns were obtained with the XbaI restriction enzyme, and pulse times were ramped from 2.2 to 63.8 s during an 18 h run at 6.0 V/cm.
Results
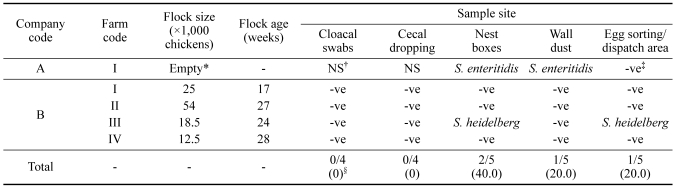
Table 1 shows the results of Salmonella isolation from five breeder farms. One farm of company A was sampled after cleansing and disinfecting because birds were fully removed, but S. enteritidis was found in the residual dust of the nest box and on the wall. In one of four farms of integrated broiler company B, S. heidelberg was only found in one nest box and in the egg sorting and dispatch area.
Table 1.
Distribution and serotypes of Salmonella spp. in breeder farms of two integrated broiler companies
*The litter on which the birds were kept was fully removed, and cleaning and disinfection of the house were carried out.
†NS, not sampled.
‡-ve, negative results in Salmonella culture.
§Number of isolates that were positive for Salmonella/number of farms tested (%).
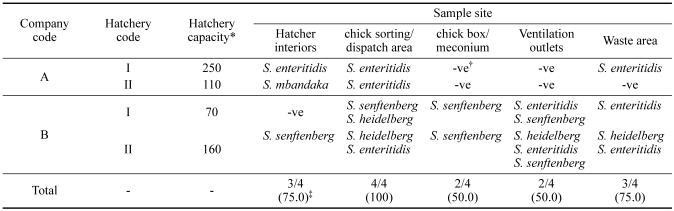
Table 2 shows the results of Salmonella isolation from four hatcheries. Salmonella isolates were recovered from all of the hatcheries. In one of two hatcheries of company A, S. enteritidis was found in the hatcher interior, chick sorting area, and waste area. In another hatchery, S. mbandaka was found in the hatcher interior, whereas S. enteritidis was also found in the chick sorting area. A total of three different serotypes, S. enteritidis, S. heidelberg, and S. senftenberg, were consistently found in the hatcheries of integrated broiler company B. For the four hatcheries, the samples types with the greatest frequency of Salmonella were obtained from the chick sorting and dispatch areas (100%). The frequency of Salmonella in the hatcher interiors, chick boxes and meconium, and waste area were 75, 50, and 75%, respectively.
Table 2.
Distribution and serotypes of Salmonella spp. in hatcheries of two integrated broiler companies
*×1,000 eggs/week.
†-ve, negative results in Salmonella culture.
‡Number of isolates that were positive for Salmonella/number of hatcheries tested (%).
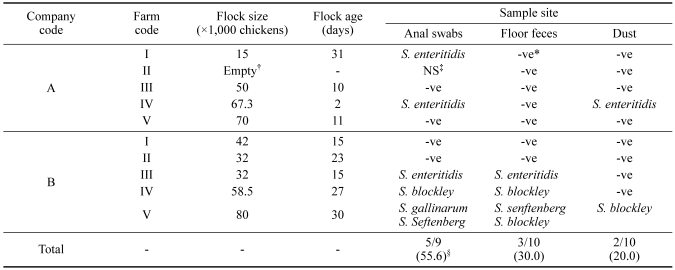
Table 3 shows the results of isolation for Salmonella at a total of ten separate broiler commercial farms owned by two companies. Of the five farms owned by company A, S. enteritidis was found on two farms. Of the farms owned by company B, two of the five farms tested positive for Salmonella. A wide variety of Salmonella serotypes was present on the farms. S. enteritidis and S. blockley were found on one of the farms. On another farm, three Salmonella serotypes, S. gallinarum, S. blockley, and S. senftenberg, were obtained from cloacal swabs, cecal droppings, and dust on the wall, respectively. The frequencies of Salmonella isolates found by sample type for cloacal swabs, cecal droppings, and dust were 55.6, 30, and 20%, respectively.
Table 3.
Distribution and serotypes of Salmonella spp. in commercial broiler farms of two integrated broiler companies
*-ve, negative results in Salmonella culture.
†The litter on which the birds were kept was fully removed, and cleaning and disinfection of the house were carried out.
‡NS, not sampled.
§Number of isolates that were positive for Salmonella/number of farms tested (%).
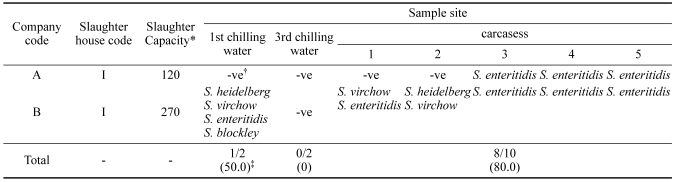
Table 4 shows the results of Salmonella isolation from chicken slaughterhouses owned by two separate companies. S. enteritidis was only found in three of five carcasses taken from the slaughterhouse of company A. No cases of Salmonella were found in the first or third chilling water. By contrast, a total of four different serotypes, S. heidelberg, S. virchow, S. enteritidis, and S. blockley were found in the first chilling water of company B. Salmonella was also found in all of the tested carcasses. S. enteritidis, S. virchow, and S. heidelberg isolates were recovered.
Table 4.
Distribution and serotypes of Salmonella spp. in chicken slaughterhouses of two integrated broiler companies
*×1,000 chickens/day.
†-ve, negative results in Salmonella culture.
‡Number of isolates that were positive for Salmonella/number of farms tested (%).
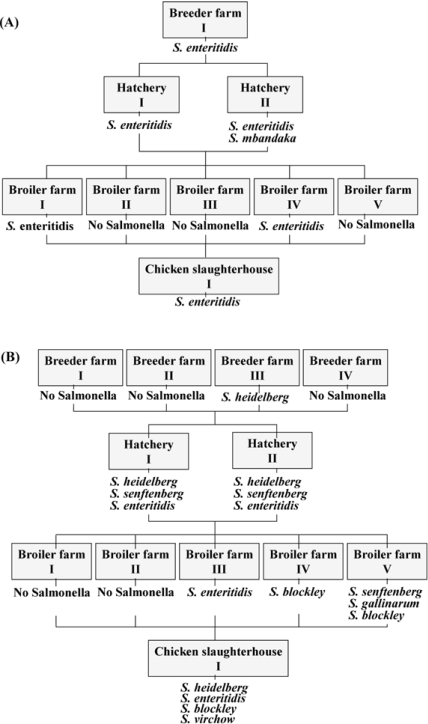
Fig. 1 shows the results of the transmission of Salmonella via an integrated broiler chicken operation. A total of two different serotypes were observed in isolates from integrated broiler chicken company A. S. enteritidis, the predominant company A isolate, was consistently found in isolates from the breeder farm, hatcheries, broiler farms, and chicken slaughterhouse. But S. mbandaka was only found at one hatchery. In company B, a total of six different serotypes, S. heidelberg, S. senftenberg, S. enteritidis, S. blockley, S. gallinarum, and S. virchow, were observed. Although S. heidelberg was not detected at the broiler farms, it was consistently found at the breeder farm, the hatcheries, and the chicken slaughterhouse. S. enteritidis was also found in the hatcheries, the broiler farm, and the chicken slaughterhouse. S. senftenberg was detected in the hatcheries and at one broiler farm, and S. blockley, which was observed at two broiler farms, was also found at the chicken slaughterhouse. S. gallinarum and S. virchow were found at one broiler farm and at the chicken slaughterhouse, respectively.
Fig. 1.
Transmission of Salmonella in the integrated broiler chicken companies. (A) The results for integrated broiler chicken company A. (B) The results for integrated broiler chicken company B.
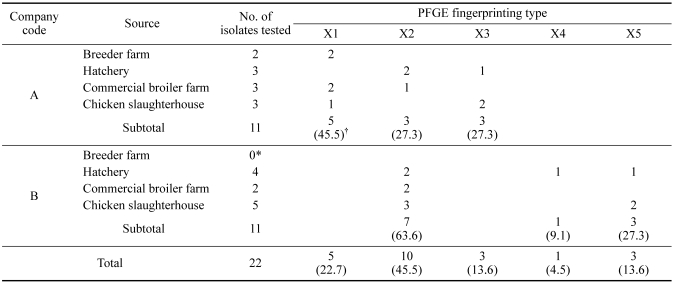
In order to determine the genetic clonality, chromosomal DNAs of 11 S. enteritidis isolates originating from integrated broiler company A and 11 S. enteritidis isolates from company B were digested with XbaI and analyzed by PFGE (Fig. 2). Ten of the 22 analyzed strains belonged to a pattern termed as X2, which was the major pattern. However, the predominant pattern of company A was pattern X1 (45.5%), whereas that of company B was pattern X2 (63.6%). In addition, pattern types X1 and X3 were found only in S. enteritidis of company A, and patterns X4 and X5 were observed only in company B. A difference in the PFGE pattern was found to be related to the origin of the integrated broiler operation.
Fig. 2.
Pulsed field gel electrophoresis patterns of S. enteritidis isolates obtained with the XbaI restriction enzyme. M: Lambda ladder marker for PFGE; Lane 1 to 11: S. enteritidis isolated from integrated broiler company A; Lane 12 to 22: S. enteritidis isolated from integrated broiler company B.
Discussion
Wilson [22] concluded that Salmonella infection in elite and grandparent chicken breeding flocks was extremely rare and was not considered to be a source of infection for the industry as a whole. However, a small number of cases of Salmonella have occurred in parent flocks in recent years [3], and previous research has demonstrated the potential for the spread of infection on both national and international scales [5,15]. In the structure of the chick supply and distribution chain, a single infected breeding flock may have a significant effect on the level of infection in commercial flocks [21].
In this study, Salmonella was found in breeder farms, hatcheries, commercial broiler farms, and chicken slaughterhouses. Davies et al. [10] investigated a company experiencing repeated S. enteritidis infection at broiler breeder sites, and revealed a variety of routes by which infection may have been re-circulating within the company. Even one infected breeding flock is capable of causing widespread distribution of contamination before it is detected [21]. Thus, the presence of several infected flocks increases this risk.
The critical role of the hatchery in disseminating Salmonella to commercial birds and possibly exposing parent flocks to contamination on egg trays, trolleys, and vehicles has also been described previously [8-10]. Most of these works have focused on the potential for crosscontamination and infection caused by a low number of organisms in chicks during incubation [13]. Problems with the washing and disinfection of crates in hatcheries, although not as severe as the problems observed in poultry abattoirs [7], have also been noted previously, as has long-term persistence of Salmonella in hatchery incubator ventilation ducting [9]. In the current study, all of four hatcheries tested were contaminated with Salmonella, although formaldehyde evaporation is normally used during hatching.
The persistence of a low level of Salmonella in the commercial broiler flocks, despite antibiotic and competitive exclusion treatment, demonstrates the importance of preventing infection rather than attempting to control it, and affects chicken slaughterhouses. This involves the development of a rational, risk-based approach to monitor and prevent infection throughout the entire breeding and production chain [3,18].
Other investigators have found the role of the hatchery to be less important. Although Lahellec and Colin [16] found a considerable amount of Salmonella in the hatchery when isolates were serotyped, they found those isolates originating from the hatchery to be less important in the final product than those present in the grow-out house prior to the placement of young chicks, or those introduced into the grow-out house by vectors during rearing. Bailey et al. [3] identified many sources of Salmonella throughout the breeding and production chain, but they did not determine the contribution of the previous grow-out environment.
In this study, S. enteritidis was isolated from one breeder farm of integrated broiler chicken company A, as well as from two hatcheries, two commercial broiler farms, and a chicken slaughterhouse. For company B, S. heidelberg was found at one breeder farm, but was not found at the five commercial broiler farms. S. heidelberg was found at two hatcheries and one chicken slaughterhouse. S. enteritidis was found in hatcheries, and was also discovered at the broiler farm and slaughterhouse, but was not found at the breeder farms. These results show that breeder farms and hatcheries play an important role in the epidemiology of Salmonella contamination within the poultry industry.
In the current study, S. enteritidis was found in the dust of nest boxes and on the walls of a breeder farm, which were cleaned and disinfected after the litter fully removed. Previous studies have shown that Salmonella can survive for long periods in contaminated livestock houses [2,4], and S. enteritidis PT4 has been shown to persist for at least a year in depopulated poultry houses and for 26 months in artificially-contaminated poultry feed [11]. In another study, S. dublin survived for nearly 6 years in manure that was artificially contaminated with 107 colony-forming units per g [19]. Although Salmonella can survive desiccation better than most other coliforms [17], overall survival in dust in the current study was lower than that seen in floor-level samples. This may have been the result of lower Salmonella numbers found in dust from non-intensively housed flocks compared with residual fecal and floor materials. In addition, S. enteritidis can survive longer in chicken houses than in open paddocks. This is likely to be related to protection from sunlight, as Salmonella in contaminated material that is placed in shady areas survives for much longer than in materials exposed to sunlight [9].
The present investigation also suggested that the strains of S. enteritidis isolated in Korea have somewhat different PFGE patterns according to the origin of the integrated broiler operation. Clearly, these data support the critical need to control Salmonella in breeder farms and hatcheries, and demonstrate important points for the control of infection in large-scale poultry operations in Korea.
Table 5.
Distributions of the S. enteritidis PFGE patterns of the integrated broiler chicken companies
*S. enteritidis was not isolated from the source.
†No. of isolates typed (%).
References
- 1.Angen Ø, Skov MN, Chriel M, Agger JF, Bisgaard M. A retrospective study on Salmonella infection in Danish broiler flocks. Prev Vet Med. 1996;26:223–237. [Google Scholar]
- 2.Bailey JS. Control of Salmonella and Campylobacter in poultry production. A summary of work at Russell Research Center. Poult Sci. 1993;72:1169–1173. doi: 10.3382/ps.0721169. [DOI] [PubMed] [Google Scholar]
- 3.Bailey JS, Cox NA, Craven SE, Cosby DE. Serotype tracking of Salmonella through integrated broiler chicken operations. J Food Prot. 2002;65:742–745. doi: 10.4315/0362-028x-65.5.742. [DOI] [PubMed] [Google Scholar]
- 4.Bale MJ, Bennett PM, Beringer JE, Hinton M. The survival of bacteria exposed to desiccation on surfaces associated with farm buildings. J Appl Bacteriol. 1993;75:519–528. doi: 10.1111/j.1365-2672.1993.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 5.Baumler AJ, Hargis BM, Tsolis RM. Tracing the origins of Salmonella outbreaks. Science. 2000;287:50–52. doi: 10.1126/science.287.5450.50. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Standardized Molecular Subtyping of Foodborne Bacterial Pathogens by Pulsed-Field Gel Electrophoresis. Atlanta: National Center for Infectious Disease; 2000. [Google Scholar]
- 7.Corry JEL, Allen VM, Hudson WR, Breslin MF, Davies RH. Sources of Salmonella on broiler carcasses during transportation and processing: modes of contamination and methods of control. J Appl Microbiol. 2002;92:424–432. doi: 10.1046/j.1365-2672.2002.01543.x. [DOI] [PubMed] [Google Scholar]
- 8.Cox NA, Bailey JS, Berrang ME. Bactericidal treatment of hatching eggs I. chemical immersion treatments and Salmonella. J Appl Poult Res. 1998;7:347–350. [Google Scholar]
- 9.Davies R, Breslin M. Environmental contamination and detection of Salmonella enterica serovar enteritidis in laying flocks. Vet Rec. 2001;149:699–704. [PubMed] [Google Scholar]
- 10.Davies RH, Nicholas RAJ, McLaren IM, Corkish JD, Lanning DG, Wray C. Bacteriological and serological investigation of persistent Salmonella enteritidis infection in an integrated poultry organisation. Vet Microbiol. 1997;58:277–293. doi: 10.1016/s0378-1135(97)00157-0. [DOI] [PubMed] [Google Scholar]
- 11.Davies RH, Wray C. An approach to reduction of Salmonella infection in broiler chicken flocks through intensive sampling and identification of cross-contamination hazards in commercial hatcheries. Int J Food Microbiol. 1994;24:147–160. doi: 10.1016/0168-1605(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 12.Gast RK. Understanding Salmonella enteritidis in laying chickens: the contributions of experimental infections. Int J Food Microbiol. 1994;21:107–116. doi: 10.1016/0168-1605(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 13.Holt PS, Gast RK, Porter RE, Jr, Stone HD. Hyporesponsiveness of the systemic and mucosal humoral immune systems in chickens infected with Salmonella enterica serovar enteritidis at one day of age. Poult Sci. 1999;78:1510–1517. doi: 10.1093/ps/78.11.1510. [DOI] [PubMed] [Google Scholar]
- 14.Keller LH, Schifferli DM, Benson CE, Aslam S, Eckroade RJ. Invasion of chicken reproductive tissues and forming eggs is not unique to Salmonella enteritidis. Avian Dis. 1997;41:535–539. [PubMed] [Google Scholar]
- 15.Lachoncha I, Baggesen DL, Rementeria A, Garaizar J. Genotypic characterisation by PFGE of Salmonella enterica serotype Enteritidis phage types 1, 4, 6, and 8 isolated from animal and human sources in three European countries. Vet Microbiol. 2000;75:155–165. doi: 10.1016/s0378-1135(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 16.Lahellec C, Colin P. Relationship between serotypes of Salmonellae from hatcheries and rearing farms and those from processed poultry carcases. Br Poult Sci. 1985;26:179–186. doi: 10.1080/00071668508416802. [DOI] [PubMed] [Google Scholar]
- 17.Mackey BM, Derrick CM. The effect of sublethal injury by heating, freezing, drying and gamma radiation on the duration of the lag phase of Salmonella typhimurium. J Appl Bacteriol. 1982;53:243–251. doi: 10.1111/j.1365-2672.1982.tb04683.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien JPD. Aspect of Salmonella enteritidis control in poultry. Worlds Poult Sci J. 1990;46:119–124. [Google Scholar]
- 19.Plym-Forshell L, Ekesbo I. Survival of Salmonellas in urine and dry faeces form cattle-an experimental study. Acta Vet Scand. 1996;37:127–131. doi: 10.1186/BF03548104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose N, Beaudeau F, Drouin P, Toux JY, Rose V, Colin P. Risk factors for Salmonella enterica subsp. enterica contamination in French broiler-chicken flocks at the end of the rearing period. Prev Vet Med. 1999;39:265–277. doi: 10.1016/s0167-5877(99)00002-1. [DOI] [PubMed] [Google Scholar]
- 21.van de Giessen AW, Peters R, Berkers PA, Jansen WH, Notermans SH. Salmonella contamination of poultry flocks in The Netherlands. Vet Q. 1991;13:41–46. doi: 10.1080/01652176.1991.9694283. [DOI] [PubMed] [Google Scholar]
- 22.Wilson S. Control of Salmonella enteritidis in poultry. Vet Rec. 1989;125:465–466. doi: 10.1136/vr.125.18.465. [DOI] [PubMed] [Google Scholar]